Plasmodium falciparum parasites express variant adhesion molecules on the surface of infected erythrocytes (IEs), which act as targets for natural protection. Recently it was shown that IE sequestration in the placenta is mediated by binding to chondroitin sulfate A via the duffy binding-like (DBL)–γ3 domain ofP falciparum erythrocyte membrane protein 1 (PfEMP1CSA). Conventional immunization procedures rarely result in the successful production of monoclonal antibodies (mAbs) against such conformational vaccine candidates. Here, we show that this difficulty can be overcome by rendering Balb/c mice B cells tolerant to the surface of human erythrocytes or Chinese hamster ovary (CHO) cells before injecting P falciparum IEs or transfected CHO cells expressing the chondroitin sulfate A (CSA)–binding domain (DBL-γ3) of the FCR3varCSA gene. We fused spleen cells with P3U1 cells and obtained between 20% and 60% mAbs that specifically label the surface of mature infected erythrocytes of the CSA phenotype (mIECSA) but not of other adhesive phenotypes. Surprisingly, 70.8% of the 43 mAbs analyzed in this work were IgM. All mAbs immunoprecipitated PfEMP1CSA from extracts of125I surface-labeled IECSA. Several mAbs bound efficiently to the surface of CSA-binding parasites from different geographic areas and to placental isolates from West Africa. The cross-reactive mAbs are directed against the DBL-γ3CSA, demonstrating that this domain, which mediates CSA binding, is able to induce a pan-reactive immune response. This work is an important step toward the development of a DBL-γ3–based vaccine that could protect pregnant women from pathogenesis.
Introduction
Plasmodium falciparum parasites express variant antigens on the surface of infected erythrocytes (IEs), which act as targets for natural protection.1 The immunodominant surface antigen P falciparum erythrocyte membrane protein 1 (PfEMP1) is involved in several adhesive interactions resulting in parasite sequestration. PfEMP1 is encoded by members of a multigene family, the var genes, which are responsible for antigenic variation.2 Adhesion to host cells is essential for parasite survival because it prevents destruction in the spleen. These surface antigens also play a role in severe malaria and the observed acquisition of immunity makes these antigens prime candidates for the development of new intervention strategies, specifically aimed at preventing adhesion and protecting against disease. For example, IE sequestration in the placenta mediated by binding of the duffy binding-like (DBL)–γ3 domain of PfEMP1CSAto the chondroitin sulfate A (CSA) receptor, is correlated with maternal morbidity, premature delivery, spontaneous abortion, and low birth weight in first pregnancies in African women.3Recent research has shown that an antiadhesion antibody immune response develops against CSA-binding parasites in multiparous women,4 suggesting that it may be possible to develop an anti-CSA adhesive phenotype vaccine based on the corresponding PfEMP1/varCSA-encoded IE surface antigen despite the genetic variability that has been observed in vargenes.2-5
An essential step in the development of novel intervention methods based on PfEMP1 is the development of tools for identification of the critical sites of PfEMP1 molecules involved in adhesion and investigation of the degree of cross-reactivity with other parasite isolates from different endemic regions. Monoclonal antibodies (mAbs) directed against various domains of PfEMP1 would be a valuable tool. However, attempts to develop mouse mAbs directed against native and conformational parasite antigens on the surface of IEs have rarely been successful. Despite intensive research in a large number of laboratories over the last 15 years, only a very limited number of mAbs against surface neoantigens of P falciparum IEs have been produced.6 7 This lack of success may be due to the abundance of immunodominant host cell surface molecules, which induce an overwhelming antibody response in mice and make it extremely difficult to develop mAbs against minor or weakly immunogenic antigens or against conformational epitopes.
In this study we have developed a new technique for overcoming the difficulties encountered in producing mAbs against minor and conformational parasite surface antigens. We tested the hypothesis that distinct isolates involved in human placental infection share common surface epitopes. Balb/c mice were rendered B-cell tolerant to human erythrocytes or Chinese hamster ovary (CHO) cells. We then injected these mice with intact IEs of the CSA-binding phenotype or CHO cells expressing a single domain (DBL-γ3CSA) of the FCR3varCSA gene. A large number of specific mAbs that specifically recognized the P falciparum CSA ligand at the surface of the IE were obtained for each fusion. Several of these mAbs bound strongly to the surface of CSA-binding parasites from different geographic regions and to placental isolates from central African women via the DBL-γ3CSA domain. This study gives strong support for the development of an antimalaria vaccine based on the FCR3 DBL-γ3CSA domain to protect pregnant women against disease.
Materials and methods
Parasites
We cultured and maintained P falciparum strains B358, BXII, FCBR, Suk, H, IBR, and FCR3 under standard culture conditions as previously described,9 replacing 10% human serum with 5% Albumax. Tissue cryosections of 6 P falciparum–infected placentas from Cameroonian women (no. 24, 42, 42DJ, 193, 939, and 940) have been described elsewhere.8
CHO-transfectant
The CHO-745 cells and a transfectant of this cell line expressing the DBL-γ3 domain of varCSA at its surface were obtained and maintained as previously described.10
Placenta cryosections
Fresh malaria placenta biopsy samples about 5 × 5 × 5 mm in size were obtained from the same 6 Cameroonian women from whom the parasite populations listed above were obtained by flushing with CSA.8 They were snap-frozen immediately after delivery and stored in liquid nitrogen until use. For liquid-phase immunofluorescence assay (L-IFA), we used 7-μm unfixed placenta cryosections mounted on standard microscope slides.
Selection of CSA, CD36, and intercellular adhesion molecule 1 adhesive phenotype
Highly synchronized (4 ± 2 hours) parasites in mature blood stage-infected erythrocytes of the CSA adhesive phenotype (mIECSA) were obtained by regular panning on Sc17Saimiri brain microvascular endothelial cells as described elsewhere,11 and successive sorbitol treatments.12 We investigated the adhesive specificity of such mIECSA of the FCR3 strain by using concentrated synchronized parasites obtained by gelatin flotation using Plasmagel (Fresnius France Pharma, Couvier, France).13 These parasites were incubated with a CSA chain bearing recombinant human thrombomodulin-coated magnetic beads (Dynabeads M450; Dynal, Oslo, Norway), as described elsewhere.14,15 Bound mIEs were expanded in culture and cytoadhesion inhibition assays were regularly performed16 to assess the specificity of binding to CSA. Typically, the adhesion of mIEs selected in this way was inhibited, by more than 95%, by 100 μg/mL soluble CSA (Fluka, l'Isle Abeau Chesnes, France) or prior 1 U/mL chondroitinase ABC treatment of the endothelial cells used for the assay. We obtained mIECD36 and mIEICAM-1 by panning FCR3 IE preparations enriched by gelatin flotation on ScC2 and Sc3A4Saimiri brain microvascular endothelial cells, which express either CD36 or intercellular adhesion molecule 1 (ICAM-1), as described elsewhere.11 Placenta parasite populations that bound CSA on endothelial cells and placenta syncytiotrophoblasts were obtained by flushing 6 full-term placentas from Cameroonian women with malaria with a soluble 50-kd CSA.8
Induction of B cell–mediated tolerance to CHO cells and normal human erythrocytes in mice
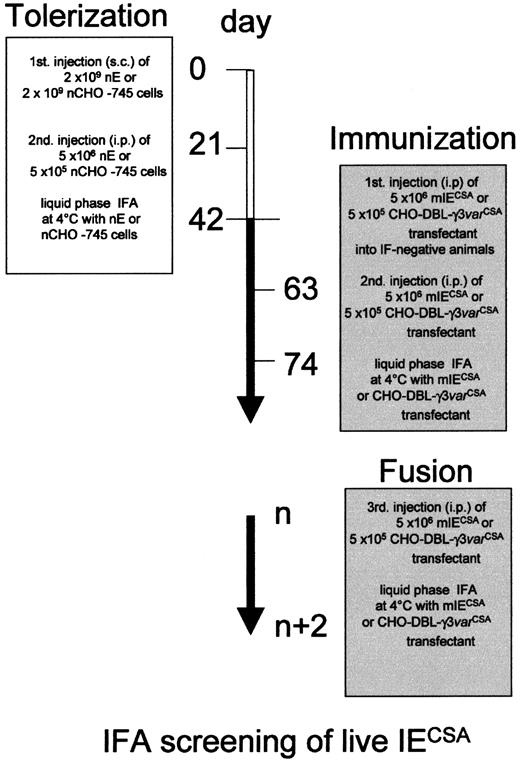
We rendered the B cells of 24- to 48-hour-old Balb/c mice (Iffa Credo, L'Arbresle, France) tolerant to normal human O−erythrocytes (nEs) or normal CHO-745 cells (nCHOs) by antigenic overload, as described in Figure 1.
Procedure for the development of mAbs specific for
P falciparum IE surface antigens. B cells of 24- to 48-hour-old Balb/c mice were rendered tolerant to nEs and CHO-745 by subcutaneous injection of 2 × 109 cells. Mice were given a boost 21 days later by intraperitoneal injection of 5 × 106 nEs or 5 × 105 nCHO-745 cells. Tolerant animals were identified by L-IFA with a 1:10 dilution of serum, 21 days after the boost (day 42). Animals given no IF or only weak IF were then immunized and boosted with 5 × 106mIECSA or 5 × 105 CHO–DBL-γ3 transfectant. Spleen cells from animals that responded (day 74) to mIECSA or DBL-γ3 were then used to raise mAbs by fusing these cells with P3U1 cells. The specific antiparasite surface immune responses were assessed by L-IFA.
Procedure for the development of mAbs specific for
P falciparum IE surface antigens. B cells of 24- to 48-hour-old Balb/c mice were rendered tolerant to nEs and CHO-745 by subcutaneous injection of 2 × 109 cells. Mice were given a boost 21 days later by intraperitoneal injection of 5 × 106 nEs or 5 × 105 nCHO-745 cells. Tolerant animals were identified by L-IFA with a 1:10 dilution of serum, 21 days after the boost (day 42). Animals given no IF or only weak IF were then immunized and boosted with 5 × 106mIECSA or 5 × 105 CHO–DBL-γ3 transfectant. Spleen cells from animals that responded (day 74) to mIECSA or DBL-γ3 were then used to raise mAbs by fusing these cells with P3U1 cells. The specific antiparasite surface immune responses were assessed by L-IFA.
The subcutaneous injection into the dorsal region of 2 × 109 nEs or CHO-745 cells suspended in 0.2 mL 0.9% NaCl was sufficient to induce B cell-mediated tolerance to these cells. We gave a booster injection of 5 × 106 nEs or 5 × 105 CHO-745 cells suspended in 0.4 mL 0.9% NaCl 21 days after the initial injection. Three weeks later, we tested mice for antibodies directed against surface antigens of nEs or nCHO cells, by L-IFA with a 1:10 dilution of serum.
Immunization of tolerant mice with P falciparum IEs and CHO cells expressing DBL-γ3CSA
Mice with B-mediated cell tolerance, for which no signal or only faint immunofluorescence (IF) was observed with nEs or nCHO cells, were selected for the specific immunization protocol. Approximately 5 × 106 highly synchronized mIECSA or 5 × 105 of transfected CHO cells expressing the DBL-γ3 domain of varCSA were injected into each mouse as described in Figure 1.
Development of mAbs
Mice giving positive IFA results with mIECSA or CHO–DBL-γ3 were used for the development of mAbs. We produced mAbs by fusing mouse spleen cells with P3U1 cells as described elsewhere.17 18 IFA+ cells were cloned by limiting dilution and reassessed by L-IFA; positive clones of interest were recloned by limiting dilution. The mAbs that reacted strongly with the cell surface were expanded and isotyped by enzyme-linked immunosorbent assay (ELISA), using the ImmunoPure Monoclonal Antibody Isotyping Kit (Pierce, Rockford, IL).
Indirect L-IFA and air-dried IFA
We used 2 different types of indirect IFA for assessing the polyclonal antibody responses of mice and for the initial screening of mAbs: with thin air-dried infected blood smears (AD-IFA) and L-IFA performed at 4°C to prevent endocytosis with nEs or nCHO cells and asynchronous and synchronized mIECSA, mIECD36, mIEICAM-1, and CHO–DBL-γ3/varCSA transfectants. Air-dried infected blood smears and fresh placenta cryosections were washed twice with phosphate-buffered saline (PBS; pH 7.4). Smears were incubated for 30 minutes at room temperature with 1 μg/mL 4,6-diamidino-2-phenyl-indole dihydrochloride (DAPI; Molecular Probes, Eugene, OR) for nuclear staining and with mAbs containing culture supernatants or 10 μg/mL purified mAbs. The smears were washed and incubated with a goat (Fab′)2 Alexa Fluor 488-labeled antimouse immunoglobulin (Ig) G or IgM (Molecular Probes) at a dilution of 1:200 for an additional 30 minutes at room temperature. The slides were then washed and mounted in 30% (vol/vol) glycerol in PBS. For L-IFA, we washed 10 μL nEs or asynchronous or synchronized mIECSA, mIECD36, mIEICAM-1 twice with culture medium without Albumax and incubated these cells in 5 μg/mL DAPI at 37°C for 45 minutes. The nEs and IEs were washed and incubated with culture supernatant or 10 μg/mL purified mAb at 4°C for 30 minutes, washed twice, and incubated at 4°C for an additional 30 minutes with a goat (Fab′)2 Alexa Fluor 488-labeled antimouse IgG or IgM (Molecular Probes) at a dilution of 1:200. In some cases, mIECSA were incubated with 100 μg/mL trypsin or chymotrypsin before the addition of mAbs, as previously described.19 For the staining of sequestrated mIEs in placenta cryosections from women with malaria, we used the AD-IFA procedure with Evans blue counterstaining (1:10 000 dilution) and simultaneous incubation with goat (Fab′)2 Alexa Fluor 488-labeled antimouse IgG or IgM (Molecular Probes) at a dilution of 1:200. Immunofluorescence staining was analyzed with a Nikon E800 microscope and images were acquired with a DDx Nikon camera (Tokyo, Japan).
ELISA
The ELISA was performed with a slightly modified version of a published protocol.20 Briefly, 96-well polystyrene microtiter plates (Nunc-Polylabo, Strasbourg, France) were coated with 10 μg/mL recombinant DBL-γ/3varcsa of the FCR3 strain (rDBL-γ/3varcsa) produced in an insect cell expression system (Fusai et al, manuscript in preparation). The plates were incubated overnight at 4°C, and unbound antigen was removed by washing with 0.05% Tween-20 in PBS (PBST). Possible residual free sites were saturated by treatment with 1% bovine serum albumin (BSA) in PBS for 1 hour at 37°C, and the plates were washed 4 times with PBST. We then added 100 μL mAb supernatant or 10 μg/mL purified mAb to duplicate wells, and incubated the plates for 2 hours at 37°C. Wells were washed with PBST and the plates were incubated at 37°C for 1 hour with a peroxidase-labeled goat antimouse IgG (Sigma, l'Isle Abeau Chesnes, France) diluted 1:4000 in PBST. Bound immunocomplexes were detected with ο-phenylenediamine (Sigma). Absorbance was read at 405 nm on a Multiskan Ascent ELISA reader (Labsystem, Helsinki, Finland). A positive result was considered to have been obtained for a mAb (+) (Table1) if the OD value was above the cutoff point set at 3 SDs above the mean background absorbance of P3U1 supernatant or unrelated mouse IgG isotypes or IgM.
Characterization of anti-mIECSA and anti–DBL-γ3CSA mAbs by IFA and ELISA
| mAbs . | L-IFA . | AD-IFA . | ELISA . | |||||
|---|---|---|---|---|---|---|---|---|
| Isotype . | CSA . | CD36 . | ICAM-1 . | CSA . | CD36 . | ICAM-1 . | rDBL-γ3 . | |
| Anti-mIECSA | ||||||||
| 3F3/C2/C1 | IgG2a | + | − | − | + | − | − | + |
| 1H6/A4 | IgG1 | + | − | − | + | − | − | + |
| 2H5/F10 | IgM | + | − | − | + | − | − | − |
| 1E5/D6 | IgM | + | − | − | + | − | − | + |
| 1E5/E4 | IgM | + | − | − | + | + | + | + |
| 2A11/E4 | IgM | + | − | − | + | + | + | + |
| Anti-CHO-DBL-γ33 | ||||||||
| 1B4/D4 | IgG2a | + | − | − | + | − | − | + |
| 1B11/A5 | IgM | + | − | − | + | − | − | + |
| 1C5/D12 | IgM | + | − | − | + | − | − | − |
| 2F5/G10 | IgM | + | − | − | + | − | − | + |
| 4F10/C8 | IgM | + | − | − | + | − | − | − |
| mAbs . | L-IFA . | AD-IFA . | ELISA . | |||||
|---|---|---|---|---|---|---|---|---|
| Isotype . | CSA . | CD36 . | ICAM-1 . | CSA . | CD36 . | ICAM-1 . | rDBL-γ3 . | |
| Anti-mIECSA | ||||||||
| 3F3/C2/C1 | IgG2a | + | − | − | + | − | − | + |
| 1H6/A4 | IgG1 | + | − | − | + | − | − | + |
| 2H5/F10 | IgM | + | − | − | + | − | − | − |
| 1E5/D6 | IgM | + | − | − | + | − | − | + |
| 1E5/E4 | IgM | + | − | − | + | + | + | + |
| 2A11/E4 | IgM | + | − | − | + | + | + | + |
| Anti-CHO-DBL-γ33 | ||||||||
| 1B4/D4 | IgG2a | + | − | − | + | − | − | + |
| 1B11/A5 | IgM | + | − | − | + | − | − | + |
| 1C5/D12 | IgM | + | − | − | + | − | − | − |
| 2F5/G10 | IgM | + | − | − | + | − | − | + |
| 4F10/C8 | IgM | + | − | − | + | − | − | − |
Eleven mAbs have been analyzed in detail.
L-IFA indicates liquid immunofluorescence assay using intact IEs; AD-IFA, air-dried immunofluorescence assay of IEs; (+) positive; (−) negative.
Immunoprecipitation of 125I surface-labeled mIECSA
We used mAbs to immunoprecipitate the corresponding proteins from surface 125I-labeled synchronized IECSAtrophozoite stage parasite extracts, as previously described.10 IgM mAb immune complexes were recovered by incubation with an antimouse μ chain-specific goat IgG (Sigma) followed by precipitation with protein G–Sepharose. A pool of sera from multiparous Cameroonian women8 was used as a positive control and unrelated mouse IgM and IgG isotypes were used as negative controls.
Results
Induction of B cell–mediated tolerance to human erythrocytes and CHO cells
The number of Balb/c mice found to be tolerant after 2 injections of human erythrocytes or CHO cells (details are in Figure 1) was variable. About 10% to 40% of the mice injected (depending on the series) with nEs did not develop antibodies. Another 20% to 40% gave faint IF, and the other mice presented positive IF signals of various intensities. The proportion of mice displaying B cell–mediated tolerance to nCHO cells was much lower, at 2% to 5%. The best results for the production of specific antibodies against new surface antigens were obtained with “IF-negative” animals, but satisfactory results were also achieved with animals that gave faint IF signals.
mAbs against P falciparum IE surface antigens
The scores for specific mAbs directed against surface-exposed antigens on IEs in general were high and similar for mice immunized against trophozoite-IECSA or CHO cells expressing DBL-γ3. Typically, 20% to 60% of the 460 wells screened per fusion reacted with the surface of IEs but not with nEs. The initial selection of positive wells was based on the screening by L-IFA of mature parasite stage IEs of the CSA adhesive phenotype. The 43 mAbs chosen for this study, obtained from mice immunized against DBL-γ3 and against IECSA of the trophozoite stage, gave surface positive IF signals only with mIECSA (Figure2A), but not with other parasites that express the CD36 or ICAM-1 adhesive phenotypes.
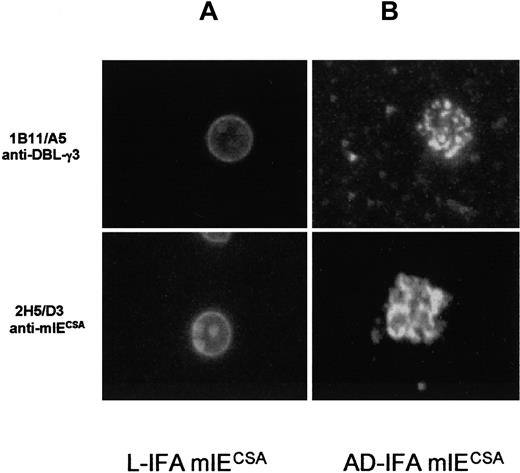
Surface staining by L-IFA and AD-IFA.
Surface staining of live FCR3 mIECSA by L-IFA with 10 μg/mL anti-mIECSA mAb 2H5/D3 and 10 μg/mL anti–DBL-γ3 mAb 1B11/A5 (A). Staining by AD-IFA of the parasitophorous vacuole and vesiclelike structures in mIECSA with 10 μg/mL anti-mIECSA mAb 2H5/D3 and anti–DBL-γ3 mAb 1B11/A5 (B).
Surface staining by L-IFA and AD-IFA.
Surface staining of live FCR3 mIECSA by L-IFA with 10 μg/mL anti-mIECSA mAb 2H5/D3 and 10 μg/mL anti–DBL-γ3 mAb 1B11/A5 (A). Staining by AD-IFA of the parasitophorous vacuole and vesiclelike structures in mIECSA with 10 μg/mL anti-mIECSA mAb 2H5/D3 and anti–DBL-γ3 mAb 1B11/A5 (B).
This IF was completely abolished by treating mIECSA with trypsin and chymotrypsin (100 μg/mL for 30 minutes at 37°C). All 43 mAbs reacted with the parasitophorous vacuole and vesiclelike structures (Maurer clefts) of mIECSA (Figure 2B). Unlike L-IFA, cross-reactivity with other adhesive phenotypes was observed for some mAbs with air-dried parasites (Table 1).
We found that, by AD-IFA, about 33% of the anti-mIECSAmAbs cross-reacted with similar cell structures in mIECD36and mIEICAM-1. This suggests the existence of cross-reactive epitopes on intra–IE-PfEMP1, which are not accessible to antibodies once the protein is exposed on the IE surface. Anti-nE mAbs were observed only at very low frequency (0.5%), demonstrating the efficacy of this novel immunization protocol.
We isotyped the mAbs used in this study and found that the anti-mIECSA and anti–DBL-γ3 mAbs were predominantly of the IgM isotype: 75% of anti–CHO-DBL-γ3CSA mAbs were IgM and 25% were IgG2a. For anti-mIECSA mAbs, 66.7% were IgM, 25% were IgG2a, and 8.3% were IgG1 (Table 1). All mAbs carried a κ light chain.
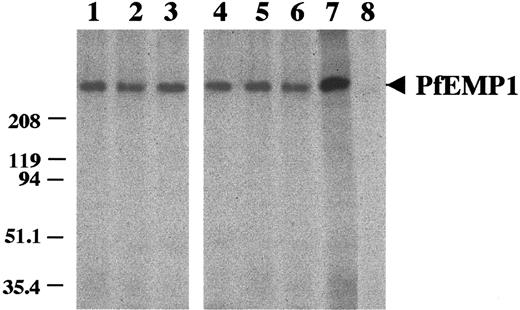
We investigated the reactivity of mAbs with parasite surface molecules, using extracts of synchronized I125 surface-labeled mIECSA. Both types of mAb, anti-mIECSA and anti–DBL-γ3, immunoprecipitated a molecule of approximately 400 kd, previously shown to correspond to PfEMP1CSA (Figure3).10
Immunoprecipitation of PfEMP1CSA from surface 125I-labeled FCR3-mIECSA extract.
Lanes 1, 2, and 3: anti-mIECSA mAbs 2H5/D3, 2A11/B7, and 2A2/G6. Lanes 4, 5, and 6: anti–DBL-γ3 mAbs 1B11/A5, 4F10/G9, and 1B4/G10. Lane 7 positive control: pool of sera from multigravidae from Cameroon. Lane 8: unrelated IgM.
Immunoprecipitation of PfEMP1CSA from surface 125I-labeled FCR3-mIECSA extract.
Lanes 1, 2, and 3: anti-mIECSA mAbs 2H5/D3, 2A11/B7, and 2A2/G6. Lanes 4, 5, and 6: anti–DBL-γ3 mAbs 1B11/A5, 4F10/G9, and 1B4/G10. Lane 7 positive control: pool of sera from multigravidae from Cameroon. Lane 8: unrelated IgM.
No other proteins, such as rifins, were detected, indicating that the immune response to the native IE is largely directed against the PfEMP1 molecule. There is no cross-reactivity with I125surface-labeled mIE of the CD36 phenotype that matches the L-IFA data.
DBL-γ3CSA is the target of most anti-mIECSA mAbs
The specificity of anti-mIECSA mAbs for PfEMP1CSA was further analyzed by testing their reactivity to the domain that binds to CSA. To this end, a rDBL-γ3/varCSA was produced by an insect cell expression system. This recombinant consisted of the same DBL-γ3 region of FCR3 expressed by the CHO transfectant that specifically binds CSA.10 The recombinant rDBL-γ3/varCSA protein inhibits the cytoadhesion of mIECSA to endothelial cells and syncytiotrophoblasts by more than 60% (100 μg/mL) and mAbs raised against it inhibit also IE adhesion to CSA by more than 90%. We assume that rDBL-γ3/varCSA protein carries conformational epitopes, because the same domain expressed as bacterial GST-fusion protein did not inhibit parasite adhesion to CSA and the antibody response in mice did not react with the native parasite molecule (Fusai et al, manuscript in preparation).
The rDBL-γ3/varCSA protein reacted specifically with 15 of 23 anti-mIECSA mAbs in ELISA. As expected, almost all anti-CHO-DBL-γ3 mAbs recognized rDBL-γ3/varCSA (85%). The intensity of surface IF and the absorbance values obtained in ELISA were not correlated (Table 1). We conclude that the DBL-γ3 domain not only mediates adhesion to CSA but also acts as an immunodominant region of PfEMP1CSA at least when using this immunization procedure described here.
Pan-reactivity of anti-CHO-DBL-γ3CSA and anti-mIECSA mAbs
Two mAbs, 2H5/D3 and 1B11/A5, respectively anti-mIECSAand anti-CHO-DBL-γ3, were arbitrarily chosen because of their typical reactivity of the other mAbs with multiple variants of a number of CSA-binding parasites from different geographic regions (Brazil, Thailand, and West Africa). Surface staining by L-IFA showed that all 7 laboratory strains analyzed (Table 2) reacted with both mAbs, 2H5/D3 and 1B11/A5, at varying degrees (2%-98%) in laboratory strains not previously selected for CSA binding (Table 2).
Analysis of pan-reactivity of anti-mIECSA (mAb 2H5/D3) and anti–DBL-γ3CSA (mAb 1B11/A5)
| Strain . | L-IFA . | % inhibition of cytoadhesion . | ||||
|---|---|---|---|---|---|---|
| 2H5/D3 . | 1B11/A5 . | CSA (100 μg/mL) . | Chondroitinase ABC (1 U/mL) . | |||
| Before panning . | After panning . | Before panning . | After panning . | |||
| B358 | 2 | > 94 | 2 | > 94 | 96 | 90 |
| BXII | 5 | > 94 | 5 | > 94 | 95 | 92 |
| FCBR | 5 | > 94 | 4 | > 94 | 95 | 96 |
| SUK | 98 | > 94 | 95 | > 94 | 90 | 91 |
| H | 2 | > 94 | 3 | > 94 | 92 | 93 |
| IBR | 97 | > 94 | 95 | > 94 | 90 | 95 |
| FCR3 | 0.3 | > 94 | 0.3 | > 94 | 91 | 96 |
| Strain . | L-IFA . | % inhibition of cytoadhesion . | ||||
|---|---|---|---|---|---|---|
| 2H5/D3 . | 1B11/A5 . | CSA (100 μg/mL) . | Chondroitinase ABC (1 U/mL) . | |||
| Before panning . | After panning . | Before panning . | After panning . | |||
| B358 | 2 | > 94 | 2 | > 94 | 96 | 90 |
| BXII | 5 | > 94 | 5 | > 94 | 95 | 92 |
| FCBR | 5 | > 94 | 4 | > 94 | 95 | 96 |
| SUK | 98 | > 94 | 95 | > 94 | 90 | 91 |
| H | 2 | > 94 | 3 | > 94 | 92 | 93 |
| IBR | 97 | > 94 | 95 | > 94 | 90 | 95 |
| FCR3 | 0.3 | > 94 | 0.3 | > 94 | 91 | 96 |
L-IFA with mIEs of 7 laboratory strains from different geographic regions. The results obtained with both mAbs are presented as the percentages of positive surface-stained mIEs in the initial population (before panning) and in the CSA-selected subpopulation (after panning on Scl7 Saimiri brain microvascular endothelial cells). The specificity of the panned cytoadhesion phenotype was confirmed by a cytoadhesion inhibition assay in the presence of soluble CSA and after chondroitinase ABC treatment of the endothelial cells. The observed inhibition of cytoadhesion was expressed as percentage inhibition with respect to the control.
Panning of each of these parasite strains on Sc17 cells, which carry CSA as the only adhesion receptor, resulted in a considerable enrichment in mIE, which reacted with both mAbs (> 94%) in most laboratory strains. Cytoadhesion inhibition assays on Sc1D cells with these 6 panned parasite subpopulations resulted in the inhibition of mIE adhesion, by 90% to 96%, by 100 μg/mL CSA or 1 U/mL chondroitinase ABC treatment of the endothelial cells (Table 2). Analysis of such CSA panned parasites by reverse transcription–polymerase chain reaction showed the expression of one type of varCSA gene10 (data not shown).
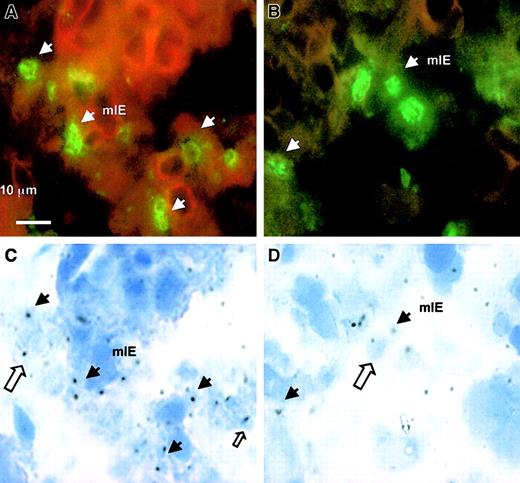
The reactivity of 2H5/D3 and 1B11/A5 with placental isolates from 6 different women infected with malaria was investigated using placental tissue cryosections. All sections showed large numbers of adhering parasites and gave strong signals with the 2 mAbs. A typical example of the antibody staining is shown in Figure4.
Reactivity of mAbs 2H5/D3 and 1B11/A5 with placental parasites using tissue cryosections of a placenta from West African women.
Positive IF staining of sequestered mIE (plain arrow) with 10 μg/mL anti-mIECSA mAb (A) and with 10 μg/mL anti–DBL-γ3 mAb 1B11/A5 (B). Panels C and D show the corresponding placental cryosections under standard light transmission microscopy. A plain arrow shows the mIE corresponding to the IF-stained mIE in the upper part of the figure. Open arrows show that some of the mIEs are not recognized by these mAbs.
Reactivity of mAbs 2H5/D3 and 1B11/A5 with placental parasites using tissue cryosections of a placenta from West African women.
Positive IF staining of sequestered mIE (plain arrow) with 10 μg/mL anti-mIECSA mAb (A) and with 10 μg/mL anti–DBL-γ3 mAb 1B11/A5 (B). Panels C and D show the corresponding placental cryosections under standard light transmission microscopy. A plain arrow shows the mIE corresponding to the IF-stained mIE in the upper part of the figure. Open arrows show that some of the mIEs are not recognized by these mAbs.
However, only a fraction of the pigmented erythrocytes in the placenta were stained with 2H5/D3 and 1B11/A5 (approximately between 40% and 60%), suggesting the presence of parasites that might bind to a distinct placental receptor such as the Fc/IgG receptor or hyaluronic acid.21 22 We conclude that the 2 mAbs, 2H5/D3 and 1B11/A5, directed against FCR3 DBL-γ3CSA, define cross-reacting epitopes that are conserved in geographically and genetically distinct CSA-binding parasite populations, including clinical isolates, and are involved in human placental infection.
Discussion
The immunization protocol developed in this work was highly efficient at generating large sets of mAbs specifically directed against the native form of present on the surface of IEs. The analysis of these mAbs led to a number of novel and important observations. First, it was noticed that mice immunized with intact parasitized erythrocytes developed mainly variant specific mAbs. No cross-reactivity was observed with the surface of IE expressing a PfEMP1 able to bind to CD36 or ICAM-1. This shows that the immune response against a native PfEMP1 molecule on the surface of IE is primarily variant specific. Although we obtained a large number of mAbs directed against the surface of mIE with this novel immunization protocol, all of those analyzed in more detail immunoprecipitate the same large molecule of approximately 400 kd, indicating that the major immunodominant surface molecule is PfEMP1 and that other molecules, such as rifins,23 24 are probably only minor targets of the antibody response against the IE surface.
Second, the mAbs against mIECSA and CHO–DBL-γ3CSA were predominantly of the IgM subclass. This result contrasts with the exclusive development of IgG mAbs against a purified recombinant DBL-γ3/varCSAproduced in insect cells (Fusai et al, manuscript in preparation), using “Titer Max” as adjuvant (Pierce) in a conventional immunization procedure. These data strongly suggest that the type of immune response to variant surface molecules depends on the context in which the antigen is presented to the immune system. It would be of interest to investigate whether the immune response in malaria patients to P falciparum IEs includes a major IgM component.
The quality of the antibody response in mice to the native PfEMP1 or DBL-γ3/varCSA domain expressed on the surface of CHO cells contrasts with the type of immune response obtained with a rDBL-γ3/varCSA domain expressed inEscherichia coli (GST fusion protein). The antibodies produced in response to the recombinant GST fusion protein (in mice, guinea pigs, or rabbits; data not shown) were not able to react with the surface of mIECSA. This result demonstrates the importance of conformational epitopes in the natural immune response to the surface of IEs. These are important considerations for epidemiologic studies attempting to correlate antibody reactivity to the var gene domains expressed in E coli with clinical immunity status in patients with malaria.
Previous work revealed that the CSA-binding region of the PfEMP1 protein might be a vaccine candidate that could protect pregnant women from malaria.25 However, it was pointed out that the genetic diversity of the DBL-γ3/varCSA domain in clinical isolates could be a major obstacle in the development of this vaccine. Our results add a new dimension to the validation of this vaccine candidate. Here we demonstrate that mAbs that react specifically with the DBL-γ3/varCSA domain have a strong pan-reactive component. Genetically different laboratory parasite strains selected on the basis of CSA binding (from Brazil, Thailand, and West Africa) and 6 placental isolates from central Africa specifically reacted with mAbs directed against the surface of DBL-γ3/varCSA of the FCR3 parasite. These data clearly demonstrate the presence of conserved conformational epitopes in CSA-binding parasites and confirm experimentally a prediction made by previous epidemiologic studies on placental malaria. This model, established by the work of Fried and Duffy,4 is based on the observation that multiparous women develop antibodies that inhibit IE-CSA adhesion in isolates from different endemic areas.
Extending this immunization procedure to other adhesive phenotypes may also provide more information concerning the involvement of other adhesive phenotypes such as CD36, ICAM-1, and platelet–endothelial cell adhesion molecule 1/CD3126,27 in adhesion-associated pathogenesis. Furthermore, our novel immunization procedure efficiently generated a large set of mAbs directed against erythrocytes infected with parasites at an early stage of development, the ring stage.28 We have developed, a large number of mAbs against IE ring-stage surface molecules (J.-B. L. D. et al, manuscript in preparation).
In conclusion, these newly generated mAbs are unique tools for screening for new Plasmodium surface antigens, mapping adhesive domains, purifying antigens, and studying the prevalence of defined adhesive phenotypes in peripheral blood and necropsies. Furthermore, the immunization method described here may be extended to any cell surface modification induced by a pathogenic process. In particular, it could be used for other Plasmodium species and human and animal pathogens that infect erythrocytes such asBartonella and Babesia and could also be extended to tumor markers on cancer cells.
We thank Catherine Lépolard and Christine Scheidig for technical assistance and Lindsay Pirrit for help with the article.
Prepublished online as Blood First Edition Paper, May 17, 2002; DOI 10.1182/blood-2002-01-0315.
Supported by grants from the European Union for Research and Technical Development (contract no. QLK2-CT2000-00109 and IC18-CT98-0362), program PAL+ 2000 of the MENRT and a DGA/PEA no. 980814. J.-B.L.D. is a doctoral fellow supported by grants from Bourses et Stages Gabon-BGE 1998-753 and FRM-FDT 20010920048/1.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jürg Gysin, Unité de Parasitologie Expérimentale, URA IPP/UNIV-MED/IMTSSA EA3282, Faculté de Médecine, Université de la Méditerranée (Aix-Marseille II), 13385 Marseille Cedex 5, France; e-mail:gysin@medecine.univ-mrs.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal