Hereditary hemochromatosis is most commonly caused by homozygosity for a point mutation (C282Y) in the human hemochromatosis gene (HFE). The mechanism by which HFEregulates iron absorption is not known, but the C282Y mutation results in loss of cell surface expression of the human hemachromatosis protein (HFE) and hyperabsorption of iron by the duodenal enterocyte. Mice homozygous for a deletion in the mouse hemochromatosis gene (Hfe) or a mutation equivalent to that seen in human hereditary hemochromatosis (C282Y) were compared with wild-type animals for their ability to regulate iron absorption. Both mutant strains hyperabsorbed 59Fe administered by gavage. Feeding a diet supplemented with carbonyl iron resulted in a more than 5-fold reduction of 59Fe absorption in both wild-type and mutant mouse strains. Similarly, the iron loading associated with age inHfe mutant mice resulted in nearly a 4-fold reduction in iron absorption. When mice were stimulated to absorb iron either by depleting iron stores or by inducing erythropoiesis, wild type andHfe mutant strains increased absorption to similar levels, approximately 5-fold over control values. Our data indicate thatHfe mutant mice retain the ability to regulate iron absorption. Mouse hemachromatosis protein (Hfe) plays a minor role in down-regulation but does not influence the up-regulation of iron absorption.
Introduction
Hereditary hemochromatosis is one of the most common inherited disorders of whites, affecting nearly 5 per 1000 people of Northern European extraction.1 The gene mutated in hereditary hemochromatosis, the human hemochromatosis gene (HFE), is a major histocompatibility complex (MHC) class I–like gene.2 The HFE mutation most frequently found in hereditary hemochromatosis results in a cysteine-to-tyrosine conversion at amino acid 282 in a region of the human hemachromatosis protein (HFE) corresponding to the α3 domain in class I proteins. This mutation results in the loss of interaction with β2-microglobulin and decreased cell surface expression of HFE.3 The HFE-β2microglobulin heterodimer interacts with the transferrin receptor (TfR), resulting in a conformational change causing a reduction in the affinity of the receptor for diferric transferrin (Tf (Fe)2).4-6 Despite increasing information regarding HFE structure and expression,7 8 the mechanism by which HFE regulates iron absorption remains undefined.
Iron absorption is mediated by the duodenal enterocyte. Absorption is affected by the rate at which iron is imported from the gut lumen into the enterocyte and the rate at which it is exported to the plasma. An importer (natural resistance–associated macrophage protein 2 (Nramp2), divalent cation transporter 1 (DCT1), divalent metal transporter 1 (DMT1)) and an exporter (ferroportin1, iron-regulated transporter 1 (Ireg1), metal transporter protein 1 (MTP1)) in enterocytes have been identified, but whether or not HFE directly affects the activity of these iron transporters is not known.9-13 The murine hemochromatosis gene (Hfe) has been altered by recombination to produce both knockout mice and mice homozygous for the C282Y mutation (knockin).14 15 Both mutant strains develop iron overload with aging and serve as useful models for hemochromatosis. We used Hfe knockout and knockin mice to determine the contribution of mouse hemachromatosis protein (Hfe) to the regulation of intestinal iron absorption in iron-replete, iron-loaded, and iron-deficient states and in mice with stimulated erythropoiesis.
Materials and methods
Mice and diets
All mice used in these experiments were of the 129/SvEvTac strain.15 Mice were maintained in the Animal Resource Center at the University of Utah following approved guidelines. For dietary studies, mice were maintained on a standard rodent diet (Harlan Teklad TD 8640) containing approximately 0.33 g of iron per kilogram, a diet supplemented with 20 g carbonyl iron per kilogram (TD 91 013), or an iron-deficient diet (TD 80 396) containing less than 0.005 g of iron per kilogram (all from Harlan Teklad, Madison, WI).
Gavage feeding of 59Fe
Mice were fasted overnight prior to gavage but allowed water ad libitum. Approximately 2.5 μCi (9.25 × 104 Bq)59Fe-HCl diluted into 0.2 mL of a solution of 0.5 M ascorbic acid, 0.15 M NaCl, and FeSO4 for a final total of 5 μg Fe was administered to each animal with an olive-tipped gavage needle. The mice were placed in a metabolic cage, supplied with water, fasted for 7 hours more, then allowed food overnight. Approximately 24 hours after gavage, the mice were euthanized by anesthesia overdose and dissected. The gastrointestinal tract, head, and lungs were removed and blood, major organs, and carcass were analyzed for radioactivity in a gamma counter (Perkin Elmer Instruments, Shelton, CT). For dietary, age-related iron loading, and stimulated erythropoiesis experiments, percentage absorption was calculated as (iron in blood, organs, and carcass/iron administered by gavage) × 100. In a second set of experiments, mice were killed 5 hours after gavage and iron absorbed was calculated as above.
Iron measurements
Transferrin saturation was determined by means of a ferrozine-based iron and total iron-binding capacity assay (Sigma Diagnostics, St Louis, MO). Liver iron content was determined by acid digestion of liver samples followed by iron quantification with atomic absorption spectroscopy.16 Blood parameters were measured with an automated cell counter calibrated for murine samples (ABX Diagnostics, Irvine, CA).
Phenylhydrazine and phlebotomy treatment
Chemically induced hemolysis was produced by a single intraperitoneal injection of 3 mg phenylhydrazine (PHZ) per 20 g body weight. Blood measurements and gavage were conducted 5 days later. The mice were phlebotomized under anesthesia by collection of approximately 0.7 mL of blood (approximately 30% of the blood volume) from the retro-orbital sinus. Blood measurements and gavage were conducted 5 days later. Reticulocyte counts were done on blood smears stained with new methylene blue.17
Results
Mouse strains with mutations in Hfe hyperabsorb iron
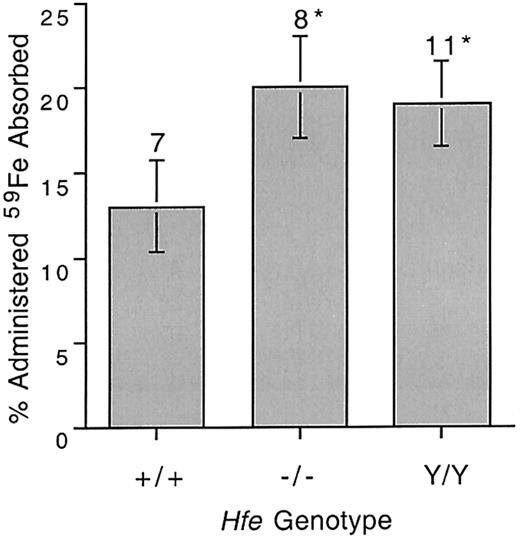
Mutations in the mouse Hfe gene result in a phenotype similar to hemochromatosis with increases in Tf saturation and liver iron content.15 We confirmed these findings in 8-week-old mice (Table 1). Iron absorption was compared in 8-week-old mice from the wild-type 129/SvEv strain (+/+), the Hfe knockout strain (−/−), and the Hfeknockin strain (Y/Y). Both Hfe knockout andHfe knockin mice absorbed significantly more iron than wild-type animals (Figure 1).
Transferrin saturation and liver iron content in control and Hfe mutant mouse strains at 8 weeks of age
| Strain . | % Tf saturation . | Liver iron (μg/g wet weight) . |
|---|---|---|
| +/+ (n = 19) | 51 ± 3 | 223 ± 11 |
| −/− (n = 14) | 83 ± 3* | 936 ± 57* |
| Y/Y (n = 19) | 78 ± 4* | 735 ± 56* |
| Strain . | % Tf saturation . | Liver iron (μg/g wet weight) . |
|---|---|---|
| +/+ (n = 19) | 51 ± 3 | 223 ± 11 |
| −/− (n = 14) | 83 ± 3* | 936 ± 57* |
| Y/Y (n = 19) | 78 ± 4* | 735 ± 56* |
+/+ indicates wild type; −/−, Hfe knockout; Y/Y,Hfe knockin for the C282Y mutation. Error presented as SEM.
Values compared with those for wild type, P< .001.
Intestinal 59Fe absorption in 8-week-old wild-type and Hfe mutant mice.
59Fe was administered by gavage and percentage administered iron absorbed was determined as described in “Materials and methods.” +/+ indicates wild type; −/−, Hfe knockout; Y/Y, C282Y knockin. Error bars represent SEM. The number of animals in each group is shown above columns. *P < .04 compared with wild type mice.
Intestinal 59Fe absorption in 8-week-old wild-type and Hfe mutant mice.
59Fe was administered by gavage and percentage administered iron absorbed was determined as described in “Materials and methods.” +/+ indicates wild type; −/−, Hfe knockout; Y/Y, C282Y knockin. Error bars represent SEM. The number of animals in each group is shown above columns. *P < .04 compared with wild type mice.
Dietary iron loading in both wild type and Hfe mutant mice results in down-regulation of iron absorption
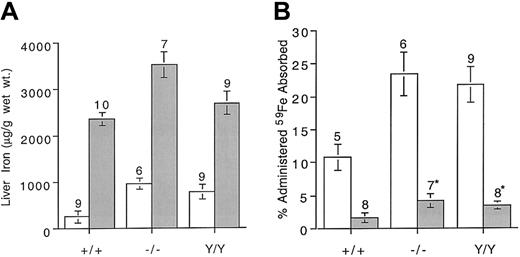
Iron loading normally leads to decreased absorption by the enterocyte. We measured absorption of 59Fe in normal and mutant mice with iron overload induced by feeding an iron-supplemented diet. Four- to 5-week-old mice were maintained for 6 weeks either on control diets or on identical formulations supplemented with 2% carbonyl iron. The mice were then killed and liver iron content was determined. Increases in liver iron content over baseline ranged from nearly 10-fold in control mice to more than 3-fold in Hfemutant mice (Figure 2A). Iron absorption in response to dietary iron supplementation in wild-type, knockout, and knockin strains was reduced 5-, 5.6-, and 6.5-fold, respectively, compared with mice maintained on control diets (Figure 2B). Although all mice down-regulated absorption, the mutant strains continued to absorb more iron than the wild type (P < .04). When Tf saturation levels are high, newly absorbed non–Tf-bound iron is rapidly cleared by the liver.18 19 The distribution of absorbed iron in blood and liver was compared in the 3 strains of mice (Table 2). As expected, a greater fraction of the absorbed iron was deposited in the livers of iron-loaded mice.
Liver iron content and 59Fe absorption in control mice and mice with dietary iron loading.
(A) Comparison of liver iron content in 5-week-old mice maintained on a control diet or a 2% carbonyl iron–supplemented diet for 6 weeks. (B) Five-week-old mice were maintained on a control diet or a 2% carbonyl iron–supplemented diet for 6 weeks. 59Fe was administered by gavage and percentage administered iron absorbed was determined as described in “Materials and methods.” Genotypes are as defined in Figure 1. Open bars indicate control diet; shaded bars, carbonyl iron diet. Error bars represent SEM. The number of animals in each group is shown above columns. * P < .04 compared with wild-type mice maintained on the carbonyl iron diet.
Liver iron content and 59Fe absorption in control mice and mice with dietary iron loading.
(A) Comparison of liver iron content in 5-week-old mice maintained on a control diet or a 2% carbonyl iron–supplemented diet for 6 weeks. (B) Five-week-old mice were maintained on a control diet or a 2% carbonyl iron–supplemented diet for 6 weeks. 59Fe was administered by gavage and percentage administered iron absorbed was determined as described in “Materials and methods.” Genotypes are as defined in Figure 1. Open bars indicate control diet; shaded bars, carbonyl iron diet. Error bars represent SEM. The number of animals in each group is shown above columns. * P < .04 compared with wild-type mice maintained on the carbonyl iron diet.
Distribution of absorbed 59Fe and percentage transferrin saturation in mice maintained on control or 2% carbonyl iron–supplemented diets
| Strain . | Diet . | Blood, % . | Liver, % . | % Tf saturation . |
|---|---|---|---|---|
| +/+ (n = 5) | Control | 33.5 ± 4.5 | 8.8 ± 0.8 | 48.0 ± 1.7 |
| +/+ (n = 8) | High Fe | 20.1 ± 5.0 | 36.0 ± 9.8* | 59.8 ± 7.0 |
| −/− (n = 6) | Control | 18.7 ± 3.7 | 43.7 ± 5.5 | 81.0 ± 5.0 |
| −/− (n = 7) | High Fe | 7.0 ± 0.9* | 67.8 ± 1.6* | 89.0 ± 4.7 |
| Y/Y (n = 9) | Control | 30.0 ± 3.0 | 29.0 ± 5.5 | 86.1 ± 2.7 |
| Y/Y (n = 8) | High Fe | 16.7 ± 2.2* | 46.8 ± 4.6* | 92 ± 3.4 |
| Strain . | Diet . | Blood, % . | Liver, % . | % Tf saturation . |
|---|---|---|---|---|
| +/+ (n = 5) | Control | 33.5 ± 4.5 | 8.8 ± 0.8 | 48.0 ± 1.7 |
| +/+ (n = 8) | High Fe | 20.1 ± 5.0 | 36.0 ± 9.8* | 59.8 ± 7.0 |
| −/− (n = 6) | Control | 18.7 ± 3.7 | 43.7 ± 5.5 | 81.0 ± 5.0 |
| −/− (n = 7) | High Fe | 7.0 ± 0.9* | 67.8 ± 1.6* | 89.0 ± 4.7 |
| Y/Y (n = 9) | Control | 30.0 ± 3.0 | 29.0 ± 5.5 | 86.1 ± 2.7 |
| Y/Y (n = 8) | High Fe | 16.7 ± 2.2* | 46.8 ± 4.6* | 92 ± 3.4 |
Mouse strains are explained in Table 1. Error presented as SEM.
Values for control vs high Fe diet, P < .05. Percentages represent the percentage of absorbed radioiron.
Iron absorption is down-regulated with age
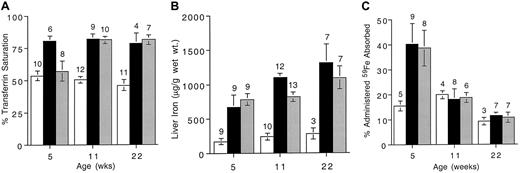
The morbidity associated with hemochromatosis increases with age and is due to the gradual accumulation of iron in parenchymal organs. The Tf saturation in Hfe mutant mice is elevated from an early age and these animals have increased liver iron stores by 4 weeks of age.15 The Tf saturation and liver iron stores were monitored for approximately 22 weeks and values in Hfemutants were compared with those of wild-type animals. As expected, Tf saturations in Hfe mutant mice were elevated at 5 weeks of age and stayed relatively constant (Figure3A). Liver iron content was higher in mutant mice as early as 5 weeks of age and continued to increase over the duration of the study (Figure 3B). In contrast, in control animals liver iron content remained constant over the 22-week study period. We then determined whether age-related iron loading resulted in down-regulation of iron absorption. Mice 5 weeks, 11 weeks, and 22 weeks of age were given 59Fe by gavage and absorption was measured. Absorption in +/+ mice was moderately reduced over time, but the Hfe mutant mouse strains showed a marked reduction in absorption with age (Figure 3C). In every case, down-regulation between 5 and 22 weeks of age was significant (P < .04). These data are consistent with the dietary iron loading results (Figure 2) and indicate that Hfe mutant mice down-regulate iron absorption when body iron stores are increased.
Iron measurements in wild-type and Hfemutant mice of different ages.
(A) Transferrin saturation was determined in 5-, 11-, and 22-week-old mice. (B) Liver iron was measured in 5-, 11-, and 22-week-old mice. (C) Five-, 11-, and 22-week-old mice were administered 59Fe by gavage and percentage administered iron absorbed was determined as described in “Materials and methods.” Open bars show results for wild-type mice (+/+); black bars, Hfe knockout mice (−/−; shaded bars, C282Y knockin (Y/Y). Error bars represent SEM. The number of animals in each group is shown above columns.
Iron measurements in wild-type and Hfemutant mice of different ages.
(A) Transferrin saturation was determined in 5-, 11-, and 22-week-old mice. (B) Liver iron was measured in 5-, 11-, and 22-week-old mice. (C) Five-, 11-, and 22-week-old mice were administered 59Fe by gavage and percentage administered iron absorbed was determined as described in “Materials and methods.” Open bars show results for wild-type mice (+/+); black bars, Hfe knockout mice (−/−; shaded bars, C282Y knockin (Y/Y). Error bars represent SEM. The number of animals in each group is shown above columns.
Wild-type and Hfe mutant mice increase iron absorption in response to reduced iron stores and accelerated erythropoiesis
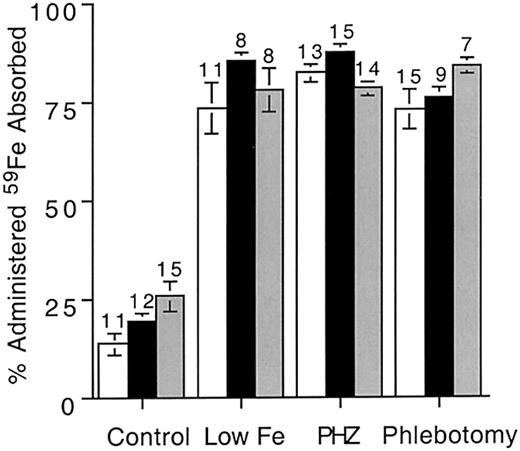
The preceding data indicate that Hfe expression is not required for the down-regulation of iron absorption. To determine the influence of Hfe on the up-regulation of iron absorption, we compared responses to different absorptive signals in wild-type andHfe mutant mice. Gastrointestinal absorption of iron increases in response to low iron stores and erythropoiesis.20 Iron stores were reduced by placing 3-week-old weanling mice on diets lacking iron for a period of 7 weeks. Erythropoiesis was induced either by treatment with PHZ or by phlebotomy (see “Materials and methods”). Blood samples were taken the day prior to 59Fe gavage, when hematocrit, hemoglobin concentration, and mean corpuscular volume (MCV) were measured. In wild-type and Hfe mutant mice maintained on iron-restricted diets, the hematocrit remained constant but the hemoglobin concentration dropped slightly (Table 3). Liver iron content was significantly reduced (P < .007) in all mice following the low-iron diet, indicating that erythropoiesis was maintained by mobilization of liver iron stores. MCV dropped in mice maintained on the low-iron diet (P < .003), suggesting that iron-limited erythropoiesis was developing. Percents of Tf saturation dropped slightly in all groups, but the differences were not statistically significant (data not shown).
Hematologic values and liver iron concentration in control and Hfe mutant mice
| . | +/+ . | −/− . | Y/Y . | |||
|---|---|---|---|---|---|---|
| Control (n = 20) . | Fe-deficient (n = 11) . | Control (n = 22) . | Fe-deficient (n = 12) . | Control (n = 18) . | Fe-deficient (n = 14) . | |
| % Hematocrit | 48.1 ± 0.8 | 48.0 ± 0.7 | 48.7 ± 0.8 | 48.2 ± 1.0 | 46.2 ± 0.8 | 44.9 ± 0.6 |
| Hemoglobin (g/dL) | 16.5 ± 0.3 | 15.4 ± 0.3 | 16.5 ± 0.3 | 14.3 ± 1.4 | 15.8 ± 0.2 | 14.9 ± 0.2 |
| MCV (fL) | 50.5 ± 0.4 | 48.2 ± 0.6 | 50.7 ± 0.2 | 47.6 ± 0.7 | 54.0 ± 0.5 | 49.7 ± 0.3 |
| Liver Fe (μg/g wet weight) | 205.1 ± 10 | 46.4 ± 6.2 | 805.8 ± 60.0 | 48 ± 5.8 | 662.8 ± 53.0 | 80.7 ± 24.7 |
| . | +/+ . | −/− . | Y/Y . | |||
|---|---|---|---|---|---|---|
| Control (n = 20) . | Fe-deficient (n = 11) . | Control (n = 22) . | Fe-deficient (n = 12) . | Control (n = 18) . | Fe-deficient (n = 14) . | |
| % Hematocrit | 48.1 ± 0.8 | 48.0 ± 0.7 | 48.7 ± 0.8 | 48.2 ± 1.0 | 46.2 ± 0.8 | 44.9 ± 0.6 |
| Hemoglobin (g/dL) | 16.5 ± 0.3 | 15.4 ± 0.3 | 16.5 ± 0.3 | 14.3 ± 1.4 | 15.8 ± 0.2 | 14.9 ± 0.2 |
| MCV (fL) | 50.5 ± 0.4 | 48.2 ± 0.6 | 50.7 ± 0.2 | 47.6 ± 0.7 | 54.0 ± 0.5 | 49.7 ± 0.3 |
| Liver Fe (μg/g wet weight) | 205.1 ± 10 | 46.4 ± 6.2 | 805.8 ± 60.0 | 48 ± 5.8 | 662.8 ± 53.0 | 80.7 ± 24.7 |
Erythropoiesis was stimulated either by PHZ treatment or by phlebotomy. Under control conditions, reticulocyte counts in all 3 strains averaged 1% (Table 4). Both PHZ and phlebotomy treatments produced a significant increase in reticulocyte counts, indicating stimulated erythropoiesis (Table 4). Hematocrit and hemoglobin concentrations were reduced by these treatments. There were no significant differences between strains. We next compared iron absorption between strains with either low iron stores or stimulated erythropoiesis. In all cases, absorption increased (an average of 4-fold compared with control values; Figure4). Hfe expression had no influence on the degree of increase, and all treatments resulted in similar absorption increases. These data indicate that Hfeexpression does not influence the increase of iron absorption induced by either low iron stores or accelerated erythropoiesis.
Hematologic values for control and Hfe mutant mice treated with PHZ or by phlebotomy
| . | +/+ . | −/− . | Y/Y . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 20) . | PHZ (n = 8) . | Phlebotomy (n = 9) . | Control (n = 22) . | PHZ (n = 7) . | Phlebotomy (n = 9) . | Control (n = 18) . | PHZ (n = 11) . | Phlebotomy (n = 5) . | |
| % Reticulocytes | 1.0 ± 0.37 | 15.0 ± 2.6 | 8.9 ± 1.3 | 1.0 ± 0.1 | 11.0 ± 0.6 | 9.8 ± 0.6 | 1.7 ± 0.3 | 13.0 ± 0.6 | 7.0 ± 0.9 |
| % Hematocrit | 48.0 ± 0.8 | 31.5 ± 2.5 | 36.2 ± 2.0 | 48.7 ± 0.8 | 35.2 ± 2.0 | 43.3 ± 1.9 | 46.2 ± 0.8 | 36.0 ± 1.4 | 40.4 ± 2.5 |
| Hemoglobin (g/dL) | 16.5 ± 0.3 | —4-150 | 13.6 ± 0.9 | 16.5 ± 0.3 | —4-150 | 14.6 ± 0.8 | 15.8 ± 0.2 | —4-150 | 13.9 ± 1.3 |
| MCV (fL) | 50.5 ± 0.4 | 56.5 ± 1.0 | 52.3 ± 0.5 | 50.7 ± 0.2 | 58.4 ± 1.0 | 51.4 ± 0.4 | 54.0 ± 0.5 | 56.5 ± 1.0 | 53.2 ± 0.4 |
| . | +/+ . | −/− . | Y/Y . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (n = 20) . | PHZ (n = 8) . | Phlebotomy (n = 9) . | Control (n = 22) . | PHZ (n = 7) . | Phlebotomy (n = 9) . | Control (n = 18) . | PHZ (n = 11) . | Phlebotomy (n = 5) . | |
| % Reticulocytes | 1.0 ± 0.37 | 15.0 ± 2.6 | 8.9 ± 1.3 | 1.0 ± 0.1 | 11.0 ± 0.6 | 9.8 ± 0.6 | 1.7 ± 0.3 | 13.0 ± 0.6 | 7.0 ± 0.9 |
| % Hematocrit | 48.0 ± 0.8 | 31.5 ± 2.5 | 36.2 ± 2.0 | 48.7 ± 0.8 | 35.2 ± 2.0 | 43.3 ± 1.9 | 46.2 ± 0.8 | 36.0 ± 1.4 | 40.4 ± 2.5 |
| Hemoglobin (g/dL) | 16.5 ± 0.3 | —4-150 | 13.6 ± 0.9 | 16.5 ± 0.3 | —4-150 | 14.6 ± 0.8 | 15.8 ± 0.2 | —4-150 | 13.9 ± 1.3 |
| MCV (fL) | 50.5 ± 0.4 | 56.5 ± 1.0 | 52.3 ± 0.5 | 50.7 ± 0.2 | 58.4 ± 1.0 | 51.4 ± 0.4 | 54.0 ± 0.5 | 56.5 ± 1.0 | 53.2 ± 0.4 |
Value outside the linear region of the detector's calibration curve.
59Fe absorption in mice with reduced iron stores or accelerated erythropoiesis.
Iron deficiency was induced by maintaining weanling mice (4 weeks of age) on iron-deficient diets for 7 weeks. Control mice of all genotypes were 10 to 11 weeks of age. Erythropoiesis was induced either by treating mice with phenylhydrazine (PHZ) or by phlebotomy.59Fe was administered by gavage and percentage administered iron absorbed was determined as described in “Materials and methods.” Open bars show results for wild-type mice (+/+); black bars, Hfe knockout mice (−/−; shaded bars, C282Y knockin (Y/Y). Error bars represent SEM. The number of animals in each group is shown above columns.
59Fe absorption in mice with reduced iron stores or accelerated erythropoiesis.
Iron deficiency was induced by maintaining weanling mice (4 weeks of age) on iron-deficient diets for 7 weeks. Control mice of all genotypes were 10 to 11 weeks of age. Erythropoiesis was induced either by treating mice with phenylhydrazine (PHZ) or by phlebotomy.59Fe was administered by gavage and percentage administered iron absorbed was determined as described in “Materials and methods.” Open bars show results for wild-type mice (+/+); black bars, Hfe knockout mice (−/−; shaded bars, C282Y knockin (Y/Y). Error bars represent SEM. The number of animals in each group is shown above columns.
Discussion
Although an interaction between the HFE-β2microglobulin complex and the TfR has been established, the mechanism by which the HFE protein influences iron absorption remains undefined. Iron absorption is regulated by both the magnitude of body iron stores and the rate of erythropoiesis.20 Iron homeostasis is achieved by precisely matching absorption and loss. A “store regulator” is thought to mediate this balance. When iron stores are reduced and erythropoiesis is normal, iron absorption is increased.21,22 The store regulator also acts to reduce absorption when iron stores are increased. Erythropoiesis clearly affects iron absorption and the effects of the “erythroid regulator” seem more pronounced than those of the store regulator.20
The availability of mice with mutated Hfe genes has enabled us to address the contribution of Hfe to the regulation of iron absorption. Mouse strains mutated at the Hfe locus display a phenotype similar to hereditary hemochromatosis in humans, namely elevated Tf saturations and liver iron content. One difference is thatHfe mutant mice do not develop fibrosis or cirrhosis due to iron overload. This may be due to the fact that humans are among the few mammals that cannot synthesize ascorbic acid.23 The antioxidant activity of ascorbic acid may provide some protection against oxidative damage resulting from iron overload.
Comparison of iron-loaded wild-type and mutant mice enabled us to determine whether Hfe expression is required to down-regulate iron absorption. In humans homozygous for the C282Y mutation, iron absorption is reduced when iron loading develops, but not to the same degree as in healthy controls.24-26 The Hfe Y/Y strain of mice was also capable of down-regulating iron absorption, but not to the same level as wild-type mice. Surprisingly, even in the absence of Hfe expression, the −/− strain retained the ability to down-regulate iron absorption, indicating that Hfe is not required for down-regulation. The relative contribution of Hfe is represented by the difference in down-regulation of absorption in iron-loaded Hfe mutant mice compared with iron-loaded wild-type mice (Figure 2B). Hfe's effect on down-regulation, although significant, is not great. HFE may be part of a signaling complex that responds to iron status, and in its absence the signal may be blunted. Alternatively, HFE may affect intracellular iron concentration, and when it is absent or expressed at low levels, the ability to detect iron overload is reduced.
Limitation of iron intake by dietary deprivation did not result in significant changes in hematocrit or hemoglobin concentration, even though decreased iron stores resulted in a slight reduction in MCV. The absence of anemia indicates that erythropoietic needs were met by mobilization of iron stores. The finding that Hfe mutant mice increased iron absorption in response to dietary iron deprivation demonstrates that Hfe expression is not required to respond to the signal for increased iron demand generated by a reduction in iron stores. Both wild-type and mutant mice up-regulated absorption to similar levels, suggesting that Hfe does not participate in this aspect of regulation.
Two other regulators of iron absorption have recently been identified. Genetically determined iron overload with a phenotype similar to that seen in HFE-mediated hemochromatosis was found to be associated with a mutation in the TfR2 gene.27-29 Juvenile hemochromatosis, an autosomal recessive trait mapped to chromosome 1q,30 has a much more severe phenotype thanHFE-mediated hemochromatosis, suggesting that this locus may be the major regulator of iron absorption. The ultimate effect of HFE on iron absorption is not determined solely by allelic differences. Mice with wild-type Hfe alleles display strain-specific differences in iron parameters such as percents of Tf saturation and liver iron content.31 The iron phenotype associated withHfe mutations also varies between strains32 and when combined with mutant alleles of other genes involved in iron metabolism.33 There is also a wide range of iron loading in humans homozygous for the C282Y mutation.34 The data for mice and for humans strongly suggest that modifier genes modulate the regulatory capacity of the hemochromatosis gene. These modifiers could interact with HFE directly or, more likely, modify iron loading through independent mechanisms to magnify or dampen the effects of HFE.
The authors wish to thank Drs Jerry Kaplan, John Phillips, and Munsey Wheby for their helpful advice and suggestions. Lynne Montross contributed to the production, characterization, and maintenance of theHfe mutant mice.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2001-11-0037.
Supported by National Institutes of Health grant R01 DK20630-21 (R.S.A. and J.P.K.) and by a fellowship from the American Society for Hematology (J.E.L.). N.C.A is an associate investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Richard S. Ajioka, Division of Hematology, University of Utah School of Medicine, 50 N Medical Dr, Salt Lake City, UT 84132; e-mail: richard.ajioka@hsc.utah.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal