Although the cellular and molecular mechanisms governing angiogenesis are only beginning to be understood, signaling through endothelial-restricted receptors, particularly receptor tyrosine kinases, has been shown to play a pivotal role in these events. Recent reports show that EphB receptor tyrosine kinases and their transmembrane-type ephrin-B2 ligands play essential roles in the embryonic vasculature. These studies suggest that cell-to-cell repellent effects due to bidirectional EphB/ephrin-B2 signaling may be crucial for vascular development, similar to the mechanism described for neuronal development. To test this hypothesis, we disrupted the precise expression pattern of EphB/ephrin-B2 in vivo by generating transgenic (CAGp-ephrin-B2 Tg) mice that express ephrin-B2 under the control of a ubiquitous and constitutive promoter, CMV enhancer-β-actin promoter-β-globin splicing acceptor (CAG). These mice displayed an abnormal segmental arrangement of intersomitic vessels, while such anomalies were not observed in Tie-2p-ephrin-B2 Tg mice in which ephrin-B2 was overexpressed in only vascular endothelial cells (ECs). This finding suggests that non-ECs expressing ephrin-B2 alter the migration of ECs expressing EphB receptors into the intersomitic region where ephrin-B2 expression is normally absent. CAGp-ephrin-B2 Tg mice show sudden death at neonatal stages from aortic dissecting aneurysms due to defective recruitment of vascular smooth muscle cells to the ascending aorta. EphB/ephrin-B2 signaling between endothelial cells and surrounding mesenchymal cells plays an essential role in vasculogenesis, angiogenesis, and vessel maturation.
Introduction
Angiogenesis, the formation of new vessels from the pre-existing primary plexus, occurs through several processes: vascular sprouting, branching and pruning, and the differential growth of blood vessels to form more mature vascular networks.1 These vascular events are required for embryogenesis and are also involved in several pathophysiologic conditions such as neovascularization in tumorigenesis, inflammatory disease, and ischemic disease in adulthood. Although the cellular and molecular mechanisms governing angiogenesis are only beginning to be understood, signaling through endothelial-restricted receptors, particularly receptor tyrosine kinases (RTKs),2,3 plays a pivotal role in these events. Three subfamilies out of at least 14 RTK subfamilies, vascular endothelial growth factor (VEGF) receptors (VEGFRs), Tie receptors, and Eph receptors are primarily expressed in the endothelial lining of blood vessels from embryonic stages to adulthood.3VEGFs,4-7 ligands for VEGFRs, angiopoietins (Angs),8 and ligands for Tie-2 receptor9-11play an essential role in vasculogenesis and angiogenesis. In contrast to VEGFs and Angs, ephrins, which were initially characterized by their roles in axon guidance and neuronal patterning,12 13cannot act as soluble mediators but rather must be membrane-bound to activate their receptors. This observation indicates that Eph/ephrin signaling is mediated by cell-to-cell interaction, resulting in repulsive or attractive signaling.
The recent observation of vascular defects in ephrin-B2 and EphB4 knockout mice strongly suggests that the interaction between the ephrin-B2 ligand and its cognate EphB4 receptor defines the boundaries of arterial-venous domains.14,15 Subsequent work demonstrating expression of ephrin-B2 and its cognate EphB receptors in mesenchymal cells adjacent to vascular endothelial cells (ECs) suggests an EphB/ephrin-B2 interaction at endothelial-mesenchymal contact zones.16 Eph receptors and ephrin ligands are divided into 2 broad subclasses, A and B, based on structural homologies and binding specificities. High redundancy exists within each subclass in terms of receptor/ligand binding. Ephrin-B ligands are transmembrane proteins that preferentially bind to receptors of the EphB subclass.3,13,17 It is notable that ephrin-B ligands not only activate their respective receptors but also are in turn activated upon engaging their receptors, as judged by tyrosine phosphorylation of the ephrin-B cytoplasmic domain.18,19 These findings and more recent reports20 indicate that ephrins may provide key bidirectional cues in an obligate cell-to-cell, contact-dependent fashion in the vascular system. The mechanism of Eph/ephrin-B2 signaling in the vasculature is presently not understood, although ephrins are repulsive guidance cues for navigating neural cells in the developing nervous system.12,13,17 More recently, our in vitro culture analysis demonstrated that EphB4/ephrin-B2 signaling between endothelial cells and their surrounding stromal cells regulates the proliferation and mobility of endothelial cells.21
In this study, we conduct functional studies on the role of ephrin-B2 in formation of the vascular system. To examine the role of ephrin-B2 in the vascular system in vivo, we disrupt the complementary EphB/ephrin-B2 expression pattern by generating transgenic (Tg) mice that constitutively and ubiquitously express ephrin-B2 under regulation of the actin promoter (CAGp-ephrin-B2 Tg mice). In these transgenics, abnormal ephrin-B2 signaling leads to aberrant vessel projection, abnormal vascular network formation, and defective recruitment of smooth muscle progenitor cells to the ascending aorta.
Materials and methods
Plasmids
The plasmid pCAG-pA was constructed by introducing the CMV enhancer-β-actin promoter-β-globin splicing acceptor (CAG) promotor22 (kindly provided by Dr Jun-ichi Miyazaki, Osaka University, Japan) into the expression vector, pSV2neopA (Kaken-Seiyaku, Tokyo, Japan). To generate Tg mice overexpressing ephrin-B2 ubiquitously, the HindIII-XhoI fragment of mouse ephrin-B2 cDNA (kindly provided by Dr John G. Flanagan, Harvard Medical School, Boston, MA), which includes the region of the open reading frame for ephrin-B2, was inserted at theHindIII-SalI site of pCAG-pA.
For generating inducible ephrin-B2 Tg mice (CAG-IND-ephrin-B2 Tg) in which ephrin-B2 expression is promoted by Cre-induced loxP sequence-specific recombination, a transgene containing the CAG-promoter-loxP-blasticidin(bsr)-pA-loxP sites, mouse ephrin-B2 cDNA, an internal ribosomal entry site (IRES), and a βgeo-pA sequence was generated. In brief, an IRES-βgeo-pA sequence from pGT1.8Iresβgeo23 (kindly provided by Dr Austin Smith, University of Edinburgh, United Kingdom) and a fragment containing mouse ephrin-B2 cDNA were inserted into a vector containing the CAGp-loxP-bsr-pA-loxP sequence.24
Generation and identification of CAGp-ephrin-B2 transgenic (Tg) mice
The plasmid pCAG-ephrin-B2-pA was purified using GENECLEAN II (BIO 101, Vista, CA) and suspended in 1 mM Tris-HCl (pH 7.5), 0.1 mM EDTA at a concentration of 5 μg/mL for microinjection to establish a transgenic mouse line. A total of 300 pronuclear injections in C57BL/6 mice were performed, resulting in the generation of 52 pups. Transgenic founder mice were identified by analyzing genomic DNA isolated from tail biopsies as described elsewhere.25 Primers spanning a 639–base pair (bp) segment of the mouse ephrin-B2 cDNA were (sense primer) 5′-CTCAACTGTGCCAGACCAGA-3′ and (antisense primer) 5′-ATAGTCCCCG-CTGACCTTCT-3′. Primers amplifying a 720-bp segment of the mouse ephrin-B2 cDNA plus the pA sequence were (sense primer) 5′-CTCAACTGTGCCAG-ACCAGA-3′ and (antisense primer) 5′-GGC-AGTGCTGGGTGATTTAT-3′. These reactions for amplification consisted of 28 cycles of denaturation at 94°C for 60 seconds, annealing at 58°C for 90 seconds, and extension at 72°C for 90 seconds. Screening of 50 surviving pups identified 3 as founders. To examine expression of the transgene, Northern analysis of total ear RNA was performed on the selected 3 lines, using mouse ephrin-B2 cDNA as a probe. Two lines expressing the transgene were selected for further analysis. To maintain an isogenic strain, all mice were propagated as heterozygous Tg mice by breeding with wild-type C57BL/6 mice. F2-, F3-, or F4-generation mice were used in all studies.
Generation of CAGp-IND-ephrin-B2 Tg mice
TT2 embryonic stem (ES) cells (kindly provided by Dr Shinichi Aizawa, Kumamoto University, Japan) were maintained as described previously.26,27 The conditional ephrin-B2 expression vector, CAGp-loxP-bsr-pA-loxP-ephrin-B2-IRES-βgeo-pA (CAGp-IND-ephrin-B2), linearized with XhoI, was introduced into ES cells by electroporation as previously described.27 Transfected ES cells were screened in 4 μg/mL Blasticidin S (Kaken-Seiyaku) for 5 days. To evaluate Cre-induced recombination in these ES cells, a CAG-Cre plasmid28 was introduced into the transfected ES cells, and X-gal staining and Southern and Western blot analyses were performed, as described elsewhere.27 Clones without Cre-induced recombination were used as a control. For further in vivo analysis, 2 mouse lines from each chimeric mouse were generated using independent ES clones, and F1-generation mice were used in all studies.
Western blotting
To detect ephrin-B2 protein in CAG-ephrin-B2 Tg mice, homogenates from murine spleen were prepared as described.29 Cell lysates were cleared by centrifugation for 15 minutes at 15 000g at 4°C, and immunoblotting was performed as previously described.29 Membranes were incubated for 1 hour at room temperature with anti–ephrin-B2 polyclonal antibody (1:300; Santa Cruz Biotechnology, Santa Cruz, CA). Proteins were visualized using the ECL Detection System (Amersham, Arlington Heights, IL).
Immunohistochemistry and X-gal staining
For whole-mount staining of embryos and ear tissues, specimens were fixed in 4% paraformaldehyde at 4°C for 10 minutes. Anti–platelet endothelial cell adhesion molecule (PECAM)-1 antibody (MEC13.3, rat anti-mouse monoclonal; Pharmingen, San Diego, CA) was used to stain endothelial cells in embryos and tissues. Procedures for immunohistochemistry and X-gal staining have been described elsewhere.30 31 Tissue sections were counterstained with nuclear fast red.
Cell culture
To examine the recruitment of the vascular smooth muscle progenitor cells into the vessel walls, explants of digested aorta (ascending, arch, and descending portion) were cultured in Dulbecco modified Eagle medium (DMEM; Gibco BRL, Gaithersburg, MD) plus 10% fetal calf serum (FCS; JRH Bioscience, Lenexa, KS) plus 2 mercaptoethanol (ME). After 7days in culture, anti–α-smooth muscle actin antibody conjugated with horseradish peroxidase (HRP) (Dako Japan, Kyoto, Japan) was used to stain vascular smooth muscle cells (SMCs). To analyze the growth of vascular SMCs, cells were harvested after 7days from cultures of digested aorta explants and 103 SMCs were recultured. After 3, 7, and 12 days in culture, cells were harvested and counted.
Results
Ephrin-B2 is expressed in both arterial endothelial cells and their surrounding mesenchymal cells
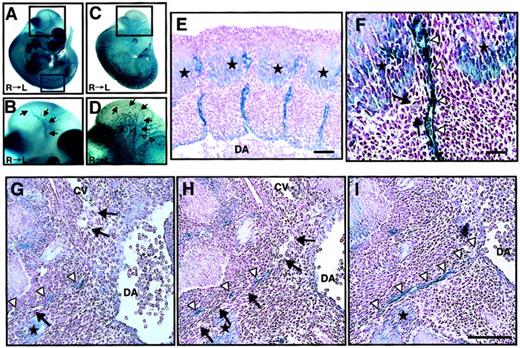
We analyzed ephrin-B2 expression in embryonic days E11.5 embryos of ephrin-B2 promoter (p)-LacZ heterozygous mice (ephrin-B2LacZ/+),32 in which thelacZ gene is inserted into the ephrin-B2 genomic locus by homologous recombination. X-gal staining revealed that ephrin-B2 was expressed in arterial endothelial cells in the vascular system and broadly in many mesenchymal cells (Figure1A,B), while Tie-2, which is a vascular endothelial maker, was specifically expressed in endothelial cells of large artery, vein, and vascular networks in Tie-2p-LacZ Tg mice (Figure 1C,D). In the trunk region of E11.5 embryos, intersomitic ephrin-B2− vessels existed between ephrin-B2+somites and arteries (Figure 1E,F). Moreover, histologic analysis revealed that ephrin-B2− vessels were sprouted from the cardinal vein, suggesting that the vessels are vein-expressing EphB4 receptors. In addition, they existed between ephrin-B2+ mesenchymal cells and arteries (Figure 1G,I). These findings suggest that an EphB4/ephrin-B2 interaction in ECs and surrounding mesenchymal cells may regulate vessel projections.
Ephrin-B2 expression in the embryonic vascular system.
(A-B) Ephrin-B2 expression was detected by whole-mount X-gal staining of ephrin-B2LacZ/+ mouse embryos32 at E11.5. (C-D) The vascular network of E11.5 mouse embryos is visualized by Tie-2 expression. To detect Tie-2 expression, whole-mount X-gal staining of Tie-2 promoter(p)-LacZ mouse embryos generated from crossing CAG-CAT-Z indicator TG mice42 and Tie-2p Cre mice was performed.33 Panels B and D show higher magnification views of the black square in the head region of panels A and C, respectively. Arrows in panels B and D indicate internal carotid artery (ICA), and open arrowheads in panel D indicate anterior cardinal vein (ACV). Panels E and F show X-gal staining of cross sections of embryos representing the blue square in panel A. Stars show ephrin-B2 expression in the caudal region of somites. A higher magnification of the intersomitic region is shown in panel F. Arrows indicate that there is no ephrin-B2 staining in vessels between ephrin-B2+arteries (arrowheads) and ephrin-B2+ somites (stars). (G-I) Serial cross sections of the E11.5 mouse vascular system in trunk region of ephrin-B2LacZ/+ mouse. X-gal staining shows that ephrin-B2+ vessel (arrowheads) is sprouted from dorsal artery (DA), while ephrin-B2− vessel (arrows) is sprouted from cardinal vein (CV). Sections were counterstained with nuclear fast red. Bars indicate 100 μm in panels E and G-I; 50 μm in panel F.
Ephrin-B2 expression in the embryonic vascular system.
(A-B) Ephrin-B2 expression was detected by whole-mount X-gal staining of ephrin-B2LacZ/+ mouse embryos32 at E11.5. (C-D) The vascular network of E11.5 mouse embryos is visualized by Tie-2 expression. To detect Tie-2 expression, whole-mount X-gal staining of Tie-2 promoter(p)-LacZ mouse embryos generated from crossing CAG-CAT-Z indicator TG mice42 and Tie-2p Cre mice was performed.33 Panels B and D show higher magnification views of the black square in the head region of panels A and C, respectively. Arrows in panels B and D indicate internal carotid artery (ICA), and open arrowheads in panel D indicate anterior cardinal vein (ACV). Panels E and F show X-gal staining of cross sections of embryos representing the blue square in panel A. Stars show ephrin-B2 expression in the caudal region of somites. A higher magnification of the intersomitic region is shown in panel F. Arrows indicate that there is no ephrin-B2 staining in vessels between ephrin-B2+arteries (arrowheads) and ephrin-B2+ somites (stars). (G-I) Serial cross sections of the E11.5 mouse vascular system in trunk region of ephrin-B2LacZ/+ mouse. X-gal staining shows that ephrin-B2+ vessel (arrowheads) is sprouted from dorsal artery (DA), while ephrin-B2− vessel (arrows) is sprouted from cardinal vein (CV). Sections were counterstained with nuclear fast red. Bars indicate 100 μm in panels E and G-I; 50 μm in panel F.
Generation of CAGp- and CAGp-IND-ephrin-B2 transgenic mice
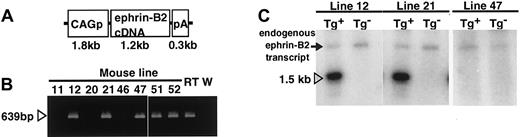
To investigate the role of an EphB/ephrin-B2 interaction in ECs and surrounding mesenchymal cells, we disrupted the complementary pattern of EphB/ephrin-B2 expression by generating Tg mice expressing ephrin-B2 constitutively and ectopically under the regulation of CAG promoter (Figure 2A). Of the 50 mice that survived to 3 weeks of age, 3 transgenic founders were generated as confirmed by polymerase chain reaction (PCR) analysis of genomic DNA (Figure 2B). All 3 founders were bred with wild-type C57BL/6 mice and transmitted the transgene following a Mendelian distribution. To analyze transgene expression in the F1 offspring from each founder, Northern analysis of total ear RNA was performed using mouse ephrin-B2 cDNA as a probe (Figure 2C). An ephrin-B2 transgene of the expected size was observed in 2 lines,12 21 whereas the third line47 was a nonexpressing line. β-actin (1:5000; Sigma, St Louis, MO) blotting on the same membrane demonstrated equal loading in each lane (data not shown). In addition, Northern blot analysis of total RNA from various tissues in 8-week-old mice revealed that the transgene was broadly expressed in both lines 12 and 21 Tg mice, indicating that the broad expression of transgene disrupted the ephrin-B2 expression pattern seen in wild-type mice (data not shown). Based on these observations, lines 12 and 21 were chosen for further study, and both showed essentially equivalent phenotypes.
Generation of CAGp-ephrin-B2 Tg mice.
(A) The structure of the CAGp-ephrin-B2-pA transgene cassettes used to generate transgenic mice. CAGp indicates the CAG promoter and pA the polyadenylation signal. (B) To detect the transgene, PCR analysis of genomic DNA was performed. Bands of the predicted size were detected in mouse lines 12, 21, and 47 and in the 2 dead founders, no. 51 and no. 52, indicating that they are transgenic. As a positive control, cDNA from E9.5 mouse embryos (RT) was used, while genomic DNA from wild-type mice (W) was used as a negative control. (C) Total RNA was prepared from the ear of F1 offspring of the lines 12, 21, and 47 of CAGp-ephrin-B2 Tg mice, respectively. Twenty micrograms RNA was loaded in each lane, and the blot was probed with mouse ephrin-B2 cDNA. Lines 12 and 21 represent high-expressing lines (arrowhead in C), while line 47 represents a nonexpressing line. There is no transgene expression in the ear tissue of nontransgenic littermates of these lines. An arrow indicates the endogenous ephrin-B2 transcript.
Generation of CAGp-ephrin-B2 Tg mice.
(A) The structure of the CAGp-ephrin-B2-pA transgene cassettes used to generate transgenic mice. CAGp indicates the CAG promoter and pA the polyadenylation signal. (B) To detect the transgene, PCR analysis of genomic DNA was performed. Bands of the predicted size were detected in mouse lines 12, 21, and 47 and in the 2 dead founders, no. 51 and no. 52, indicating that they are transgenic. As a positive control, cDNA from E9.5 mouse embryos (RT) was used, while genomic DNA from wild-type mice (W) was used as a negative control. (C) Total RNA was prepared from the ear of F1 offspring of the lines 12, 21, and 47 of CAGp-ephrin-B2 Tg mice, respectively. Twenty micrograms RNA was loaded in each lane, and the blot was probed with mouse ephrin-B2 cDNA. Lines 12 and 21 represent high-expressing lines (arrowhead in C), while line 47 represents a nonexpressing line. There is no transgene expression in the ear tissue of nontransgenic littermates of these lines. An arrow indicates the endogenous ephrin-B2 transcript.
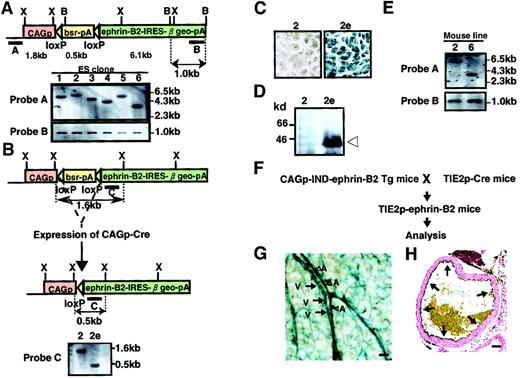
To obtain Tg mice expressing ephrin-B2 inducibly, the vector pCAG-IND-ephrin-B2 was introduced into ES cells by electroporation. Clones were isolated after positive selection in Blasticidin S, and genomic DNA was analyzed by Southern blot analysis to select cell lines with a single-copy integration pattern (Figure3A). We established 6 independent ES cell lines carrying a single copy of the vector and subsequently selected 2 ES clones among them. To test the Cre-induced recombination, we deleted the Blasticidin resistance sequence and allowed ephrin-B2 expression to occur by electroporation of the vector pCAG-Cre into these 2 ES cells (Figure 3B). As expected, recombination allowed these cells to express ephrin-B2, β-galactosidase, and resistance to neomycin (G418). We selected recombined clones in G418 and confirmed this event by Southern blotting analysis and X-gal staining (Figure3B,C and data not shown). As expected, Western blot analysis revealed a high level of ephrin-B2 expression in these ES clones (Figure 3D and data not shown). Using these selected clones, we generated 2 independent lines of Tg mice inducibly expressing ephrin-B2 (CAGp-IND-ephrin-B2 Tg). We checked the germline transmission in mice by Southern blot analysis (Figure 3E). To analyze Tg mice expressing ephrin-B2 in vascular endothelial cells, CAGp-IND-ephrin-B2 Tg mice were mated with Tie-2p-Cre mice33 (Figure 3F). X-gal staining showed the expected Tie-2 Cre-induced recombination, indicating that ephrin-B2 is expressed specifically in both arterial and venous endothelial cells in these obtained mice (Tie-2p-ephrin-B2 Tg mice) (Figure 3G,H).
Generation of CAGp-IND-ephrin-B2 Tg mice.
(A) Representation of the Southern blot analysis of ES cell clone nos. 1-6. ES cell lines carrying a single copy of the CAGp-IND-ephrin-B2 vector fragment were established by Southern blot analysis ofXbaI-digested (upper) and BamHI-digested (lower) genomic DNA from each ES cell clone. Shown are restriction enzyme sites (B, BamHI; X, XbaI). Bars A and B indicate the location of probes for Southern blot analysis. Horizontal open arrowheads represent loxP sites. CAGp, CAG promoter; bsr, Blasticidin resistance gene; pA, polyadenylation signals. (B) To allow ephrin-B2 expression, selected cell clones were electroporated with the Cre expression vector, CAGp-Cre, and selected in G418. Representation of the Southern blot analysis ofXbaI-digested genomic DNA from the parental no. 2 clone (left) and its no. 2e subclone (right) in which the Blasticidin resistance gene is deleted by Cre-induced recombination. The expected fragment sizes are indicated in panels A and B. (C) X-gal staining of ES clone and its subclone shown in panel B. (D) Immunodetection of ephrin-B2 protein of ES clones (2 and subclone 2e) shown in panels B and C. Extracts (10 μg protein) from ES cells were used for Western blotting with an anti–ephrin-B2 antibody. (E) Southern blot analysis of XbaI-digested genomic DNA from F1 male mouse of lines no. 2 and no. 6 using probe A and B in panel A for analyzing the germline transmission. (F) Schematic representation of the experiment. To allow ephrin-B2 expression regulated by the Tie-2 promoter in vivo, CAGp-IND-ephrin-B2 Tg mice were crossed with Tie-2p-Cre mice.33 (G) X-gal staining of vessels in the skin of Tie-2p-ephrin-B2 Tg mice. Blue color indicates that ephrin-B2 is expressed in both arteries (A and arrowheads) and veins (V and arrows). (H) X-gal staining of the ascending aorta of Tie-2p-ephrin-B2 Tg mice. Arrows indicate that ephrin-B2 (blue color) was specifically expressed in endothelial cells. Bars indicate 50 μm.
Generation of CAGp-IND-ephrin-B2 Tg mice.
(A) Representation of the Southern blot analysis of ES cell clone nos. 1-6. ES cell lines carrying a single copy of the CAGp-IND-ephrin-B2 vector fragment were established by Southern blot analysis ofXbaI-digested (upper) and BamHI-digested (lower) genomic DNA from each ES cell clone. Shown are restriction enzyme sites (B, BamHI; X, XbaI). Bars A and B indicate the location of probes for Southern blot analysis. Horizontal open arrowheads represent loxP sites. CAGp, CAG promoter; bsr, Blasticidin resistance gene; pA, polyadenylation signals. (B) To allow ephrin-B2 expression, selected cell clones were electroporated with the Cre expression vector, CAGp-Cre, and selected in G418. Representation of the Southern blot analysis ofXbaI-digested genomic DNA from the parental no. 2 clone (left) and its no. 2e subclone (right) in which the Blasticidin resistance gene is deleted by Cre-induced recombination. The expected fragment sizes are indicated in panels A and B. (C) X-gal staining of ES clone and its subclone shown in panel B. (D) Immunodetection of ephrin-B2 protein of ES clones (2 and subclone 2e) shown in panels B and C. Extracts (10 μg protein) from ES cells were used for Western blotting with an anti–ephrin-B2 antibody. (E) Southern blot analysis of XbaI-digested genomic DNA from F1 male mouse of lines no. 2 and no. 6 using probe A and B in panel A for analyzing the germline transmission. (F) Schematic representation of the experiment. To allow ephrin-B2 expression regulated by the Tie-2 promoter in vivo, CAGp-IND-ephrin-B2 Tg mice were crossed with Tie-2p-Cre mice.33 (G) X-gal staining of vessels in the skin of Tie-2p-ephrin-B2 Tg mice. Blue color indicates that ephrin-B2 is expressed in both arteries (A and arrowheads) and veins (V and arrows). (H) X-gal staining of the ascending aorta of Tie-2p-ephrin-B2 Tg mice. Arrows indicate that ephrin-B2 (blue color) was specifically expressed in endothelial cells. Bars indicate 50 μm.
Abnormal vascular structure of the aorta in CAGp-ephrin-B2 Tg mice
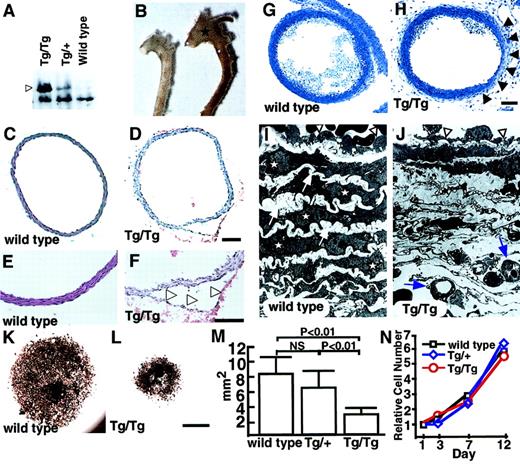
Two founders (no. 51 and no. 52) (Table1, Figure 2B) died suddenly as neonates because of a dissecting aneurysm. Although we could not examine ephrin-B2 expression in these lines, the lethality suggests that vascular morphologic phenotypes may be sensitive to ephrin-B2 doses. To investigate this possibility, we analyzed homozygous CAGp-ephrin-B2 Tg (Tg/Tg) mice, which were generated from crosses of both line12 and line 21 mice. Western blotting revealed that Tg/Tg mice expressed twice the level of ephrin-B2 protein than did heterozygous CAGp-ephrin-B2 Tg (Tg/+) mice (Figure4A). All Tg/Tg mice died of acute aortic dissecting aneurysms at neonatal stages (Figure 4B-F, Table 1). To analyze whether defects existed in vascular structures, we performed histological analyses of the vascular morphology of E19Tg/Tg, Tg/+, and wild-type littermates. Compared to the vascular structure of Tg/+ and wild-type mice, Tg/Tg mice exhibited a thin vascular wall in the ascending aorta and aortic arch (Figure 4G-H). Furthermore, electron microscope analysis revealed that in Tg/Tgmice, ECs have a flat morphology with a budlike structure, while ECs in Tg/+ and wild-type mice show a round morphology. In addition, SMCs and elastic bands were missing inTg/Tg mice compared to wild-type mice, and new microvessels were seen in these spaces (Figure 4I-J). This abnormal vasculature was observed in the ascending and arch portions of the aorta, while a normal vascular structure was detected in the descending aorta in Tg/Tg mice. To investigate whether recruitment of SMCs was defective, explants of aorta (ascending, arch, and descending portions) were cultured. InTg/Tg mice, migrating and proliferating SMCs from explants of ascending (Figure 4K-M) and arch portions (data not shown) of aorta were significantly decreased compared to those of wild-type littermates and Tg/+ mice, while no differences among the 3 groups were observed in cultures from explants from descending aorta (data not shown). To examine whether these findings depend on defects in recruitment of smooth muscle progenitor cells rather than cell growth, identical numbers of SMCs derived from ascending aorta explant cultures of Tg/Tg,Tg/+, and wild-type littermates were recultured, and their growth curves were analyzed. No differences in cell growth were observed among the 3 genotypes (Figure 4N). No defective vascular structures were observed in either Tie-2p-ephrin-B2 Tg mice specifically expressing ephrin-B2 in ECs (Figure 3H) or endothelin promoter-ephrin-B2 Tg mice, which express ephrin-B2 in both ECs and SMCs (our unpublished data, 2001). These results indicate that defective vessel structures seen in ascending and arch portions of aorta are at least associated with failure of smooth muscle progenitor cells to migrate into these regions because of abnormal ephrin-B2 expression.
Numbers of surviving CAGp-ephrin-B2 mice
| Days . | Mouse line . | |||||||
|---|---|---|---|---|---|---|---|---|
| Line 12(F1 × F1) . | Line 21(F1 × F1) . | Line 51(F0) . | Line 52(F0) . | |||||
| Tg/Tg . | Tg/+ . | +/+ . | Tg/Tg . | Tg/+ . | +/+ . | |||
| 0 | 7 | 14 | 6 | 6 | 11 | 8 | 1 | 1 |
| 14 | 7 | 14 | 6 | 2 | 11 | 8 | 0 | 1 |
| 17 | 6 | 14 | 6 | 1 | 11 | 8 | 0 | 0 |
| 21 | 1 | 14 | 6 | 0 | 11 | 8 | 0 | 0 |
| 28 | 0 | 14 | 6 | 0 | 11 | 8 | 0 | 0 |
| Days . | Mouse line . | |||||||
|---|---|---|---|---|---|---|---|---|
| Line 12(F1 × F1) . | Line 21(F1 × F1) . | Line 51(F0) . | Line 52(F0) . | |||||
| Tg/Tg . | Tg/+ . | +/+ . | Tg/Tg . | Tg/+ . | +/+ . | |||
| 0 | 7 | 14 | 6 | 6 | 11 | 8 | 1 | 1 |
| 14 | 7 | 14 | 6 | 2 | 11 | 8 | 0 | 1 |
| 17 | 6 | 14 | 6 | 1 | 11 | 8 | 0 | 0 |
| 21 | 1 | 14 | 6 | 0 | 11 | 8 | 0 | 0 |
| 28 | 0 | 14 | 6 | 0 | 11 | 8 | 0 | 0 |
In mouse lines 12 and 21, the total numbers of surviving mice were counted from 3 independent crosses between F1 Tg/+ male and Tg/+ female mice.
Abnormal vascular morphology in CAGp-ephrin-B2 Tg mice.
(A) Western blotting of extracts (20 μg protein) from the spleens of neonatal ephrin-B2 Tg mice with anti–ephrin-B2 polyclonal antibody. Arrowhead indicates overexpressed ephrin-B2 protein in CAGp-ephrin-B2 Tg mice. (B) Vascular phenotypes of wild-type (left) and CAGp-ephrin-B2Tg/Tg mice (right) at neonatal stages. The dilated aorta is observed, especially in the ascending portion (stars). (C-F) Cross sections of the ascending aorta stained with hematoxylin and eosin were examined after the aortic dissection event. Tg/Tg mice (D, F) with aortic dissection (arrowheads in F). Bars indicate 100 μm. (G-J) Light and electron micrographs comparing wild-type (G, I) andTg/Tg mouse embryos (H, J) at E19. Sections were stained with toluidine blue. Arrowheads in panel H show a thin vascular wall. Bars indicate 150 μm (G, H). Electron micrographs of the same vessel (G, H) at magnification × 1700 (I, J). In electron micrographs, SMCs (stars) and elastic bands (arrows) have shown the complementary arrangement pattern in wild-type mice (I). In contrast, they have disappeared, and new microvessels (arrows) are observed in this space (J). ECs in Tg/Tg mice have a flat morphology with a budlike structure (arrowheads in J), while ECs in wild-type mice show a round morphology (arrowheads in I). (K-M) Explant culture of ascending aorta from a CAGp-ephrin-B2 Tg/Tg mouse (L) and a wild-type littermate (K) were examined by staining with the anti–smooth muscle actin antibody. Bars indicate 1 mm. (M) Quantitative analysis of the area of smooth muscle actin+ cells was performed with the NIH image computer analyzing system. Each column represents the mean area, and the error bars indicate SD (n = 8). (N) In vitro proliferation analysis. Identical numbers of cells of each genotype were plated. The growth of these cultures was measured by counting the total number of cells on each of the indicated days. A representative experiment is shown. Similar results were obtained from SMCs derived from all 3 genotypes.
Abnormal vascular morphology in CAGp-ephrin-B2 Tg mice.
(A) Western blotting of extracts (20 μg protein) from the spleens of neonatal ephrin-B2 Tg mice with anti–ephrin-B2 polyclonal antibody. Arrowhead indicates overexpressed ephrin-B2 protein in CAGp-ephrin-B2 Tg mice. (B) Vascular phenotypes of wild-type (left) and CAGp-ephrin-B2Tg/Tg mice (right) at neonatal stages. The dilated aorta is observed, especially in the ascending portion (stars). (C-F) Cross sections of the ascending aorta stained with hematoxylin and eosin were examined after the aortic dissection event. Tg/Tg mice (D, F) with aortic dissection (arrowheads in F). Bars indicate 100 μm. (G-J) Light and electron micrographs comparing wild-type (G, I) andTg/Tg mouse embryos (H, J) at E19. Sections were stained with toluidine blue. Arrowheads in panel H show a thin vascular wall. Bars indicate 150 μm (G, H). Electron micrographs of the same vessel (G, H) at magnification × 1700 (I, J). In electron micrographs, SMCs (stars) and elastic bands (arrows) have shown the complementary arrangement pattern in wild-type mice (I). In contrast, they have disappeared, and new microvessels (arrows) are observed in this space (J). ECs in Tg/Tg mice have a flat morphology with a budlike structure (arrowheads in J), while ECs in wild-type mice show a round morphology (arrowheads in I). (K-M) Explant culture of ascending aorta from a CAGp-ephrin-B2 Tg/Tg mouse (L) and a wild-type littermate (K) were examined by staining with the anti–smooth muscle actin antibody. Bars indicate 1 mm. (M) Quantitative analysis of the area of smooth muscle actin+ cells was performed with the NIH image computer analyzing system. Each column represents the mean area, and the error bars indicate SD (n = 8). (N) In vitro proliferation analysis. Identical numbers of cells of each genotype were plated. The growth of these cultures was measured by counting the total number of cells on each of the indicated days. A representative experiment is shown. Similar results were obtained from SMCs derived from all 3 genotypes.
Regulation of vessel projections by EphB/ephrin-B2 signaling between endothelial cells and surrounding mesenchymal cells
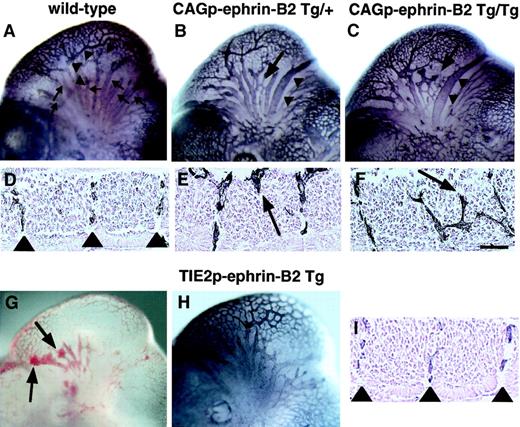
To investigate the role of EphB/ephrin-B2 signaling between endothelial cells and surrounding mesenchymal cells in vascular formation, we compared vascular network formation in CAGp-ephrin-B2 Tg mice to that of Tie-2p-ephrin-B2 Tg mice. In the head region of wild-type embryos, a highly organized vascular system with branches extending from the anterior cardinal vein (ACV) and internal carotid artery (ICA) was observed (Figure 5A), and ephrin-B2 expression was restricted to the artery (Figure 1B). Notably, CAGp-ephrin-B2 Tg mice exhibited dilation and truncation of venous vessels (Figure 5B,C). In contrast, these abnormal findings were not observed in Tie-2p-ephrin-B2 Tg mice, while some Tie-2p-ephrin-B2 Tg mice exhibited cranial hemorrhages (Figure 5G,H). As the trunk region of wild-type embryos at E11.5 contains intersomitic vessels arranged in segments located between somite boundaries (Figure 5D, Figure 1E,F), we analyzed the arrangement of intersomitic vessels in CAGp-ephrin-B2 Tg mice. As predicted, the intersomitic vessels were disorganized and penetrated aberrantly into neighboring somites in CAGp-ephrin-B2 Tg mice (Figure 5E,F), while no abnormality of somite boundaries was detected. In contrast, these abnormal vessel projections were not observed in Tie-2p-ephrin-B2 Tg mice (Figure 5I).
Aberrant vessel projection in CAGp-ephrin-B2 Tg mice.
Embryonic vasculature in the head region of an E11 wild-type littermate (A), a CAGp-ephrin-B2 Tg/+ embryo (B), a CAGp-ephrin-B2 Tg/Tg embryo (C), and a Tie-2p-ephrin-B2 Tg embryo (H) was visualized by whole-mount immunohistochemistry with the anti–PECAM-1 antibody. (A) Wild-type embryo showing well-developed vascular networks of branches from the ICA (arrowheads) and branches of the ACV (arrows). Note disorganization of the ACV (arrow in B and C) and large-diameter vessels (arrowheads in B and C) in the head of the CAGp-ephrin-B2 Tg mouse. (G) Hemorrhage (arrows) was observed in Tie-2p-ephrin-B2 Tg mice. (H) No disorganization of the ACV and large-diameter vessels in the head of Tie-2p-ephrin-B2 Tg mice. Intersomitic vessels of the trunk at lumbar levels were visualized with the PECAM-1 antibody (D-F, I). A wild-type embryo showing a segmental pattern of intersomitic vessels (arrowheads in D). CAGp-ephrin-B2 Tg mice show the abnormal vessel sprouts penetrating into somites (arrows in E and F), while Tie-2p-ephrin-B2 Tg mice show a normal segmental pattern of intersomitic vessels (arrowheads in I). Top in panels D-F and I is the ventral side. Bars indicate 50 μm.
Aberrant vessel projection in CAGp-ephrin-B2 Tg mice.
Embryonic vasculature in the head region of an E11 wild-type littermate (A), a CAGp-ephrin-B2 Tg/+ embryo (B), a CAGp-ephrin-B2 Tg/Tg embryo (C), and a Tie-2p-ephrin-B2 Tg embryo (H) was visualized by whole-mount immunohistochemistry with the anti–PECAM-1 antibody. (A) Wild-type embryo showing well-developed vascular networks of branches from the ICA (arrowheads) and branches of the ACV (arrows). Note disorganization of the ACV (arrow in B and C) and large-diameter vessels (arrowheads in B and C) in the head of the CAGp-ephrin-B2 Tg mouse. (G) Hemorrhage (arrows) was observed in Tie-2p-ephrin-B2 Tg mice. (H) No disorganization of the ACV and large-diameter vessels in the head of Tie-2p-ephrin-B2 Tg mice. Intersomitic vessels of the trunk at lumbar levels were visualized with the PECAM-1 antibody (D-F, I). A wild-type embryo showing a segmental pattern of intersomitic vessels (arrowheads in D). CAGp-ephrin-B2 Tg mice show the abnormal vessel sprouts penetrating into somites (arrows in E and F), while Tie-2p-ephrin-B2 Tg mice show a normal segmental pattern of intersomitic vessels (arrowheads in I). Top in panels D-F and I is the ventral side. Bars indicate 50 μm.
Regulatory roles of ephrin-B2 in network formation of capillary-sized vessels
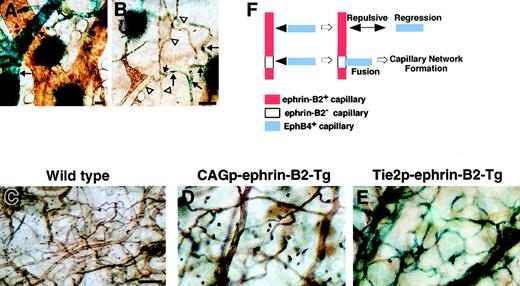
To analyze the role of EphB/ephrin-B2 signaling in network formation of capillary vessels, we investigated the pattern of ephrin-B2 expression in capillary-sized networks of 14-day-old mice (P14) using the ephrin-B2LacZ/+ mice. Sporadic ephrin-B2 was expressed in capillary networks, while ephrin-B2 expression was uniform in arterioles (Figure6A,B). These findings suggest that the signaling of EphB/ephrin-B2 may be important for capillary formation. To examine this hypothesis, we performed a lectin binding assay34 35 to analyze capillary network formation in both CAGp- and Tie-2p-ephrin-B2 Tg mice. Capillary network formation in both CAGp- and Tie-2p-ephrin-B2 Tg mice was markedly decreased compared to that of wild-type littermates, in which capillary formation was dense (Figure 6C-E). An ephrin-B2− site in capillary is essential to bind with EphB4+ capillary, because EphB4+ capillary cannot bind to ephrin-B2+capillary because of a repulsive effect. Here, we propose a model for capillary network formation that is regulated by EphB/ephrin-B2 signaling (Figure 6F).
Defects in capillary formation in both CAGp- and Tie-2p-ephrin-B2 Tg mice.
(A, B) Expression of ephrin-B2 in capillary-sized vessels in the P14 neonatal mouse. Before lectin staining (brown color), X-gal staining was performed. X-gal staining of ephrin-B2LacZ/+ mice indicates that ephrin-B2 (blue color) was detected sporadically in capillary-sized vessels (arrows in B), while ephrin-B2 was strongly expressed in the large-sized arterial vessels (arrows in A). Arrowheads in panel B indicate ephrin-B2− ECs in capillary-sized vessels. Comparison of microvessel morphology of the ear in lectin-stained whole mounts of ear skin (C-E). A marked decrease in capillary-sized vessels was seen in CAGp-ephrin-B2 Tg mice and Tie-2p-ephrin-B2 Tg mice compared to wild-type littermates. X-gal staining indicates that ephrin-B2 (blue color) was expressed in arteries, veins, and capillary-sized vessels in Tie-2p-ephrin-B2 Tg mice (E). A model of capillary formation is shown in panel F. All bars indicate 50 μm. In lines 12 and 21, the total numbers of surviving mice were counted from 3 independent crosses between F1 Tg/+ male and Tg/+ female mice.
Defects in capillary formation in both CAGp- and Tie-2p-ephrin-B2 Tg mice.
(A, B) Expression of ephrin-B2 in capillary-sized vessels in the P14 neonatal mouse. Before lectin staining (brown color), X-gal staining was performed. X-gal staining of ephrin-B2LacZ/+ mice indicates that ephrin-B2 (blue color) was detected sporadically in capillary-sized vessels (arrows in B), while ephrin-B2 was strongly expressed in the large-sized arterial vessels (arrows in A). Arrowheads in panel B indicate ephrin-B2− ECs in capillary-sized vessels. Comparison of microvessel morphology of the ear in lectin-stained whole mounts of ear skin (C-E). A marked decrease in capillary-sized vessels was seen in CAGp-ephrin-B2 Tg mice and Tie-2p-ephrin-B2 Tg mice compared to wild-type littermates. X-gal staining indicates that ephrin-B2 (blue color) was expressed in arteries, veins, and capillary-sized vessels in Tie-2p-ephrin-B2 Tg mice (E). A model of capillary formation is shown in panel F. All bars indicate 50 μm. In lines 12 and 21, the total numbers of surviving mice were counted from 3 independent crosses between F1 Tg/+ male and Tg/+ female mice.
Discussion
The ephrin-B2 ligand that signals through cell-surface EphB receptors is itself a transmembrane protein, suggesting that ephrin-B2 signaling may play an important role in cell-to-cell contact interaction. The phenotypes of mice lacking ephrin-B2, EphB4, and EphB2/B3 indicate that ephrin-B2 and its cognate EphB receptors are essential for early vascular development.14-16 20 However, the early embryonic lethality of these knockout mice has prevented further analysis of the role of EphB/ephrin-B2 signaling in the vasculature.
In this study, we focused on defining the role of EphB/ephrin-B2 interaction between ECs and mesenchymal cells in the formation of the vascular system. During embryogenesis, ephrin-B2 is broadly expressed in arterial endothelial cells, the surrounding mesenchyme, somites, and neurons,36 while the expression pattern of Eph receptors is still controversial. Although initial work focused on the reciprocal distribution of EphB4 in the venous ECs of the embryo, recent findings indicate that other EphB receptors are widely expressed and could have specific ligand-receptor interactions to arterial ephrin-B2.15,16,36 These observations suggest that EphB/ephrin-B2 may regulate precise vessel projections through interactions between ECs and surrounding cells during embryogenesis, like an axonal guidance in neurons is regulated by Eph/ephrin signaling by providing a repulsive or attractive cue.37 38
To test this hypothesis, we disrupted the precise expression pattern of EphB/ephrin-B2 in vivo by generating Tg mice that express ephrin-B2 under the control of a ubiquitous and constitutive promoter, CAGp. These mice displayed an abnormal segmental arrangement of intersomitic vessels, while such anomalies were not observed in Tie-2p-ephrin-B2 Tg mice, in which ephrin-B2 was overexpressed in only vascular ECs. This finding suggests that surrounding cells expressing ephrin-B2 ectopically alter the migration of ECs expressing EphB receptors into the intersomitic region where ephrin-B2 expression is normally absent. Similarly, ectopic expression of ephrin-B ligands leads to aberrant intersomitic vein projection in Xenopus laevis,39 and disorganized intersomitic vessels are seen in mouse mutants in EphB415 and ephrin-B2.16 20 These previous findings also supported our hypothesis that precise guidance of EphB4+ vascular ECs occurs because of repellent cues mediated by ephrin-B2+ cells. However, whether Eph/ephrin signaling in the vascular system parallels mechanisms observed in the nervous system remains to be determined. Further elucidation of the molecular mechanism how repulsive or attractive cell-to-cell interactions control vascular formation is essential. As vessel projection is the initial event in many pathologic conditions, it may provide new strategies in (anti)angiogenic treatment.
Although EphB4/ephrin-B2 interaction is known to be involved in defining boundaries between arteries and veins, the mechanism of capillary formation is not clear. More recently, we analyzed the function of EphB4/ephrin-B2 signals using in vitro stromal cells and a para-aortic splanchnopleura coculture system.21 This demonstrated that the proliferation and migration of ECs were suppressed when the ephrin-B2+ cells and EphB4+ cells contact each other at the boundary in vitro vascular network formation. In this study, we found a sporadic pattern of ephrin-B2 expression in cells involved in capillary formation in vivo. In addition, our morphological analysis reveals defects in capillary-sized arterial-venous boundary formation in the circulatory system of both CAGp- and Tie-2p-ephrin-B2 Tg mice. This indicates that disruption of the precise expression pattern of EphB/ephrin-B2 in the circulatory system caused the failure of capillary formation, suggesting the important role of EphB/ephrin-B2 signaling at arterial-venous boundary formation. Some Tie-2p-ephrin-B2 Tg mice also showed intracerebral bleeding, which may have been caused by the abnormalities of capillary formation. In the nervous system, repulsive cues promoted by contact between Eph+ cells and ephrin+ cells induce withdrawal of the ephrin+axons.40 If similar contact-mediated repellent effects exist in the vascular system, such sporadic ephrin-B2 expression in capillaries could mediate fusion of distinct types of endothelial cells and play a role in network formation. Indeed, more recently we found that EphB4+ ECs were inhibited to spread and grow on the ephrin-B2-Fc–coated dishes (K.H. et al, manuscript in preparation). Given this set of findings, precise ephrin-B2 expression is essential for capillary formation.
Neural crest cells migrate along specific pathways to the branchial arches and differentiate into vascular SMCs in the vascular system.41,42 Only in CAGp-ephrin-B2 Tg mice was defective recruitment of smooth muscle progenitor cells in the ascending aorta and aortic arch detected, suggesting that disruption of normal EphB/ephrin-B2 signaling induced by ubiquitous ephrin-B2 expression caused abnormal migration of neural crest cells. A similar conclusion has been reached for trunk neural crest migration. Krull et al43 demonstrated that the migration of neural crest cells expressing EphB3 is regulated by ephrin-B1+ cells in the trunk region. In their studies, the addition of soluble ephrin-B1 disrupted the patterned migration of neural crest cells in a whole- trunk explant. It is notable that these effects were dependent on the dose of ephrin-B ligand. In addition, Wang et al37demonstrated that neural crest cell migration was precisely regulated by EphB/ephrin-B2 repulsive effect on the stripes where ephrin-B2+ and ephrin-B2− stripes were alternatively present in in vitro functional assays. These reports and our findings in CAGp-ephrin-B2 Tg mice suggest that the precise expression pattern and dose of ephrin-B2 may be critical in mediating neural crest cell migration to ascending and arch portions of the aorta, where these cells differentiate into vascular smooth muscle progenitor cells. In addition, we found an absence of elastic fibrillar structures in the aortic wall. This suggests that correct interactions between SMCs and extracellular matrix play an important role in developing the vascular wall. However, it remains to be clarified what role ephrin-B2 signaling plays in this interaction.
In summary, our in vivo analysis demonstrated that EphB/ephrin-B2 signaling between ECs and their surrounding mesenchymal cells regulates the vessel formation through promoting or suppressing the proliferation and migratory activity of both ECs and SMCs.
We thank Dr John G. Flanagan for providing us with full-length ephrinB2 cDNA; Ms T. Keida, M. Tokushima, and I. Kawasaki for experimental assistance; and Dr A. Hirata for technical support in the lectin-staining experiment.
Supported by Grants-in-Aid for Scientific Research on Priority Areas from the Ministry of Education, Culture, Sports, Science and Technology; Grants-in-Aid for Scientific Research from Japan Society for promotion of Science; and a grant from the Takeda science foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Toshio Suda, The Sakaguchi Laboratory, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo, 160-8582, Japan; e-mail: sudato@sc.itc.keio.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal