The endothelium expresses a large repertoire of genes under apparent transcriptional control of biomechanical forces, many of which are neither cell-type nor flow specific. We set out to identify genes that are uniquely flow responsive in human vascular endothelial cells. Transcriptional profiling using commercial DNA microarrays identified 12 of 18 000 genes that were modulated at least 5-fold after 24 hours of steady laminar flow (25 dyne/cm2). After a 7-day exposure to unidirectional pulsatile flow (19 ± 12 dyne/cm2), only 3 of 12 remained elevated at least 5-fold. A custom microarray of ∼300 vascular cell–related gene fragments was constructed, and expression analysis revealed that many flow-induced genes are also induced by at least one of the following agents: tumor necrosis factor–α (TNF-α), interleukin-1β (IL-1β), transforming growth factor-β, vascular endothelial growth factor, or thrombin, indicating a more general role in adaptive or stress responses. Most flow-induced genes were also induced by TNF-α but not IL-1β, suggesting the involvement of reactive oxygen species. A limited panel of genes that are unique for flow-exposed cultures was identified, including lung Krüppel-like factor (LKLF/KLF2) and cytochrome P450 1B1 (CYP1B1). In marked contrast, both these genes were substantially repressed by TNF-α. LKLF but not CYP1B1 mRNA was detected exclusively in the vascular endothelium of healthy human aorta by in situ hybridization and appeared to be flow regulated. To date LKLF is the first endothelial transcription factor that is uniquely induced by flow and might therefore be at the molecular basis of the physiological healthy, flow-exposed state of the endothelial cell.
Introduction
Atherosclerosis is a chronic multifactorial disease of the arteries that initiates and develops from young age until it manifests itself clinically later in human life. Although the risk factors for atherosclerosis are of a systemic nature, the localization of lesions is confined to specific and reproducible positions in the arterial tree. The hypothesis that this focality of atherosclerosis is caused by local blood flow turbulence at vessel bifurcations and curvatures has been firmly supported over the last decades.1,2 Lowering of the shear stress on the vascular endothelium at sites of flow turbulence, creating steep shear-stress gradients,3 is now believed to be one of the initiating factors in atherogenesis. Consequently, the ability of the atheroprotective force of shear stress to modulate the transcription of genes expressed by endothelial cells, denoted endothelial genes, has triggered ample research effort on the identification of shear-stress–regulated genes.2,4 In contrast to the protective function of shear stress, the cytokine tumor necrosis factor–α (TNF-α) is an important mediator of the inflammatory process that occurs during the progression of atherosclerosis.5 Produced by macrophages that have infiltrated the lesion, cytokines like TNF-α are known to induce the expression of many endothelial genes that contribute to the complex processes that are involved in atherogenesis.6 In contrast to shear stress, cytokines are therefore mostly considered to be proatherogenic factors.5
Several studies have been aimed at the identification of endothelial genes that are regulated by arterial levels of shear stress.7,8 These studies have typically focused on the identification of genes that are selectively induced by laminar but not by turbulent flow. Well-known examples include the transcriptional regulation of various adhesion molecules like vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) and several atheroprotective genes involved in, for instance, handling oxidative stresses; for example, superoxide dismutases.7,9,10 Most of these genes can be considered atheroprotective solely on the basis of their function and thus would be significant in a physiological context. Their regulation patterns, however, reveal that they are expressed in a variety of cell types and are also induced in response to various cytokines believed to be primarily atherogenic, including TNF-α.6
In this study, we took an alternative approach to identify genes that are consistently expressed in endothelial cells exposed to prolonged laminar flow and not in static cultures. Thus, we aimed at identifying those genes that are truly discriminative for the physiological long-term flow-exposed state of the endothelial cell and potentially lie at the basis of the phenotypic changes induced by laminar flow. This rationale is essentially based on the contrasting difference between the in vivo protective effect of shear stress and the atherogenic potential of inflammatory cytokines exclusively at sites of flow turbulence in healthy and atherosclerotic tissue, respectively. Our stringent selection scheme consisted of preselecting flow-regulated candidate genes and focusing on genes that are still induced after exposing endothelial cells to flow for 7 days. Absence of a transcriptional response to a variety of atherosclerosis-related stimuli was taken as a final criterion. Our results confirm the existence of a set of highly flow-induced flow- and endothelial-specific genes and demonstrate that the expression of one such gene, the transcription factor lung Krüppel-like factor (LKLF), occurs only with prolonged shear stress in vitro and in vivo.
Materials and methods
Cell culture and shear-stress experiments
Human umbilical vein endothelial cells (HUVECs) were isolated as described6,11 and cultured in Medium-199 (GIBCO-BRL, Paisley, Scotland), supplemented with 20% (vol/vol) fetal bovine serum, 50 μg/mL heparin (Sigma, St Louis, MO), 6-25 μg/mL endothelial cell growth supplement (ECGS; Sigma), and 100 U/mL penicillin/streptomycin (GIBCO-BRL). Cells at passage levels 1 to 2 were plated on fibronectin-coated Thermanox coverslips (NUNC, Napierville, IL) in growth medium containing 6 μg/mL ECGS 24 hours before the exposure to shear stress. Shear stress was applied in a parallel plate-type flow chamber as described with either steady flow12 (25 dyne/cm2) or pulsatile flow (12 ± 7 dynes/cm2), using a CellMax Quad positive-displacement pump (Cellco, Germantown, MD). Afterward, total RNA was isolated using TRIZOL (GIBCO-BRL) according to the manufacturer's instructions. Alternatively, HUVECs were seeded into fibronectin-coated artificial capillary cartridges (Polypropylene 70, Cat No. 400-025; Cellco) in medium containing 8 μg/mL ECGS.13 Using the CellMax Quad pump system, flow was gradually increased to correspond to a pulsatile shear stress of 19 ± 12 dynes/cm,2 which was maintained over the next 7 days with intermediary medium changes. Flow pulsatility was assessed using a T206 Transonic flow meter (Transonic Systems, Ithaca, NY). Simultaneously, inlet pressure of the culture chambers was measured by a dome-type pressure transducer connected to a Nihon-Kohden AP-621G bridge amplifier (Foothill Ranch, CA). Both signals were digitized at a sampling rate of 40 Hz, using Powerlab equipment (Grand Junction, CO). HL-60 and HeLa cells were cultured as described.14 15
Cytokine stimulation
Media on confluent HUVEC cultures (passages 1-2) were replaced with fresh full-growth medium containing 6 μg/mL ECGS 24 hours before cytokine stimulation. The human cytokines/growth factors TNF-α (50 ng/mL), interleukin-1β (IL-1β; 15 ng/mL), transforming growth factor–β1 (TGF-β1; 10 ng/mL) (R&D Systems, Minneapolis, MN), vascular endothelial growth factor (VEGF; 50 ng/mL) (PeproTech, Rocky Hill, NJ), or α-thrombin (5 U/mL; Sigma) were added directly to the culture medium. Total RNA was isolated using TRIZOL after incubation times of 2, 6, and 24 hours.
RNA amplification
Total RNA (∼4 μg) from shear-stress–exposed and static HUVEC cultures was amplified essentially as described.16Double-stranded cDNA was prepared using the SuperScript Plasmid System for cDNA Synthesis (GIBCO-BRL) with a T7(dT)15 primer. The cDNA was transcribed using the AmpliScribe T7 Transcription Kit (Epicentre Technologies, Madison, WI). The yield and size distribution of the resulting amplified antisense RNA (aRNA) was determined by measuring A260 and by electrophoresis on a 1% (wt/vol) agarose gel.
Probe synthesis and cDNA array hybridizations
For the 4-hour flow experiments, probes labeled with α-33P-dCTP (Amersham, Piscataway, NJ) were directly reverse transcribed from 4 μg aRNA using 3 μg random hexamers. Probes of the 24-hour flow experiments were transcribed from unlabeled first-strand cDNA (transcribed from 4 μg aRNA using 3 μg random hexamers) using 1 μg T7(dT)15 primer and SuperScript II RT (GIBCO-BRL). These first- and second-strand cDNA probes were hybridized for 60 hours to the filter-based Gene Discovery Array (GDA) I Version 1.3 (Incyte Genomics, Palo Alto, CA), according to the manufacturer's instructions. The filters were imaged on a Storm PhosphorImager (Molecular Dynamics, Sunnyvale, CA), scanned with 50-micron resolution, and quantified at Incyte Genomics. Selected differential clones were obtained from Incyte Genomics and sequence verified.
Construction of custom microarrays
A custom cardiovascular glass-based microarray was constructed containing a comprehensive set of prominent endothelial genes and general vascular genes from previous studies, for example, genes encoding various proteoglycans; factors involved in thrombosis/fibrinolysis, cell shape, and vesicular transport/transcytosis; and control genes for normalization purposes.6,7,17,18 Polymerase chain reaction (PCR)–amplified gene-specific fragments of these genes, deprived of any repetitive or highly homologous sequences, were spotted on glass microscope slides in triplicate and hybridized with fluorescent cDNA probes using the methods previously described by Brown and coworkers (detailed protocols taken from the web sitehttp://cmgm.stanford.edu/pbrown/protocols/index.html).19Arrays were scanned on a ScanArray 3000 microarray scanner (GSI Lumonics, Bedford, MA), and fluorescence signals were background corrected and normalized for glyceraldehyde phosphate dehydrogenase (GAPDH). All analyses were performed with the Eisen software package Scanalyze, Cluster, and TreeView, as described (http://rana.lbl.gov).20
Semiquantitative real-time RT-PCR
Reverse transcription of 3 μg of total RNA was performed with 1 μg (dT)12-18 primer (GIBCO-BRL) using SuperScript II. Real-time reverse transcriptase–polymerase chain reactions (RT-PCRs) were performed using the FastStart DNA Master SYBR Green I kit (Roche, Mannheim, Germany) in the LightCycler System (Roche). Primers were LKLF, (forward) 5′-GCACGCACACAGGTGAGAAG-3′ and (reverse) 5′-ACCAGTCACAGTTTGGGAGGG-3′; CYP1B1, (forward) 5′-CTCCTCCTCTTCACCAGGTATCC-3′ and (reverse) 5′-AACCACAGTGTCCTTGGGAATG-3′. The PCR efficiency, determined for each primer pair separately using a series of cDNA dilutions, was used to calculate relative differences between samples, which were expressed as ratios compared to the static controls.
Nonradioactive mRNA in situ hybridization
Vascular tissues were fixed and paraffin embedded as described,6 and 16 μm sections were mounted onto SuperFrost Plus microscope slides (Menzel-Gläser, Braunschweig, Germany). The in situ hybridizations were performed as described.21 Riboprobes were derived from the following cDNA fragments: 460–base pair (bp) BstNI-BstNI fragment of the LKLF cDNA (GenBank, H28611), entire 1350-bp insert of a human claudin-5 cDNA clone (GenBank, R60153), entire 1790-bp insert of a CYP1B1 cDNA clone (GenBank, N72909), 192-bp fragment of human von Willebrand factor cDNA 8239-8442 (GenBank, X04385). All cDNA clones were obtained from either Incyte Genomics or as IMAGE-consortium cDNA clones22 from the United Kingdom Human Genome Mapping Project Resource Centre (Cambridge, United Kingdom).
Results
Identification of shear-stress–responsive endothelial genes
We first set out to identify endothelial genes that are highly shear-stress–regulated. For that purpose we have performed transcriptional profiling using commercially available cDNA arrays containing approximately 18 000 cDNA clones. Primary HUVECs were subjected to laminar flow in a parallel plate-type perfusion chamber, generating a shear stress of 25 dyne/cm.2 For use as controls, HUVECs were cultured in parallel under static conditions. Cells were exposed to flow for 4 or 24 hours to evaluate early responses versus the effect of longer-term flow exposures. Subsequently, total RNA was isolated and amplified in one round of T7 RNA polymerase-based amplification yielding antisense RNA (aRNA).16 Reverse transcription of the aRNA was performed to obtain 33P-labeled cDNA probes, which were hybridized with the cDNA arrays. The hybridized arrays were quantified, background corrected, and normalized. A plot was constructed comparing the signal intensities of all spots in the shear-exposed situation to the static cultures (Figure 1A). This plot shows that the vast majority of the genes present on the array are only marginally modulated after a 24-hour flow exposure, and lie between the 5-fold up-down- regulation lines. Approximately 4,000 of 18 000 genes (∼20%) were significantly expressed, of which about 230 (∼5.7%) appeared more than 2-fold induced or repressed. Next, for each gene on the array the ratios were calculated of the hybridization signal intensities of the 4- and 24-hour shear experiments over their corresponding static controls. To score genes as significantly shear-stress–regulated, 2 stringent selection criteria were used. First, genes had to be at least 5-fold differentially expressed, that is, ratios > 5 or < 0.2. Second, background-corrected hybridization signals had to be above the selected intensity threshold in either the shear-exposed or static conditions, that is, > 1.5 · 104 for the 4-hour and > 5 · 103 for the 24-hour shear experiments (outside the gray area in Figure 1A). Of 18 000 GDA filter array clones, only 29 met these criteria. As the GDA arrays contain nonsequence verified clones, with an estimated 30% incorrect annotations, all 29 clones were ordered and their identity established by resequencing, and PCR fragments were analyzed by filter-based spot blot analysis. Thus, we could confirm shear responsiveness for 12 of these genes, of which the expression kinetics are shown in Figure 1B-D. Three different kinetic classes of gene regulation can be distinguished: genes that are quickly induced and reach a maximum response within 6 hours (Figure 1C), genes that have a delayed response and continue to increase in a linear fashion beyond 24 hours of shear-stress exposure (Figure 1B), and the down-regulated gene claudin-5 (Figure 1D).
Gene expression profile of laminar flow–exposed HUVECs determined by cDNA array hybridization.
(A) The plot shows a log-log comparison of the 24-hour shear-stress–exposed versus static cultures of background corrected and normalized hybridization signals of all ∼18 000 cDNAs on the array. Diagonal lines represent the following: dashed line, unity line with equal expression in both conditions; +5/−5 and +10/−10 labeled lines, 5- and 10-fold up- and down-regulation. Gray boxes represent hybridization signals that are below the limits of significant expression (<5 × 103). Clones more than 5-fold differentially expressed were picked, re-arrayed on a custom microarray, checked for reproducibility of induction (B-D), and sequence verified. (B-D) The kinetics of transcriptional regulation by steady laminar flow (25 dyne/cm2) for 0, 2, 6, and 24 hours were determined for the 12 highly shear-stress–responsive genes (Table1). (B) Late flow-responsive genes DIA4 (diaphorase),SAT (spermidine/spermine N1-acetyltransferase),FTL (ferritin light polypeptide), TXNRD1(thioredoxin reductase 1), AF1Q (ALL1-fused gene from chromosome 1q), SLC7A11 (solute carrier family 7), and GenBank T80319 (hypothetical protein). (C) Immediate-early induced genes KLF2 (LKLF), CYP1B1 (cytochrome P450 1B1),CDKN1A (cyclin-dependent kinase inhibitor 1A [p21]), andGJA5 (connexin-40). (D) Down-regulated gene CLDN5(claudin-5). Hybridization signals shown are the mean ± SD of duplo spots and expressed as percentage of the GAPDH signal.
Gene expression profile of laminar flow–exposed HUVECs determined by cDNA array hybridization.
(A) The plot shows a log-log comparison of the 24-hour shear-stress–exposed versus static cultures of background corrected and normalized hybridization signals of all ∼18 000 cDNAs on the array. Diagonal lines represent the following: dashed line, unity line with equal expression in both conditions; +5/−5 and +10/−10 labeled lines, 5- and 10-fold up- and down-regulation. Gray boxes represent hybridization signals that are below the limits of significant expression (<5 × 103). Clones more than 5-fold differentially expressed were picked, re-arrayed on a custom microarray, checked for reproducibility of induction (B-D), and sequence verified. (B-D) The kinetics of transcriptional regulation by steady laminar flow (25 dyne/cm2) for 0, 2, 6, and 24 hours were determined for the 12 highly shear-stress–responsive genes (Table1). (B) Late flow-responsive genes DIA4 (diaphorase),SAT (spermidine/spermine N1-acetyltransferase),FTL (ferritin light polypeptide), TXNRD1(thioredoxin reductase 1), AF1Q (ALL1-fused gene from chromosome 1q), SLC7A11 (solute carrier family 7), and GenBank T80319 (hypothetical protein). (C) Immediate-early induced genes KLF2 (LKLF), CYP1B1 (cytochrome P450 1B1),CDKN1A (cyclin-dependent kinase inhibitor 1A [p21]), andGJA5 (connexin-40). (D) Down-regulated gene CLDN5(claudin-5). Hybridization signals shown are the mean ± SD of duplo spots and expressed as percentage of the GAPDH signal.
Gene transcription levels after long-term adaptation to flow
Currently, no conclusive data are available on the time required for endothelial cells to fully adapt to flow in vitro. Life-long exposure of the endothelium to flow in vivo inhibits proliferation and lowers the metabolic rate, which is consistent with a resting phenotype.23 Therefore, sustained flow-regulated expression of the 12 flow-responsive and additional vascular genes was studied in an artificial capillary flow system using custom-made cardiovascular microarrays. This flow system allows culturing of endothelial cells under continuous unidirectional pulsatile laminar flow for long periods of time.13 In addition, cells are grown in a polar fashion on the luminal surface of permeable hollow fibers, which are in contact with a separate extracapillary (subendothelial) compartment. Thus, a more physiological model system is created compared to endothelial cells cultured on flat, solid supports. The time-dependent change of flow in the system was accurately measured and revealed a pulsatile, unidirectional flow profile generating a minimal, maximal, and mean wall shear stress of 8, 32, and 19 dynes/cm2 (19 ± 12 dynes/cm2), respectively (Figure 2). The capillaries were seeded with primary HUVECs and exposed to flow for 7 days after a 24-hour period of gradual flow increase, allowing cell attachment and growth. Next, total RNA was extracted and used in hybridization experiments with custom microarrays (construction and content is described in “Materials and methods” and footnotes of Table1). These arrays comprise a selection of ∼300 genes, facilitating a simultaneous quantitative analysis of the expression of the 12 shear-stress–responsive genes identified in this study and various additional genes/pathways that play prominent roles in endothelial cell biology. Table 1 shows the expression ratios calculated from 3 independent sustained-flow experiments and their control static cultures only for the 68 genes whose expression level exceeded 5% of the hybridization signal of GAPDH in at least the flow or static condition. Overall, the expression of 9 of the ∼300 genes that are present on the array was increased at least 3-fold by sustained flow compared to static cultures. These expression analyses also show that 3 of the 12 genes, which were identified in this study after a 24-hour exposure to flow, were no longer significantly “differential” after chronic flow exposure. The induction levels of 6 genes had dropped to between 2- and 5-fold, but the expression of 3 genes remained elevated at least 5-fold after prolonged flow: lung Krüppel-like factor (LKLF/KLF2), cytochrome P450 1B1 (CYP1B1), and diaphorase 4 (DIA4/NQO1).
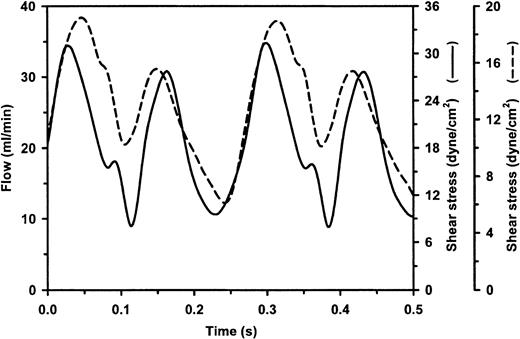
Characterization of unidirectional pulsatile flow in the artificial capillary flow system and parallel plate flow chamber.
The time-dependent change in flow was measured in the artificial capillary flow system (solid line) and parallel plate-type flow chamber (dashed line) as described in “Materials and methods,” revealing a unidirectional sinusoidal pulsatile flow profile. Flow is expressed in mL/min and was used to calculate the corresponding wall shear stress in the artificial capillaries (330 μm diameter) and parallel plate chamber (550 μm height; 0.98 cm wide) expressed in dyne/cm2 on 2 separate axes. Calculated mean, minimal, and maximal shear stresses were 19, 8, and 32 dyne/cm,2respectively, for the artificial capillaries and 12, 6, and 19 dyne/cm,2 respectively, for the parallel plate flow device.
Characterization of unidirectional pulsatile flow in the artificial capillary flow system and parallel plate flow chamber.
The time-dependent change in flow was measured in the artificial capillary flow system (solid line) and parallel plate-type flow chamber (dashed line) as described in “Materials and methods,” revealing a unidirectional sinusoidal pulsatile flow profile. Flow is expressed in mL/min and was used to calculate the corresponding wall shear stress in the artificial capillaries (330 μm diameter) and parallel plate chamber (550 μm height; 0.98 cm wide) expressed in dyne/cm2 on 2 separate axes. Calculated mean, minimal, and maximal shear stresses were 19, 8, and 32 dyne/cm,2respectively, for the artificial capillaries and 12, 6, and 19 dyne/cm,2 respectively, for the parallel plate flow device.
Genes expressed by endothelial cells and their transcriptional regulation by prolonged unidirectional pulsatile flow
| Protein name . | Gene name . | GenBank* . | Ratio ± SEM† . | Cat‡ . | Protein function . |
|---|---|---|---|---|---|
| β-Actin | ACTB | NM_001101 | 1.1 ± 0.07 | F | Cytoskeletal protein |
| Activin βA | INHBA | NM_002192 | 3.1 ± 1.29 | E | Vascular growth factor |
| ALL1-fused gene from chromosome 1q | AF1Q | NM_006818 | 1.4 ± 0.43 | A | Unknown |
| Amyloid β A4 precursor protein | APP | NM_000484 | 2.3 ± 0.49 | D | Alzheimer disease/hemostasis |
| Annexin II | ANXA2 | NM_004039 | 1.2 ± 0.06 | D | Membrane-binding protein |
| Annexin V | ANXA5 | NM_001154 | 2.2 ± 0.14 | D | Anticoagulant protein |
| ApoL1 | APOL1 | NM_003661 | 2.0 ± 0.19 | C | HDL-associated plasma protein |
| ApoL2 | APOL2 | NM_030882 | 1.7 ± 0.42 | C | Homolog of ApoL1 |
| ApoL3 (CG12_1) | APOL3 | NM_014349 | 3.9 ± 1.40 | C | Homolog of ApoL1 |
| AT7_4_3 | No match | 0.4 ± 0.10 | C | Unknown | |
| Biglycan | BGN | NM_001711 | 1.3 ± 0.41 | D | Extracellular matrix constituent |
| Caveolin 1 | CAV1 | NM_01753 | 0.6 ± 0.13 | D | Main component of caveolae |
| Claudin 5 | CLDN5 | NM_003277 | 2.1 ± 0.43 | A | Tight junction protein |
| Cockayne syndrome, type I | CKN1 | NM_000082 | 5.7 ± 2.35 | C | RNA polymerase II transcription |
| Connexin 40 | GJA5 | NM_005266 | 3.2 ± 0.93 | A | Gap junction protein |
| Cyclin-dependent kinase inhibitor 1A (p21) | CDKN1A | NM_000389 | 1.7 ± 0.62 | A | Cell-cycle regulation |
| Cytochrome P450 1B1 | CYP1B1 | NM_000104 | 26.9 ± 0.08 | A | Hormone metabolism |
| Dermatan sulphate proteoglycan 3 | DSPG3 | NM_004950 | 0.7 ± 0.05 | D | Extracellular matrix constituent |
| Diaphorase | DIA4/NQO1 | NM_000903 | 10.9 ± 5.93 | A | Detoxification/nitric oxide synthesis |
| DKFZp564F053 cDNA clone | AA166719 | 3.7 ± 1.24 | C | Unknown | |
| Early growth response 1 | EGR1 | NM_001964 | 1.2 ± 0.17 | B/D | Transcription factor |
| Endothelial protein C receptor | PROCR | NM_006404 | 1.1 ± 0.24 | D | Anticoagulation |
| eNOS | NOS3 | NM_000603 | 4.0 ± 0.73 | B/D | Nitric oxide synthesis |
| EST 15 | R96525 | 1.7 ± 0.25 | C | Unknown | |
| EST 23 | AI632668 | 1.2 ± 0.30 | C | Unknown | |
| EST 35 | AA455259 | 1.8 ± 0.21 | C | Unknown | |
| EST 41 | AI140281 | 1.7 ± 0.42 | C | Unknown | |
| Ferritin, heavy polypeptide | FTH1 | NM_002032 | 2.5 ± 0.48 | C | Intracellular iron storage |
| Ferritin, light polypeptide | FTL | NM_000146 | 2.0 ± 0.12 | A | Intracellular iron storage |
| Fibronectin 1 | FN1 | X02761 | 3.9 ± 0.59 | D | Extracellular matrix protein |
| G1 to S phase transition 1 | GSPT1 | NM_002094 | 0.5 ± 0.08 | C | Cell-cycle regulation |
| Galectin 8 | LGALS8 | NM_006499 | 4.3 ± 0.73 | C | Carbohydrate binding protein |
| GAPDH | GAPD | NM_002046 | 1.0 ± 0.00 | F | Carbohydrate metabolism |
| Hypothetical protein | NM_014108 | 2.3 ± 1.00 | E | Unknown | |
| Hypothetical protein | FLJ23514 | NM_021827 | 0.6 ± 0.09 | C | Unknown |
| Hypothetical protein | FLJ20003 | N47844 | 1.8 ± 0.30 | C | Unknown |
| Hypothetical protein | T80319 | 2.9 ± 0.58 | A | Unknown | |
| Hypothetical protein | BC004930 | 0.4 ± 0.23 | C | Unknown | |
| Interleukin 8 | IL8 | M28130 | 1.0 ± 0.16 | D/E | Inflammatory cytokine |
| LOX-1 | OLR1 | NM_079167 | 1.0 ± 0.45 | D | Oxidized LDL internalization |
| Lung Krüppel-like factor | KLF2 | NM_016270 | 4.9 ± 1.74 | A | Transcription factor |
| Matrix Gla protein | MGP | NM_000900 | 3.7 ± 1.21 | E | Inhibits extracellular matrix calcification |
| MnSOD | SOD2 | NM_000636 | 1.1 ± 0.61 | B/D | Free radical scavenging |
| Multimerin | MMRN | NM_007351 | 2.0 ± 0.54 | D | Hemostasis |
| PAI-1 | SERPINE1 | NM_000602 | 1.4 ± 0.71 | D | Fibrinolysis/proliferation/migration |
| PECAM-1 | PECAM1 | NM_000442 | 2.1 ± 0.37 | D | (Leukocyte) adhesion receptor |
| Prefoldin 2 | PFDN2 | NM_012394 | 2.2 ± 0.44 | C | Membrane-binding protein/autoantigen |
| Protease nexin 1 | GDN | M17783 | 6.4 ± 3.18 | D | Serine protease inhibitor |
| Rabkinesin 6 | RAB6KIFL | NM_005733 | 3.0 ± 0.55 | C | Intracellular transport/cell division |
| Ras-related C3 botulinum toxin substrate 3 | RAC3 | NM_005052 | 1.6 ± 0.07 | C | Ras-related GTPase |
| Regulator of G protein signaling | RGS5 | R38334 | 1.4 ± 0.77 | C | GTPase activator |
| 28S ribosomal RNA | M11167 | 0.9 ± 0.19 | F | Translation | |
| Ribosomal protein L30 | RPL30 | NM_000989 | 1.1 ± 0.16 | F | Translation |
| Ribosomal protein S11 | RPS11 | NM_001015 | 0.7 ± 0.13 | C | Translation |
| Solute carrier family 7, member 11 | SLC7A11 | NM_014331 | 2.1 ± 0.06 | A | Amino acid transporter |
| Spermidine/spermine N1-acetyltransferase | SAT | NM_002970 | 1.0 ± 0.12 | A | Polyamine metabolism |
| Stromal cell-derived factor 2 | SDF2 | AA343837 | 2.4 ± 0.20 | C | Secretory protein |
| Thioredoxin reductase 1 | TXNRD1 | NM_003330 | 2.0 ± 0.08 | A | Intracellular redox control |
| Thrombin receptor 1 | F2R | NM_001992 | 2.0 ± 0.18 | D | Mitogenic signaling |
| Thrombomodulin | THBD | NM_000361 | 2.3 ± 0.11 | B/D | Anticoagulation |
| Thyroid hormone receptor interactor 7 | TRIP7 | L40357 | 2.5 ± 0.67 | C | Thyroid hormone signal transduction |
| Tissue factor | F3 | NM_001993 | 0.4 ± 0.03 | B/D | Extrinsic coagulation |
| Tissue factor pathway inhibitor 1 | TFPI | NM_006287 | 1.8 ± 0.77 | D | Extrinsic coagulation |
| Translation elongation factor 1 α 2 | EEF1A2 | NM_001958 | 1.1 ± 0.05 | F | Translation |
| α-Tubulin | TUBA3 | NM_006009 | 1.2 ± 0.04 | F | Intracellular transport |
| VCAM | VCAM1 | NM_001078 | 0.6 ± 0.02 | B/D | Leukocyte adhesion receptor |
| Vinculin | VCL | NM_003373 | 1.6 ± 0.63 | D | Cytoskeletal/adherens junction protein |
| Von Willebrand Factor | VWF | NM_000552 | 2.4 ± 0.18 | D | Platelet adhesion (hemostasis) |
| Protein name . | Gene name . | GenBank* . | Ratio ± SEM† . | Cat‡ . | Protein function . |
|---|---|---|---|---|---|
| β-Actin | ACTB | NM_001101 | 1.1 ± 0.07 | F | Cytoskeletal protein |
| Activin βA | INHBA | NM_002192 | 3.1 ± 1.29 | E | Vascular growth factor |
| ALL1-fused gene from chromosome 1q | AF1Q | NM_006818 | 1.4 ± 0.43 | A | Unknown |
| Amyloid β A4 precursor protein | APP | NM_000484 | 2.3 ± 0.49 | D | Alzheimer disease/hemostasis |
| Annexin II | ANXA2 | NM_004039 | 1.2 ± 0.06 | D | Membrane-binding protein |
| Annexin V | ANXA5 | NM_001154 | 2.2 ± 0.14 | D | Anticoagulant protein |
| ApoL1 | APOL1 | NM_003661 | 2.0 ± 0.19 | C | HDL-associated plasma protein |
| ApoL2 | APOL2 | NM_030882 | 1.7 ± 0.42 | C | Homolog of ApoL1 |
| ApoL3 (CG12_1) | APOL3 | NM_014349 | 3.9 ± 1.40 | C | Homolog of ApoL1 |
| AT7_4_3 | No match | 0.4 ± 0.10 | C | Unknown | |
| Biglycan | BGN | NM_001711 | 1.3 ± 0.41 | D | Extracellular matrix constituent |
| Caveolin 1 | CAV1 | NM_01753 | 0.6 ± 0.13 | D | Main component of caveolae |
| Claudin 5 | CLDN5 | NM_003277 | 2.1 ± 0.43 | A | Tight junction protein |
| Cockayne syndrome, type I | CKN1 | NM_000082 | 5.7 ± 2.35 | C | RNA polymerase II transcription |
| Connexin 40 | GJA5 | NM_005266 | 3.2 ± 0.93 | A | Gap junction protein |
| Cyclin-dependent kinase inhibitor 1A (p21) | CDKN1A | NM_000389 | 1.7 ± 0.62 | A | Cell-cycle regulation |
| Cytochrome P450 1B1 | CYP1B1 | NM_000104 | 26.9 ± 0.08 | A | Hormone metabolism |
| Dermatan sulphate proteoglycan 3 | DSPG3 | NM_004950 | 0.7 ± 0.05 | D | Extracellular matrix constituent |
| Diaphorase | DIA4/NQO1 | NM_000903 | 10.9 ± 5.93 | A | Detoxification/nitric oxide synthesis |
| DKFZp564F053 cDNA clone | AA166719 | 3.7 ± 1.24 | C | Unknown | |
| Early growth response 1 | EGR1 | NM_001964 | 1.2 ± 0.17 | B/D | Transcription factor |
| Endothelial protein C receptor | PROCR | NM_006404 | 1.1 ± 0.24 | D | Anticoagulation |
| eNOS | NOS3 | NM_000603 | 4.0 ± 0.73 | B/D | Nitric oxide synthesis |
| EST 15 | R96525 | 1.7 ± 0.25 | C | Unknown | |
| EST 23 | AI632668 | 1.2 ± 0.30 | C | Unknown | |
| EST 35 | AA455259 | 1.8 ± 0.21 | C | Unknown | |
| EST 41 | AI140281 | 1.7 ± 0.42 | C | Unknown | |
| Ferritin, heavy polypeptide | FTH1 | NM_002032 | 2.5 ± 0.48 | C | Intracellular iron storage |
| Ferritin, light polypeptide | FTL | NM_000146 | 2.0 ± 0.12 | A | Intracellular iron storage |
| Fibronectin 1 | FN1 | X02761 | 3.9 ± 0.59 | D | Extracellular matrix protein |
| G1 to S phase transition 1 | GSPT1 | NM_002094 | 0.5 ± 0.08 | C | Cell-cycle regulation |
| Galectin 8 | LGALS8 | NM_006499 | 4.3 ± 0.73 | C | Carbohydrate binding protein |
| GAPDH | GAPD | NM_002046 | 1.0 ± 0.00 | F | Carbohydrate metabolism |
| Hypothetical protein | NM_014108 | 2.3 ± 1.00 | E | Unknown | |
| Hypothetical protein | FLJ23514 | NM_021827 | 0.6 ± 0.09 | C | Unknown |
| Hypothetical protein | FLJ20003 | N47844 | 1.8 ± 0.30 | C | Unknown |
| Hypothetical protein | T80319 | 2.9 ± 0.58 | A | Unknown | |
| Hypothetical protein | BC004930 | 0.4 ± 0.23 | C | Unknown | |
| Interleukin 8 | IL8 | M28130 | 1.0 ± 0.16 | D/E | Inflammatory cytokine |
| LOX-1 | OLR1 | NM_079167 | 1.0 ± 0.45 | D | Oxidized LDL internalization |
| Lung Krüppel-like factor | KLF2 | NM_016270 | 4.9 ± 1.74 | A | Transcription factor |
| Matrix Gla protein | MGP | NM_000900 | 3.7 ± 1.21 | E | Inhibits extracellular matrix calcification |
| MnSOD | SOD2 | NM_000636 | 1.1 ± 0.61 | B/D | Free radical scavenging |
| Multimerin | MMRN | NM_007351 | 2.0 ± 0.54 | D | Hemostasis |
| PAI-1 | SERPINE1 | NM_000602 | 1.4 ± 0.71 | D | Fibrinolysis/proliferation/migration |
| PECAM-1 | PECAM1 | NM_000442 | 2.1 ± 0.37 | D | (Leukocyte) adhesion receptor |
| Prefoldin 2 | PFDN2 | NM_012394 | 2.2 ± 0.44 | C | Membrane-binding protein/autoantigen |
| Protease nexin 1 | GDN | M17783 | 6.4 ± 3.18 | D | Serine protease inhibitor |
| Rabkinesin 6 | RAB6KIFL | NM_005733 | 3.0 ± 0.55 | C | Intracellular transport/cell division |
| Ras-related C3 botulinum toxin substrate 3 | RAC3 | NM_005052 | 1.6 ± 0.07 | C | Ras-related GTPase |
| Regulator of G protein signaling | RGS5 | R38334 | 1.4 ± 0.77 | C | GTPase activator |
| 28S ribosomal RNA | M11167 | 0.9 ± 0.19 | F | Translation | |
| Ribosomal protein L30 | RPL30 | NM_000989 | 1.1 ± 0.16 | F | Translation |
| Ribosomal protein S11 | RPS11 | NM_001015 | 0.7 ± 0.13 | C | Translation |
| Solute carrier family 7, member 11 | SLC7A11 | NM_014331 | 2.1 ± 0.06 | A | Amino acid transporter |
| Spermidine/spermine N1-acetyltransferase | SAT | NM_002970 | 1.0 ± 0.12 | A | Polyamine metabolism |
| Stromal cell-derived factor 2 | SDF2 | AA343837 | 2.4 ± 0.20 | C | Secretory protein |
| Thioredoxin reductase 1 | TXNRD1 | NM_003330 | 2.0 ± 0.08 | A | Intracellular redox control |
| Thrombin receptor 1 | F2R | NM_001992 | 2.0 ± 0.18 | D | Mitogenic signaling |
| Thrombomodulin | THBD | NM_000361 | 2.3 ± 0.11 | B/D | Anticoagulation |
| Thyroid hormone receptor interactor 7 | TRIP7 | L40357 | 2.5 ± 0.67 | C | Thyroid hormone signal transduction |
| Tissue factor | F3 | NM_001993 | 0.4 ± 0.03 | B/D | Extrinsic coagulation |
| Tissue factor pathway inhibitor 1 | TFPI | NM_006287 | 1.8 ± 0.77 | D | Extrinsic coagulation |
| Translation elongation factor 1 α 2 | EEF1A2 | NM_001958 | 1.1 ± 0.05 | F | Translation |
| α-Tubulin | TUBA3 | NM_006009 | 1.2 ± 0.04 | F | Intracellular transport |
| VCAM | VCAM1 | NM_001078 | 0.6 ± 0.02 | B/D | Leukocyte adhesion receptor |
| Vinculin | VCL | NM_003373 | 1.6 ± 0.63 | D | Cytoskeletal/adherens junction protein |
| Von Willebrand Factor | VWF | NM_000552 | 2.4 ± 0.18 | D | Platelet adhesion (hemostasis) |
GenBank accession numbers for access to mRNA sequence or spotted cDNA fragment.
Ratios of the expression level in flow-exposed cultures over static cultures, as determined by microarray hybridization, are expressed as the mean of 3 independent 7-day shear-stress experiments ± SEM.
Genes on the custom microarray are organized in the following categories (Cat): (A) 12 shear-stress–responsive genes identified in this study, (B) 10 established shear-stress–responsive genes,7,17 (C) 106 TNF-α–responsive genes,6 (D) prominent endothelial (-specific) genes, (E) 40 smooth muscle activation–specific genes,24 and (F) control genes for normalization purposes. The sets of TNF-α–responsive endothelial genes and activated SMC genes were identified in previous studies from our group using differential display RT-PCR (DD/RT-PCR).6,24 The recently available University of California Santa Cruz (UCSC) draft version of the human genome was used to annotate those differential display (DD) fragments that had not yet been mapped to a named gene.44
Endothelial cells differentially respond to a panel of atherosclerosis-related stimuli
The qualification of the 3 genes KLF2,CYP1B1, and DIA4 as being highly responsive to prolonged shear stress prompted us to study the stimulus and cell-type specificity of their transcriptional regulation. Therefore, the custom microarrays were used to study the effect under static conditions of a set of established modulators of endothelial gene expression, including the cytokines/growth factors TNF-α, IL-1β, TGF-β, VEGF, as well as thrombin, on the expression of the selected endothelial genes and flow-responsive genes identified in this study. To that end, HUVECs were cultured in the continuous presence of these agents for 2, 6, and 24 hours to allow identification of early- and late-induced genes, whereas nonstimulated controls were taken at 0 and 24 hours. The human cell lines HL-60 (myelomonocytic) and HeLa (cervical carcinoma) were included in the analysis to provide additional information on cell type–specific expression. Total RNA was isolated from these cultures and enriched for polyA+, which was then used to produce Cy3-labeled cDNA probes that were hybridized to the arrays. The fluorescence intensities were quantified for each individual gene under the various conditions, and medians of the triplicates were used to automatically exclude infrequent spotting artifacts, as medians were, in most cases, close to the mean of the triplicate signals. Subsequently, stringent data-filtering was applied by including only those genes in the analysis that had hybridization signals above 10% of the GAPDH signal with at least one of the stimuli used. Filtered expression data were median centered, and genes were arranged using a self-organizing map.20 Finally, these results were subjected to complete linkage clustering with uncentered correlation to produce a hierarchical tree (Figure3A).20
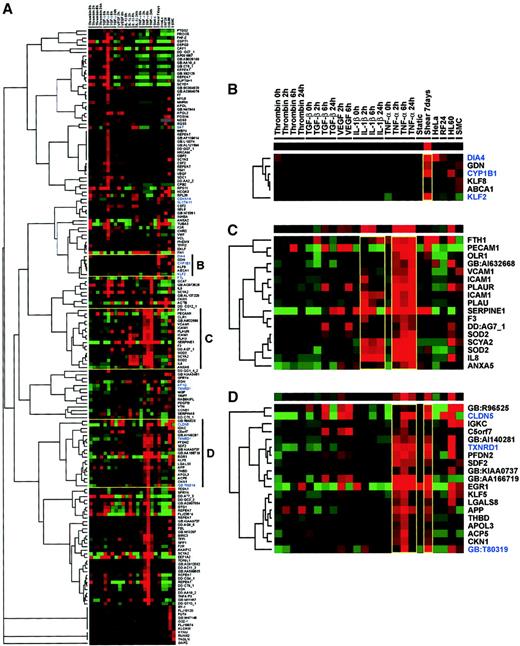
One-way complete linkage clustering of microarray data.
The custom microarray expression data of endothelial cells exposed to a variety of modulators was clustered on the basis of similar regulation patterns using GeneCluster/TreeView.20 HUVECs were exposed to a pulsatile shear stress of 19 ± 12 dyne/cm2 in an artificial capillary system. Static cultures were stimulated with TNF-α, IL-1β, TGF-β, VEGF, or thrombin for 2, 6, and 24 hours. Expression profiles for HL-60, HeLa, and SMC cells were included after normalization to GAPDH. (A) The self-organizing map and hierarchical tree show the total cluster of all genes after data filtering, only including genes in the analysis that had hybridization signals above 10% of the GAPDH signal with at least one of the stimuli/conditions used. The 12 flow-responsive genes identified in this study are shown in blue. Specific clusters shown are genes induced exclusively by shear stress (B), genes induced by IL-1β and TNF-α (C), and genes induced by TNF-α and shear stress (D). Clusters shown in panels B-D are headed by a single row of colored squares representing the general trend of the cluster. Colors of the squares represent the relative expression of the genes in the condition given in the column heading as established after median centering of normalized hybridization signals. Red and green represent higher and lower expression than the median for that particular gene, respectively. Color intensity is related to the difference with the median (black).
One-way complete linkage clustering of microarray data.
The custom microarray expression data of endothelial cells exposed to a variety of modulators was clustered on the basis of similar regulation patterns using GeneCluster/TreeView.20 HUVECs were exposed to a pulsatile shear stress of 19 ± 12 dyne/cm2 in an artificial capillary system. Static cultures were stimulated with TNF-α, IL-1β, TGF-β, VEGF, or thrombin for 2, 6, and 24 hours. Expression profiles for HL-60, HeLa, and SMC cells were included after normalization to GAPDH. (A) The self-organizing map and hierarchical tree show the total cluster of all genes after data filtering, only including genes in the analysis that had hybridization signals above 10% of the GAPDH signal with at least one of the stimuli/conditions used. The 12 flow-responsive genes identified in this study are shown in blue. Specific clusters shown are genes induced exclusively by shear stress (B), genes induced by IL-1β and TNF-α (C), and genes induced by TNF-α and shear stress (D). Clusters shown in panels B-D are headed by a single row of colored squares representing the general trend of the cluster. Colors of the squares represent the relative expression of the genes in the condition given in the column heading as established after median centering of normalized hybridization signals. Red and green represent higher and lower expression than the median for that particular gene, respectively. Color intensity is related to the difference with the median (black).
Thus, the comprehensive series of expression data obtained by the microarray analyses organizes into various clusters, of which 3 reveal a particularly interesting trend, that is, they reveal genes predominantly induced by shear stress (Figure 3B), genes induced by TNF-α and IL-1β (Figure 3C), and genes induced by TNF-α and shear stress but not by IL-1β (Figure 3D). Furthermore, this analysis showed that the expression of 10 of the 12 flow-modulated genes identified in this study was substantially increased by at least one of the applied stimuli. In the shear-specific cluster, 2 genes, LKLF(KLF2) and cytochrome P450 1B1 (CYP1B1), were expressed only at high levels in endothelial cells exposed to prolonged pulsatile flow and not induced by TNF-α or any of the other agents tested. Furthermore, in static HUVEC cultures, the expression level of LKLF in HL-60 and HeLa cells was around or below the detection limit of the microarray experiments. In marked contrast, substantial expression of CYP1B1 was also found in HL-60 cells and moderate expression in HeLa cells, whereas DIA4 was expressed vice versa.
Kinetics of LKLF and CYP1B1 induction by flow and inverse regulation by TNF-α
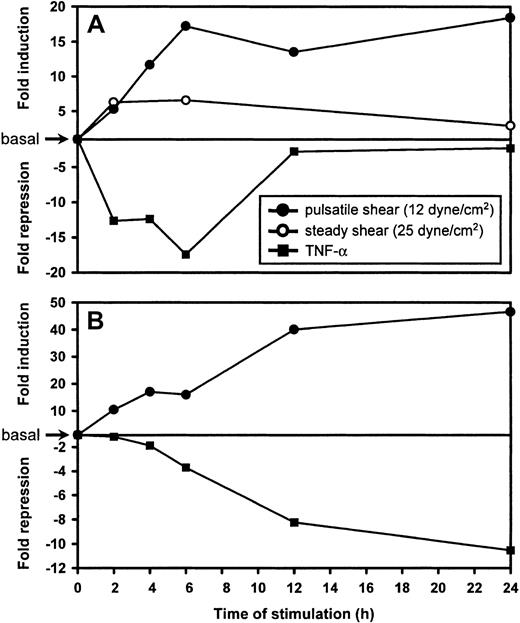
Semiquantitative real-time RT-PCR was used to verify the microarray expression data, to more accurately determine the induction/repression levels, and to determine the expression kinetics of LKLF and CYP1B1, which both have low basal expression levels in cultured cells. Pulsatile flow experiments (12 ± 7 dynes/cm2; see Figure 2 for flow profile), using a parallel-plate flow chamber, and separate TNF-α stimulations were performed on HUVECs for 2, 4, 6, 12, and 24 hours, with static nonstimulated controls taken at 0, 6, and 24 hours. In addition, HUVECs were exposed to a steady laminar flow for 2, 6, and 24 hours (25 dyne/cm2). Relative expression levels of LKLF andCYP1B1 were determined using real-time RT-PCR and expressed as ratios over the controls (Figure 4). LKLF and CYP1B1 were induced by flow to maximum levels within 6 hours and 12 hours, respectively. Interestingly, compared to steady laminar flow, pulsatile flow resulted in an additional 3-fold increase in LKLF expression. These expression levels were sustained well beyond 24 hours of flow exposure. In contrast, both LKLF andCYP1B1 were significantly down-regulated by TNF-α within 6 hours. LKLF expression remained at levels that were still 2.5-fold lower than in static cultures, whereas repression of CYP1B1by TNF-α was sustained.
Kinetics of LKLF and CYP1B1 differential expression as determined by real-time semi-quantitative RT-PCR.
In a parallel plate-type flow chamber, HUVECs were exposed to either steady laminar flow generating a shear stress of 25 dyne/cm2 (○) or unidirectional pulsatile laminar flow generating a shear stress of 12 ± 7 dyne/cm2 (●) for various time intervals. Static HUVECs cultured in parallel were treated with 50 ng/mL TNF-α (▪). Ratios of the relative expression levels for LKLF (A) and CYP1B1 (B) determined by semiquantitative real-time RT-PCR over static cultures were calculated and expressed as fold induction or fold repression.
Kinetics of LKLF and CYP1B1 differential expression as determined by real-time semi-quantitative RT-PCR.
In a parallel plate-type flow chamber, HUVECs were exposed to either steady laminar flow generating a shear stress of 25 dyne/cm2 (○) or unidirectional pulsatile laminar flow generating a shear stress of 12 ± 7 dyne/cm2 (●) for various time intervals. Static HUVECs cultured in parallel were treated with 50 ng/mL TNF-α (▪). Ratios of the relative expression levels for LKLF (A) and CYP1B1 (B) determined by semiquantitative real-time RT-PCR over static cultures were calculated and expressed as fold induction or fold repression.
Human vascular expression of LKLF, cytochrome P450 1B1, and claudin-5
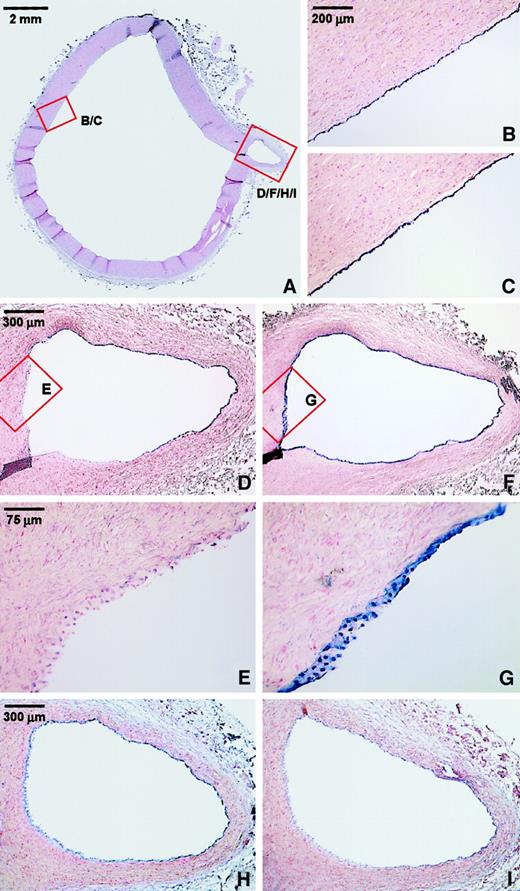
The microarray data have aided us in limiting the extensive (endothelial) gene collection to a set of 3 genes with interesting vascular cell–type specificity and patterns of transcriptional regulation by various stimuli. In contrast to all other genes tested, up-regulation of both LKLF and CYP1B1 was largely endothelial specific, whereas LKLF was exclusively expressed in endothelial cells under flow. In contrast, claudin-5 (CLDN5)was the only gene found to be considerably down-regulated after 24 hours of exposure to laminar flow, but its expression was induced by TNF-α (Figures 1D, 3D). We therefore studied the expression of these genes in human vascular tissue. Nonradioactive mRNA in situ hybridizations were performed on various sections from our human vascular tissue collection.6,18 Figure5 shows the results obtained with the thoracic aorta taken from a 13-year-old female donor. In this tissue, no neointima was present, and inflammatory processes in the vessel wall were absent. Expression of LKLF was restricted to the endothelium and continuous throughout all the endothelial cells of the aorta itself (Figure 5A-B). In the small branch, however, substantial differences in LKLF expression were observed (Figure 5D-E). Whereas the endothelial LKLF expression at the opposite wall of the branching point was comparable to that in the aorta (Figure 5D), no LKLF mRNA was detected in the endothelium covering the area where the branch physically disconnects from the larger aorta (Figure 5E). In contrast, expression of claudin-5 also was restricted to the endothelium but was not significantly differential at this branching point, in accordance with the observation that in vitro, its transient down-regulation at 24 hours (Figure 1D) returns to baseline levels after 7 days' (Table 1) exposure to pulsatile shear (Figure 5H). Remarkably, in contrast to its high level of expression under flow in vitro (150% of GAPDH), the level of CYP1B1 mRNA detected in vivo was not significantly above background (Figure 5I), confirming earlier histochemical reports on the tissue distribution of CYP1B1expression.24-26 As a positive control for the integrity of both the endothelial cell lining and the mRNA at this site, in situ hybridizations for von Willebrand factor (VWF) mRNA were performed on consecutive sections (Figure 5C,F-G). There was a strong and uniform signal for VWF, demonstrating the presence of a continuous layer of endothelium and the good RNA quality in these tissues, as well as confirming the endothelium-specific expression of LKLF and claudin-5.
Endothelial-specific expression of LKLF and claudin-5 in the normal human aorta.
(A) Overview of a section of the thoracic aorta of a 13-year-old female stained with nuclear fast red. Nonradioactive mRNA in situ hybridizations was performed on consecutive sections, using antisense riboprobes of LKLF (B,D,E), claudin-5 (H), cytochrome P450 1B1 (I), and the endothelial-specific marker von Willebrand factor (VWF) (C,F,G). The detection of the mRNA-probe hybrid results in a blue color associated with the nuclei. Panels E and G show no significant expression of the LKLF mRNA (E), whereas VWF (G) is consistently and specifically expressed in the endothelium of the entire specimen. Stacks of nuclei are visible because of the thickness of the sections (16 μm) and the conical shape of the branching artery. Claudin-5 was specifically expressed in the endothelium (H), but detection of the cytochrome P450 1B1 mRNA was too close to background hybridization levels (I).
Endothelial-specific expression of LKLF and claudin-5 in the normal human aorta.
(A) Overview of a section of the thoracic aorta of a 13-year-old female stained with nuclear fast red. Nonradioactive mRNA in situ hybridizations was performed on consecutive sections, using antisense riboprobes of LKLF (B,D,E), claudin-5 (H), cytochrome P450 1B1 (I), and the endothelial-specific marker von Willebrand factor (VWF) (C,F,G). The detection of the mRNA-probe hybrid results in a blue color associated with the nuclei. Panels E and G show no significant expression of the LKLF mRNA (E), whereas VWF (G) is consistently and specifically expressed in the endothelium of the entire specimen. Stacks of nuclei are visible because of the thickness of the sections (16 μm) and the conical shape of the branching artery. Claudin-5 was specifically expressed in the endothelium (H), but detection of the cytochrome P450 1B1 mRNA was too close to background hybridization levels (I).
Discussion
Presently, the only cell types known to exhibit a physiologically significant response to flow are endothelial cells and osteocytes.27 Adaptation of these cells to continuous flow during their entire life span is crucial for correct functioning in their respective physiological contexts. A change of this biomechanical force likely leads to dysfunction of those processes that are specific for these cell types and are specifically under the control of flow. Studies with novel screening techniques such as DNA microarray hybridizations and differential display have shown that TNF-α and hemodynamic forces like shear stress regulate the transcription of a large and diverse set of endothelial genes in vitro.6-8This extensive, though not yet fully known, gene set, however, includes many genes that are general stress responsive or involved in handling oxidative stress. Moreover, the expression of these genes by a variety of cell types and their transcriptional regulation reveals that most of them are not involved in processes specific to the endothelium nor in the atheroprotective force of shear stress. Also, in vivo verification of the endothelial expression has been performed for only a few of these targets. In this study, we therefore aimed to extract candidates from the large set of shear-stress–responsive genes that determine the endothelium's unique capacity to modulate its phenotype in accordance with local hemodynamic factors. The results described here demonstrate that the vast majority of the genes identified in this study and by others are also induced by various other (atherogenic) stimuli and/or are expressed in other cell types.
The kinetics of steady laminar flow–induced genes identified in this study can be divided into 2 different classes: genes that are up-regulated to steady levels within 6 hours and genes that have a delayed response increasing beyond 24 hours of flow exposure. In contrast to the immediate-early induced genes, the induction of the late genes likely requires de novo protein synthesis, for example, flow-induced transcription/translation of transcription factors. Up until now, a panel of transcription factors has been demonstrated to be modestly regulated by flow, and their response elements have been identified.28-30 However, the wide patterns of expression of the transcription factors, early growth response-1 (Egr-1), nuclear factor-κB (NF-κB), c-fos, and c-jun, show that they are neither endothelium specific nor exclusively regulated by sustained flow. This view is further substantiated by the involvement of reactive oxygen species, which can be produced by endothelial cells under flow in the activation of c-fos, AP1, and NF-κB.31-33 Coordinately expressed genes, which are under the control of similar combinations of transcription factors, can be detected by clustering analysis. The clustering analysis of the microarray data from our study on a variety of modulators of endothelial cell gene expression arranged the panel of vascular genes in various clusters, of which 3 are particularly interesting: genes induced predominantly by shear stress (Figure 3B), genes induced by TNF-α and IL-1β but not by shear stress (Figure 3C), and genes induced by both shear stress and TNF-α (Figure 3D). The latter 2 panels show that genes induced by TNF-α but not by shear are also likely to be induced by IL-1β, whereas genes induced by shear and TNF-α are not likely to be induced by IL-1β. This implies the existence of some distinct overlap between the transcriptional pathways of shear stress and TNF-α on one side, and TNF-α and IL-1β on the other. The involvement of the TNF-α–related transcription factor NF-κB in activating transcription from a previously identified shear-stress–response element (SSRE)28 is in agreement with these findings and might be at the basis of the apparent cross-talk between shear stress and TNF-α signaling pathways. The indirect activation of NF-κB by flow, together with the presence of the NF-κB-binding SSRE in the gene promoters, is a potential source for this overlapping transcriptional response to flow and to TNF-α of the majority of flow-regulated genes identified in this study and by others. However, although we found up to 3 SSREs in a few of the 12 highly flow-responsive genes, no conclusions can be drawn on this subject without performing systematic promoter studies. Nevertheless, the large overlap between flow- and TNF-α–induced gene regulation patterns is presumably related to the observed increase of intracellular oxidative stress in endothelial cells that occurs both by TNF-α activation34,35 and under unidirectional pulsatile, but not oscillatory or steady flow.36 The involvement of reactive oxygen species is further substantiated by the induction of many protective flow-induced genes that handle oxidative stress, which are neither stimulus nor cell-type specific. In this respect, the regulation of Mn and Cu/Zn-superoxide dismutases by flow7,10 37 and their expression in various cell types can be complemented with the flow responsiveness of thioredoxin reductase and ferritin, identified in this study.
The long-term exposure of endothelial cells to flow in this study shows that the regulation of most of the shear-stress–responsive genes identified in this study and by others is transient. Although still elevated at 24 hours, they return to basal levels after a 7-day exposure to flow. CYP1B1 proved to be one of the few highly flow-specific and nontransiently expressed endothelial genes involved in metabolic processes. The high CYP1B1 levels found in the HL-60 cell line confirm reports that this is the major cytochrome P450 isoform in human blood monocytes.24 Also, the expression in HeLa cells is in agreement with the high CYP1B1expression levels that are observed in many human tumors.25 The lack of expression in healthy human vascular tissue suggests that its strong transcriptional induction by flow, also noted by others,8 seems restricted to the conditions in in vitro model systems, whereas its expression in vivo is regulated in a more complex manner. It can be assumed that transient effects of flow on gene expression are more generally observed for a wide variety of stimuli and are therefore general stress responses. The continuous, life-long exposure of the endothelium to flow in vivo raises the question whether in vitro general stress-responsive, and in particular, transiently flow-modulated genes would indeed be induced in a physiological context. In this view, the transient flow responsiveness of claudin-5 and connexin-40, which was found in this study, demonstrates its possible in vivo triviality, although a plausible rationale is at hand. Both claudin-5 and connexin-40 are involved in mediating endothelial cell-to-cell contacts in tight junctions and gap junctions, respectively. The process of flow-induced reorganization of endothelial cells to their stretched shape might require the transient transcriptional modulation of these genes because of the temporary relief of cell-to-cell contacts.38 In addition, in situ hybridization for claudin-5 indeed showed its continuous presence in the endothelial lining of all human vessels tested so far. Our results with both claudin-5 and CYP1B1stress the fact that in vitro data, especially when dealing with the transient application of a normally continuous force or activator, need to be backed up with in vivo confirmation.
The most notable panel from our clustering analysis identifies the small set of genes whose expression seems almost exclusively regulated by flow in vitro. The most remarkable exponent of this set is lung Krüppel-like factor (KLF2). Also, the difference in transcriptional response of LKLF to steady laminar versus unidirectional pulsatile flow is noteworthy. Exposure of HUVECs to pulsatile flow for 24 hours up to 7 days induced LKLF expression continuously as much as 20-fold, whereas steady flow induced LKLF less than 5-fold with a peak around 4 hours. In contrast to its induction by flow, the expression of LKLF in vitro was actually repressed by TNF-α and not affected by any other stimulus tested. Next, we show for the first time the endothelial-specific expression of LKLF in adult human vascular tissue. Thus far, the expression of LKLF had been described exclusively in mice and was found to be restricted to endothelial cells and naı̈ve T-cells.39,40 The flow-specific response of LKLF in vitro suggests that the substantial differences in LKLF expression that were found at a small vessel bifurcation of the thoracic aorta are also flow mediated. However, a more detailed correlation between the magnitude of local shear stress and expression of LKLF in such tissues will require an elaborate future effort. The endothelium-specific expression of LKLF during murine embryogenesis was implicated in vasculogenesis, based on the phenotype of LKLF knockout mice.40 LKLF−/− mice died in utero around embryonic day 13 because of severe vessel wall anomalies. Apparent changes in the endothelial lineage itself were not observed, nor did any other tissue or organ appear damaged. Restriction of high LKLF expression to endothelium exposed to (pulsatile) laminar flow implies the ability of shear stress to transcriptionally regulate the target genes of LKLF that are involved in the mechanisms behind these vessel abnormalities. However, none of the flow-responsive genes identified in our study fit the observed phenotype in LKLF−/− mice. This includes CYP1B1, since no apparent defects were observed in CYP1B1-null mice, excluding a crucial involvement in vasculogenesis.41Furthermore, we found insignificant expression in the vascular tissues tested thus far, in accordance with published results.25,26 Therefore, the factors that are under transcriptional control of LKLF, and thus responsible for the observed phenotype, remain unidentified. To our knowledge, LKLF is the only transcription factor known to date that is specifically expressed by endothelial cells in vivo and exclusively induced by flow. The Krüppel-like factor family members typically function as transcriptional switches in differentiation/activation processes in many cell types.42 Recently, the involvement of yet 2 other Krüppel-like factors, GKLF and BTEB2, was demonstrated in mediating TGF-β–regulated smooth muscle cell (SMC) differentiation.43 Therefore, an interesting role for the Krüppel-like factors in vascular biology is implicated. Additional studies will have to be performed to reveal the downstream target genes of LKLF and the promoter elements responsible for the flow-specific transcriptional induction of LKLF.
In conclusion, this study on the transcriptional regulation of an extensive collection of atherosclerosis-related genes by a variety of (anti-) atherogenic stimuli demonstrates that only a very limited set of endothelial genes is more or less exclusively regulated by flow and is cell-type specific. Furthermore, the induction of the majority of flow-regulated endothelial genes both by flow and by cytokines likely results from potential cross-talk between flow- and inflammatory-mediated downstream signaling mechanisms. The endothelial-specific transcription factor LKLF was identified as a promising target to aid the further elucidation of the molecular basis of the flow-mediated vascular protection from atherosclerosis.
Prepublished online as Blood First Edition Paper, May 13, 2002; DOI 10.1182/blood-2002-01-0046.
Supported by the Netherlands Heart Foundation, the Hague, by grants NHS96.094 and NHS97.209; and the Molecular Cardiology Program grant M93.007.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Anton J. G. Horrevoets, Department of Biochemistry, Academic Medical Center K1-161, Meibergdreef 15, 1105 AZ Amsterdam, The Netherlands; e-mail: a.j.horrevoets@amc.uva.nl.

![Fig. 1. Gene expression profile of laminar flow–exposed HUVECs determined by cDNA array hybridization. / (A) The plot shows a log-log comparison of the 24-hour shear-stress–exposed versus static cultures of background corrected and normalized hybridization signals of all ∼18 000 cDNAs on the array. Diagonal lines represent the following: dashed line, unity line with equal expression in both conditions; +5/−5 and +10/−10 labeled lines, 5- and 10-fold up- and down-regulation. Gray boxes represent hybridization signals that are below the limits of significant expression (<5 × 103). Clones more than 5-fold differentially expressed were picked, re-arrayed on a custom microarray, checked for reproducibility of induction (B-D), and sequence verified. (B-D) The kinetics of transcriptional regulation by steady laminar flow (25 dyne/cm2) for 0, 2, 6, and 24 hours were determined for the 12 highly shear-stress–responsive genes (Table1). (B) Late flow-responsive genes DIA4 (diaphorase),SAT (spermidine/spermine N1-acetyltransferase),FTL (ferritin light polypeptide), TXNRD1(thioredoxin reductase 1), AF1Q (ALL1-fused gene from chromosome 1q), SLC7A11 (solute carrier family 7), and GenBank T80319 (hypothetical protein). (C) Immediate-early induced genes KLF2 (LKLF), CYP1B1 (cytochrome P450 1B1),CDKN1A (cyclin-dependent kinase inhibitor 1A [p21]), andGJA5 (connexin-40). (D) Down-regulated gene CLDN5(claudin-5). Hybridization signals shown are the mean ± SD of duplo spots and expressed as percentage of the GAPDH signal.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/5/10.1182_blood-2002-01-0046/3/m_h81723062001.jpeg?Expires=1767736060&Signature=tmYPVlcMY9B-GI3Xo~Kd0NdiaQijf-1TrhPNo~9K3x23yx~iFoEB4Q~PR2FvPFKILOzwUfoccVdcPEH-qFGWRZMEUXSZq6WT3r-npNp0J8-0bjbpwxBAvOYhPNGi7Xe28Ag~JD1OCkoR~gB9uAwTH-1tw3qMSXid836XcyEaRyGuzLRlISqLZl8BMY9wSy3lcMyVE~gr7c69iRElkvSXblLTHZvGecZtKrQYWGDPOgoG43uIIkdb6BSoLQRHpkfIufb~tsOC5awbTeTQtlxJCM6VfCKte7VW~FdRBim2ySgr-XQ8i1ongkjPl7vH0aEGNvwqpn7mCJmoq-y1hBsBWw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal