Recently, Yagi et al1 reported on expression of Ikaros isoforms in patients with childhood acute myeloid leukemia (AML). Ikaros expression was assessed by nested polymerase chain reaction (PCR) and immunoblotting. The authors found that Ikaros isoform 6 (Ik-6) was detected in 7 of 10 cases of M4 and M5, but in none of the remaining FAB (French-American-British) subtypes. They conclude that the pathogenesis of myelomonocytic/monocytic AML may involve aberrant regulation of apoptosis by Bcl-XL up-regulation due to unscheduled expression of Ik-6.
Over the past several years, there has been a controversy regarding the expression of Ikaros isoforms in human leukemia. Sun et al reported that leukemic cells from infants with B-cell acute lymphoblastic leukemia (ALL) expressed dominant-negative Ikaros isoforms Ik-4, Ik-7, Ik-8, and their deletion mutants.2 They also reported similar observations with childhood T-cell ALL3 and childhood ALL4 using reverse transcriptase (RT) PCR and immunoblotting. Contrary to their reports, we demonstrated overexpression of dominant-negative Ikaros isoform Ik-6 in patients with blast crisis of chronic myelogenous leukemia (CML)5and adult B-cell ALL6 using similar methods. Recently, Payne et al reported that Ik-4, Ik-7, Ik-8, and their deletion mutants, previously linked to leukemia by Sun et al,2-4 are expressed in normal human cells.7 As I have already raised a technical concern over immunoblotting of the articles of Sun et al,8 I would like to ask a question about RT-PCR in the article of Yagi et al.
We extensively examined expression of Ikaros isoforms in patients with human hematologic malignancies and could not find any overexpression of dominant-negative isoforms in patients with AML, including 6 cases of M4 and 5 cases of M5 (T. Tabayashi, F. I., unpublished data, February 2002). Is the discrepancy between our observation and the interpretation of Yagi et al coming from the difference in patients' age (adult vs children)? I realized that Yagi et al were using nested RT-PCR for detection of Ikaros isoforms, although we are using just single RT-PCR. In general, PCR amplifies shorter products more efficiently than longer ones. I am afraid that with nested RT-PCR, by amplifying Ikaros isoforms not proportionately, we might get more shorter PCR products than expected. In case we could detect Ikaros expression by immunoblotting, we should easily amplify the products by single PCR. As shown in Table 1, Sun et al also used nested RT-PCR. The authors need to show the results of the first-round PCR to avoid skewed amplification of shorter PCR products, since the relative amount of dominant-negative isoform to full-length isoform is important to discuss the pathogenesis.
Reports of Ikaros expression in human leukemia
| Report . | PCR . | Isoform . | Disease . |
|---|---|---|---|
| Yagi et al1 | Nested | Ik6 | Childhood AML |
| Sun et al2 | Nested | Ik4, 7, 8 | Infant B-ALL |
| Sun et al3 | Nested | Ik4, 7, 8 | Childhood T-ALL |
| Sun et al4 | Nested | Ik4, 7, 8 | Childhood ALL |
| Nakayama et al5 | Single | Ik6 | CML blast crisis |
| Nakase et al6 | Single | Ik6 | Adult B-ALL |
| Report . | PCR . | Isoform . | Disease . |
|---|---|---|---|
| Yagi et al1 | Nested | Ik6 | Childhood AML |
| Sun et al2 | Nested | Ik4, 7, 8 | Infant B-ALL |
| Sun et al3 | Nested | Ik4, 7, 8 | Childhood T-ALL |
| Sun et al4 | Nested | Ik4, 7, 8 | Childhood ALL |
| Nakayama et al5 | Single | Ik6 | CML blast crisis |
| Nakase et al6 | Single | Ik6 | Adult B-ALL |
The expression of Ikaros in childhood AML
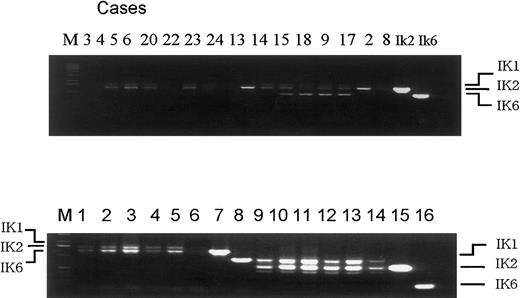
We thank Dr Ishimaru for his remarks about our article.1-1 As described, we determined the expression of Ikaros dominant-negative isoform 6 (Ik6) with use of first-round as well as nested reverse transcriptase polymerase chain reaction (RT-PCR). As a result, we detected Ik6 expression in 7 of 10 cases of M4 and M5 leukemia, but in none of the remaining French-American-British subtypes. Although only nested RT-PCR results have been shown in the article,1-1 in 5 of these 7 samples, first-round RT-PCR clearly detected Ik6 (4 of these 5 shown in Figure 1-1, upper panel). Accordingly, our observation was not limited to nested RT-PCR. In Figure 1-1, bands of Ik6 appeared to be denser than that of Ik1 or Ik2 in the Ik6-positive cases. However, for precisely quantifying the isoforms in these leukemic specimens, more meticulous procedures may be necessary. Because Dr Ishimaru and his colleagues could not detect Ik6 by single RT-PCR in adult acute myeloid leukemia (AML) cases, they raised the question whether there is a difference between adult and pediatric AML. Unfortunately, we do not have any data on adult de novo AML for comparison. Contrary to the report by Payne et al,1-2 we detected none of the dominant-negative Ik isoforms including Ik6 with either first-round or nested RT-PCR in healthy human controls. Since details of the healthy controls were not shown in the article,1-1 we present here the data, where we tested fractionated CD4+, CD8+, CD14+, CD19+ subsets in peripheral blood and placenta cDNA (CLONTECH Laboratories, Palo Alto, CA) (Figure 1-1, lower panel).
RT-PCR results in pediatric AML and healthy controls.
Upper panel: first-round RT-PCR of AML specimens. Lane number indicates the case number of Table 1 in our article. Of the 7 Ik-6 positive cases, 4 are shown here (case 10 not included, cases 11 and 12 not tested). Lower panel: healthy controls. Lanes 1-8 indicate first-round RT-PCR and lanes 9-16 indicate nested RT-PCR. Template c DNA were obtained from mononuclear cells (lanes 1, 9), CD4+ cells (lanes 2, 10), CD8+ cells (lanes 3, 11), CD14+cells (lanes 4, 12), CD19+ cells (lanes 5, 13), placenta (lanes 6, 14), positive control for Ik2 (lanes 7, 15), and positive control for Ik6 (lanes 8, 16). M indicates Kb ladder marker.
RT-PCR results in pediatric AML and healthy controls.
Upper panel: first-round RT-PCR of AML specimens. Lane number indicates the case number of Table 1 in our article. Of the 7 Ik-6 positive cases, 4 are shown here (case 10 not included, cases 11 and 12 not tested). Lower panel: healthy controls. Lanes 1-8 indicate first-round RT-PCR and lanes 9-16 indicate nested RT-PCR. Template c DNA were obtained from mononuclear cells (lanes 1, 9), CD4+ cells (lanes 2, 10), CD8+ cells (lanes 3, 11), CD14+cells (lanes 4, 12), CD19+ cells (lanes 5, 13), placenta (lanes 6, 14), positive control for Ik2 (lanes 7, 15), and positive control for Ik6 (lanes 8, 16). M indicates Kb ladder marker.
Another point Dr Ishimaru raised was the report of Sun et al.1-3 In cases of infant leukemia, Sun et al1-3reported a high incidence of detectable mutant Ik isoforms by nested RT-PCR. We found, however, that none of the dominant-negative Ik isoforms including Ik6 could be detected even with nested RT-PCR (M.T. et al, unpublished observation, 2000), indicating that there are yet-to-be-clarified differences aside from PCR technical issues that Dr Ishimaru is most concerned with. In accord with other studies in Japan,1-4,1-5 we demonstrated that among childhood lymphoid leukemia, Ik 6 was detectable in 26.3% of B-precursor acute lymphoblastic leukemia (ALL) with first-round RT-PCR and Western blot.1-6 Finally, in our article,1-1 we clearly showed that the pathogenesis of myelomonocytic/monocytic AML may involve aberrant regulation of apoptosis due to unscheduled expression of the Ik6 isoform.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal