The Cancer and Leukemia Group B conducted a phase 3 trial of the P-glycoprotein modulator PSC-833 in untreated acute myeloid leukemia patients aged 60 years and older. Patients were randomized to 1 of 2 regimens, with doses determined in a prior phase 1 study, consisting of cytarabine 100 mg/m2/d by 7-day infusion, with daunorubicin 60 mg/m2 and etoposide 100 mg/m2 daily for 3 days (ADE), or daunorubicin 40 mg/m2 and etoposide 60 mg/m2 for 3 days with PSC-833, 2.8 mg/kg over 2 hours, and then 10 mg/kg/d by 3-day infusion (ADEP). The ADEP arm was closed after randomization of 120 patients (61 to ADE and 59 to ADEP) because of excessive early mortality. Rates of complete remission, nonresponse, and death were 46%, 34%, and 20% for ADE, versus 39%, 17%, and 44% for ADEP (P = .008). Nevertheless, disease-free survival (median 7 vs 8 months; P = .38) and overall survival (approximately 33% alive at 1 year) did not differ and were similar to historical results. Although the number of patients was limited, ADE patients whose pretreatment cells exhibited PSC-833–modulated dye efflux in vitro (n = 22) had worse outcomes than those without efflux (n = 11) (complete remission, nonresponse, and death rates of 41%, 41%, and 18%, compared with 91%, 9%, and 0%; P = .03), but with ADEP outcomes were nearly identical. Moreover, for patients with PSC-833–modulated efflux, median disease-free survival was 5 months with ADE and 14 months with ADEP (P = .07). Further modulation trials in older patients must await the design of less-toxic regimens.
Introduction
Intensive treatment regimens have improved outcome in younger patients with acute myeloid leukemia (AML), but clinical trials in patients aged 60 years and older by the Cancer and Leukemia Group B (CALGB) and others have shown remarkably consistent inferior results in this age group, with complete remission (CR) rates of 45% to 55% and only 10% to 15% of patients surviving 4 years.1-5 New approaches are needed.
AML in older patients has a high frequency of expression of the multidrug resistance (MDR) mediator P-glycoprotein (Pgp), encoded by the MDR1 gene, and Pgp expression in AML cells is associated with a lower CR rate.6 Based on the frequency of Pgp-mediated MDR in AML in older patients and its association with adverse treatment outcome, Pgp represents an appropriate target for therapeutic intervention in this age group.
Pgp functions as an energy-dependent drug efflux pump whose substrates include anthracyclines and epipodophyllotoxins. A number of agents inhibit the function of Pgp, blocking Pgp-mediated efflux of drugs from the intracellular compartment.7,8 Drugs such as verapamil, quinine, and cyclosporin A (CSA) are effective Pgp modulators in vitro, but their clinical utility is limited by side effects such as hypotension with verapamil or the potential for immunosuppression and nephrotoxicity with CSA. PSC-833 is a nonimmunosuppressive, non-nephrotoxic cyclosporine analog that is 20 times more potent than CSA in increasing daunorubicin retention in MDR cells.9Moreover, PSC-833 concentrations sufficient to block Pgp function in vitro are easily achieved in patients with doses that are relatively free of side effects.10 However, a constraint on the clinical application of PSC-833 is its interference with clearance of cytotoxic drugs that are susceptible to Pgp-mediated efflux.11 12 Thus, dose reductions of these drugs are often necessary when they are administered in PSC-833–containing regimens.
CALGB chose to develop chemotherapy regimens consisting of cytarabine (Ara-C), daunorubicin, and etoposide with and without PSC-833, for comparison in AML patients aged 60 years and older. We hypothesized that using 2 drugs that are substrates for Pgp in the presence of an inhibitor of Pgp-mediated drug efflux would increase the fraction of leukemia cells killed by chemotherapy to a greater extent than would be seen with dose escalation alone. To define equitoxic regimens for comparison in a phase 3 randomized trial, CALGB conducted parallel phase 1 studies of Ara-C, daunorubicin, and etoposide (ADE) and Ara-C, daunorubicin, etoposide, and PSC-833 (ADEP) in AML patients aged 60 years and older in CALGB study 9420.13 After the doses were established in this phase 1 study, a phase 3 trial, CALGB 9720, was initiated to determine whether the addition of PSC-833 to chemotherapy improves the CR rate, disease-free survival (DFS), and survival of older AML patients. We report here the results of the phase 3 randomized trial comparing ADE and ADEP.
Patients and methods
Eligibility criteria
Eligibility criteria included a diagnosis of AML with French-American-British (FAB) types M0-M2 or M4-M7, as defined by standard morphologic and immunophenotypic criteria,14 15and age 60 years old or greater. Patients with antecedent myelodysplastic syndromes (MDS) were eligible for treatment on this trial, as were patients who had received cytotoxic therapy (chemotherapy and/or radiation therapy) for prior malignant or nonmalignant disorders. Prior MDS was defined by cytopenias and bone marrow findings establishing a diagnosis of MDS at least 3 months prior to the diagnosis of AML. Prior treatment for AML or MDS was not permitted, with the exception of growth factor or cytokine support and leukapheresis, hydroxyurea, or cranial irradiation used to manage hyperleukocytosis.
Patients with organ dysfunction were not excluded, but it was cautioned that abnormalities in hepatic or renal function were to be considered as potentially serious obstacles for safe tolerance of the therapy prescribed on the protocol. Similarly, there was no numeric cutoff for the cardiac ejection fraction, but uncontrolled or severe cardiovascular disease was an exclusion criterion for enrollment on the study.
The protocol was reviewed and approved by local institutional review boards, and written informed consent was obtained from all patients.
Registration and randomization procedures
Patients were registered and simultaneously randomized to 1 of the 2 induction regimens via a telephone call to the CALGB Statistical Center. The randomization was a balanced permuted blocks design and was stratified by type of AML (AML de novo or AML with antecedent MDS).
Induction therapy
The ADE and ADEP induction regimens are shown in Table1. All patients received Ara-C 100 mg/m2/d by continuous infusion for 7 days. Patients randomized to receive ADE also received daunorubicin 60 mg/m2/d by intravenous bolus injection and etoposide 100 mg/m2/d by 2-hour infusion, both daily for 3 days, initiated concurrently with the Ara-C. Patients randomized to receive ADEP were treated with daunorubicin 40 mg/m2/d by bolus injection and etoposide 60 mg/m2/d by 2-hour infusion, both daily for 3 days, and PSC-833 given as a 2-hour intravenous loading dose of 2.8 mg/kg immediately prior to the first daunorubicin and etoposide doses, followed by a 72-hour infusion of 10 mg/kg/d begun concurrently with the first daunorubicin and etoposide doses. This PSC-833 dose is known to produce continuous plasma levels above 2 ng/mL (1.64 nM), a concentration that consistently reverses MDR in vitro.16 The protocol specified that medications known to affect the disposition of CSA and its analogs should not be used in conjunction with PSC-833. Specifically, medications that increase CSA concentrations could not be administered for 48 hours before PSC-833 was started, during PSC-833 administration, or up to 48 hours after the end of the PSC-833 infusion. These medications include calcium-channel blockers (diltiazem, nicardipine, verapamil), antifungals (fluconazole in doses above 200 mg/d, itraconazole, ketoconazole), antibiotics (clarithromycin, erythromycin), and others (bromocriptine, danazol). Medications that decrease CSA concentrations could not be administered during the 14 days prior to the start of PSC-833 or during PSC-833 administration but could be restarted immediately after the end of the PSC-833 infusion. These medications include antibiotics (nafcillin, rifampin) and anticonvulsants (carbamazepine, phenobarbitone, phenytoin). Additionally, reduced clearance of digoxin and a decrease in its apparent volume of distribution have been observed with CSA; care was therefore urged if patients received digoxin in conjunction with PSC-833.
Remission induction regimens
| . | ADE . | ADEP . |
|---|---|---|
| Ara-C | 100 mg/m2/d × 7 | 100 mg/m2/d × 7 |
| Daunorubicin | 60 mg/m2/d × 3 | 40 mg/m2/d × 3 |
| Etoposide | 100 mg/m2/d × 3 | 60 mg/m2/d × 3 |
| PSC-833 | — | 2.8 mg/kg over 2 h, then |
| 10 mg/kg/d × 3 |
| . | ADE . | ADEP . |
|---|---|---|
| Ara-C | 100 mg/m2/d × 7 | 100 mg/m2/d × 7 |
| Daunorubicin | 60 mg/m2/d × 3 | 40 mg/m2/d × 3 |
| Etoposide | 100 mg/m2/d × 3 | 60 mg/m2/d × 3 |
| PSC-833 | — | 2.8 mg/kg over 2 h, then |
| 10 mg/kg/d × 3 |
Each drug was given on consecutive days starting on day 1.
Second induction course
Patients with persistent leukemia in the bone marrow, defined by at least 20% marrow cellularity with more than 5% blasts on day 14 or at a subsequent time point following initiation of induction therapy, received a second course of induction chemotherapy identical to the initial induction course (ADE or ADEP) except that Ara-C was given for 5 rather than 7 days, 2 rather than 3 doses of daunorubicin and etoposide were administered, and PSC-833 was administered as a 48-hour rather than 72-hour infusion following the loading dose.
Postremission therapy
Patients who achieved a CR received a single course of postremission chemotherapy identical to the initial induction regimen (ADE or ADEP) except that Ara-C was given for 5 rather than 7 days, 2 rather than 3 doses of daunorubicin and etoposide were administered, and PSC-833 was administered as a 48-hour rather than 72-hour infusion following the loading dose. Postremission chemotherapy was initiated as soon as possible following attainment of CR but no sooner than day 28 of induction therapy. Full recovery from infection and stomatitis and attainment of a CALGB performance status of 0 to 1 were required. Repeat bone marrow testing was required if postremission chemotherapy was initiated more than 4 weeks following the initial documentation of CR.
Patients who had hematologic recovery and remained free of leukemia following postremission chemotherapy underwent a second randomization to receive interleukin-2 maintenance therapy17 or no further treatment. Patients randomized to interleukin-2 received 0.9 × 106 U/m2/d by subcutaneous injection on days 1-14, 19-28, 33-42, 47-56, 61-70, and 75-90, with subcutaneous bolus doses of 12 × 106 U/m2/d on days 15-17, 29-31, 43-45, 57-59, and 71-73.
Supportive care
It is the policy of CALGB to require that all patients entered on acute leukemia treatment studies be cared for at institutions that are capable of providing adequate transfusion therapy and consultative physicians for the complications that may occur in this patient population. All patients except those with a history of allergic reactions were treated with allopurinol, and all received blood products and antibiotics as clinically indicated. Specific approaches involving oral or systemic antibiotics were neither advocated nor forbidden by the protocol. Hematopoietic growth factors were not administered routinely as part of the protocol, but they could be given to neutropenic patients with prognostic factors predictive of clinical deterioration, such as pneumonia, hypotension, multiorgan dysfunction (sepsis syndrome), or fungal infection, consistent with the American Society of Clinical Oncology guidelines.18
Quality control, quality assurance, safety monitoring, and auditing
All data forms were sent to the CALGB Statistical Center as well as to the study chair. The study chair reviewed the eligibility of each patient as well as all data forms to verify the institutional assessments of toxicity and response. Data were entered into the official CALGB database by data entry staff. The CALGB Data and Safety Monitoring Board (DSMB) periodically reviewed interim reports for the study prepared by the Statistical Center.
Members of the CALGB Data Audit Committee visit all participating institutions at least once every 3 years to verify compliance with federal regulations and protocol requirements for CALGB studies, including those pertaining to eligibility, treatment, response, and follow-up.19 Data from 32% of patients in this report have been audited.
Outcome and toxicity measurements
CR was defined according to the criteria established by the National Cancer Institute workshop.20 It was required that these findings persist for at least 4 weeks in the absence of further treatment. Relapse of AML was defined as marrow infiltration by more than 5% blasts not attributable to another cause or as the development of extramedullary disease in a patient previously in CR. In the absence of circulating blasts, marrow infiltration with 5% to 20% blasts required confirmation with a repeat marrow testing at least 1 week later.
Outcomes were compared between patients with de novo AML (no cytotoxic therapy for prior malignant or nonmalignant conditions and no antecedent MDS), AML with prior cytotoxic therapy for a different malignancy with or without antecedent MDS, and AML with antecedent MDS with no prior cytotoxic therapy.
Toxicity was graded according to the CALGB expanded common toxicity criteria.
Cytogenetic analysis
Cytogenetic analysis was performed as part of a prospective karyotyping study, CALGB 8461, “Cytogenetic Studies in Acute Leukemia,” which was a mandatory companion study for CALGB 9720. Bone marrow samples were processed for cytogenetic analysis by standard techniques using direct and short-term (24 to 48 hours) unstimulated cultures. Chromosomes were G- or Q-banded. Karyotypes were designated according to the International System for Cytogenetic Nomenclature.21 Two karyotypes from each clone were centrally reviewed. For a patient to be designated as having a normal karyotype, a minimum of 20 bone marrow metaphases had to have been examined.
Patients were assigned risk for the attainment of CR based on pretreatment cytogenetic analysis.22 Low-risk patients (those with a high probability of attaining CR) consisted of cases with t(8;21), inv(16), or t(16;16). High-risk patients consisted of those with complex karyotypes (at least 3 abnormalities), abn(12p), inv(3), or t(3;3). Intermediate-risk patients included those with normal karyotypes, del(5q), −7, +8, t(9;11), del(9q), del(11q), +11, or −Y, unless categorized as low or high risk based on the criteria above. This risk classification was used in the analysis but not in the assignment of treatment regimens.
Pgp expression and function
Pgp expression and function were studied in pretreatment bone marrow or blood samples of patients enrolled on CALGB 9720 as part of an optional companion study, CALGB 9760, “Multidrug Resistance Studies in Acute Leukemia.” Mononuclear cells isolated by density centrifugation were cryopreserved and thawed using standard methodology. To ensure comparability of results across cooperative groups, methodology was standardized with that of the Southwest Oncology Group.6 23-25
Briefly, Pgp function was studied by efflux of the fluorescent dye 3,3′-diethyloxacarbocyanine iodide (DiOC2(3); Aldrich Chemical, St Louis, MO)26 in the presence and absence of PSC-833 (Novartis Pharmaceutical, East Hanover, NJ). To allow dye uptake, cells were incubated at a density of 1 × 106/mL in RPMI 1640 with 10% fetal calf serum and 0.6 ng/mL DiOC2(3) at 37°C for 30 minutes. Following uptake, cells were washed with phosphate-buffered saline and resuspended in medium. An aliquot of cells was placed on ice to measure DiOC2(3) uptake; the remaining cells were allowed to efflux DiOC2(3) over 90 minutes in the presence and absence of 2.5 μM PSC-833. Following efflux, the cells were washed, resuspended, and kept on ice until analyzed. As a control, rhodamine-123 efflux was also studied using an identical experimental design.
Pgp expression was studied with the MRK16 monoclonal antibody (Kamiya Biomedical, Tukwila, WA) labeled with biotinylated goat antimouse F(ab′)2 antibody (Southern Biotech, Birmingham, AL) and detected with streptavidin conjugated to Red 670 (Gibco/Life Technologies, Grand Island, NY). The MRK16 isotype control was mouse immunoglobulin G2a (Caltag, San Francisco, CA). Samples were studied by 3-color flow cytometry with fluorescein isothiocyanate–conjugated CD34 and phycoerythrin-conjugated CD33 antibodies (Becton Dickinson, San Jose, CA). Single-color flow cytometric studies were also performed with MRK16 labeled with biotinylated goat anti-mouse F(ab′)2 antibody detected with streptavidin conjugated to Red 670 as well as with phycoerythrin-conjugated goat anti-mouse antibody (Caltag).
Samples were analyzed on a FACScan flow cytometer (Becton Dickinson) using CellQuest software (Becton Dickinson). The Kolmogorov-Smirnov (KS) statistic27 was used to compare efflux in the presence and absence of PSC-833 as well as labeling with MRK16 and with isotype control. Samples were considered positive for efflux modulated by PSC-833 if the KS D value was at least 0.20 and for Pgp expression if the KS D value was at least 0.10.
The 8226 and 8226/Dox6 myeloma cells (provided by Dr W. Dalton, H. Lee Moffitt Cancer Center and Research Institute, Tampa, FL) served as negative and positive controls, respectively, for DiOC2(3) efflux and for Pgp expression; 8226/Dox6 cells have low-level Pgp-mediated MDR, consistent with the levels seen in clinical samples.28
Statistical design and analysis
The objective of the induction phase of this study was the comparison of ADE and ADEP with respect to CR rates and survival (measured from date of first randomization) using the intent-to-treat principle in which all randomized patients are included in the analysis in their assigned treatment group.
The original design had a target accrual of 400 patients to address the objectives regarding postremission therapy. With approximately 200 patients on each of the induction arms, we would have a power of 0.84 to detect an increase in the CR rate from 0.50 to 0.65 (α = 0.05, 2-sided test). Because the randomization was stopped early due to excessive mortality on the ADEP arm, only 120 patients are available for analysis. The power to detect the same difference in CR rates for the induction regimens was reduced to 0.31 with this sample size.
The difference in the proportions of complete responders in each arm was analyzed with the Fisher 2-sided exact test for 2 × 2 contingency tables.29 The difference in the distribution of complete responders, patients with resistant disease, and induction deaths was analyzed with the exact test for 3 × 2 tables.30 Differences in the proportions of patients experiencing grade 3 or higher selected toxicities were also analyzed with the Fisher exact test. A logistic regression model was used to analyze the relationship between CR and the cofactors treatment and type of AML.31
The distributions of survival and DFS times were estimated using the Kaplan-Meier method, and differences were tested with the log-rank statistic stratified by type of AML (AML de novo or AML with antecedent MDS).32 33 Follow-up data available as of September 2001 were used in the analyses.
DiOC2(3) efflux modulated by PSC-833 and Pgp expression were also analyzed with respect to CR rates, DFS, and survival.
The original study design specified formal interim analyses on each anniversary of the activation date. These analyses, which included a comparison of the 2 induction regimens with respect to CR rate and details of toxicity and adverse events, were reported to the CALGB DSMB.
Results
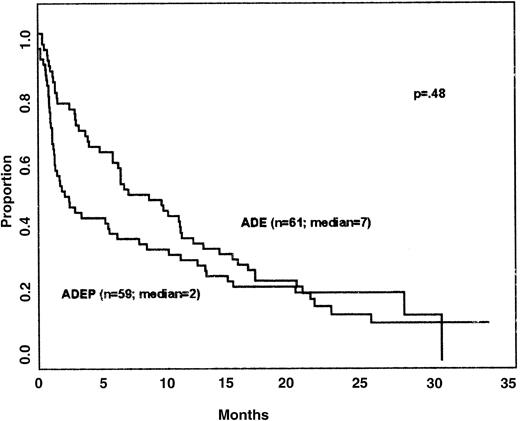
CALGB 9720 was activated in January 1998, and patient enrollment began in March 1998. In January 1999, 120 patients had been enrolled, and there had been 37 deaths, including 12 on the ADE arm and 25 on the ADEP arm. Because of concern about excessive mortality on the ADEP arm, accrual to that arm was suspended pending collection of additional data and completion of a detailed analysis to be presented to the DSMB and to the National Cancer Institute. In March 1999, following DSMB review, the ADEP arm was permanently closed to accrual. The study has since continued with all subsequently registered patients receiving ADE induction and consolidation therapy, followed by randomization to interleukin-2 maintenance therapy or observation. The 120 patients randomized to ADE or ADEP induction therapy are reported here. There were 61 patients randomized to ADE and 59 patients randomized to ADEP.
Pretreatment clinical characteristics are shown in Table2. As expected in a randomized study, the ages and sex distributions of patients were similar on the 2 arms (overall median age 70 years; 56% males). Despite the lack of numeric cutoffs for creatinine, bilirubin, and cardiac ejection fraction for patients to be eligible for this study, fewer than 5% on either arm of the study had measurements of these parameters that were outside the range required for eligibility for the prior phase 1 study, CALGB 9420 (creatinine and bilirubin less than or 1.5 times normal and left ventricular ejection fraction at least 40%).13
Pretreatment patient characteristics
| . | ADE (n = 61) . | ADEP (n = 59) . | Total (n = 120) . |
|---|---|---|---|
| Median age, y | 70 | 71 | 70 |
| Range | 60-82 | 60-84 | 60-84 |
| Sex | |||
| Male | 32 (52%) | 35 (59%) | 67 (56%) |
| Female | 29 (48%) | 24 (41%) | 53 (44%) |
| Race | |||
| White | 54 (89%) | 53 (90%) | 107 (89%) |
| Black | 4 (7%) | 2 (3%) | 6 (5%) |
| Hispanic | 2 (3%) | 4 (7%) | 6 (5%) |
| Asian | 1 (2%) | 0 (0%) | 1 (1%) |
| Organ function | |||
| Creatinine more than 1.5 × normal | 2 (3%) | 1 (2%) | 3 (3%) |
| Bilirubin more than 1.5 × normal | 3 (5%) | 1 (2%) | 4 (3%) |
| LVEF less than 40%* | 2/50 (4%) | 0/50 (0%) | 2/100 (2%) |
| . | ADE (n = 61) . | ADEP (n = 59) . | Total (n = 120) . |
|---|---|---|---|
| Median age, y | 70 | 71 | 70 |
| Range | 60-82 | 60-84 | 60-84 |
| Sex | |||
| Male | 32 (52%) | 35 (59%) | 67 (56%) |
| Female | 29 (48%) | 24 (41%) | 53 (44%) |
| Race | |||
| White | 54 (89%) | 53 (90%) | 107 (89%) |
| Black | 4 (7%) | 2 (3%) | 6 (5%) |
| Hispanic | 2 (3%) | 4 (7%) | 6 (5%) |
| Asian | 1 (2%) | 0 (0%) | 1 (1%) |
| Organ function | |||
| Creatinine more than 1.5 × normal | 2 (3%) | 1 (2%) | 3 (3%) |
| Bilirubin more than 1.5 × normal | 3 (5%) | 1 (2%) | 4 (3%) |
| LVEF less than 40%* | 2/50 (4%) | 0/50 (0%) | 2/100 (2%) |
LVEF indicates left ventricular ejection fraction.
Number with LVEF less than 40%/number with available LVEF.
Pretreatment disease characteristics, shown in Table3, were also quite similar on the 2 arms. Overall, 73% of patients were stratified as AML de novo and 27% as prior MDS. AML had occurred following chemotherapy or radiation therapy for a prior malignant or nonmalignant condition in 8% (11% of patients treated with ADE and 5% of those treated with ADEP). The distribution of karyotypes was similar on the 2 arms, as was the distribution of D values quantitating both Pgp function, measured by DiOC2(3) efflux modulated by PSC-833, and Pgp expression, measured by MRK16 antibody labeling.
Pretreatment disease characteristics
| . | ADE (n = 61) . | ADEP (n = 59) . | Total (n = 120) . |
|---|---|---|---|
| Type of AML | |||
| De novo | 45 (74%) | 43 (73%) | 88 (73%) |
| Prior MDS | 16 (26%) | 16 (27%) | 32 (27%) |
| Prior chemotherapy/radiation therapy (not for AML) | |||
| No prior MDS | 5 (8%) | 1 (2%) | 6 (5%) |
| Prior MDS | 2 (3%) | 2 (3%) | 4 (3%) |
| FAB type | |||
| M0 | 2 (3%) | 2 (3%) | 4 (3%) |
| M1 | 12 (20%) | 12 (20%) | 24 (20%) |
| M2 | 23 (38%) | 22 (37%) | 45 (38%) |
| M4 | 11 (18%) | 9 (15%) | 20 (17%) |
| M5 | 3 (5%) | 6 (10%) | 9 (8%) |
| M6 | 4 (7%) | 3 (5%) | 7 (6%) |
| M7 | 1 (2%) | 0 (0%) | 1 (1%) |
| AML, not specified | 2 (3%) | 2 (3%) | 4 (3%) |
| Other acute leukemia | 3 (5%) | 3 (5%) | 6 (5%) |
| Cytogenetic risk | |||
| Low | 3 (5%) | 3 (5%) | 6 (5%) |
| Intermediate | 29 (48%) | 30 (51%) | 59 (49%) |
| High | 7 (11%) | 8 (14%) | 15 (13%) |
| No risk assignment | 7 (11%) | 4 (7%) | 11 (9%) |
| Not evaluable | 7 (11%) | 6 (10%) | 13 (11%) |
| Not available | 8 (13%) | 8 (14%) | 16 (13%) |
| DiOC2(3) efflux ± PSC-8333-150 | |||
| Median | 0.29 | 0.25 | 0.26 |
| Range | 0.04-0.89 | 0.00-0.93 | 0.00-0.93 |
| D 0.2 or more | 22 (67%) | 21 (64%) | 43 (65%) |
| MRK16 staining3-150 | |||
| Median | 0.13 | 0.10 | 0.12 |
| Range | 0.02-0.69 | 0.03-0.53 | 0.02-0.69 |
| D 0.1 or more | 25 (76%) | 18 (55%) | 43 (65%) |
| . | ADE (n = 61) . | ADEP (n = 59) . | Total (n = 120) . |
|---|---|---|---|
| Type of AML | |||
| De novo | 45 (74%) | 43 (73%) | 88 (73%) |
| Prior MDS | 16 (26%) | 16 (27%) | 32 (27%) |
| Prior chemotherapy/radiation therapy (not for AML) | |||
| No prior MDS | 5 (8%) | 1 (2%) | 6 (5%) |
| Prior MDS | 2 (3%) | 2 (3%) | 4 (3%) |
| FAB type | |||
| M0 | 2 (3%) | 2 (3%) | 4 (3%) |
| M1 | 12 (20%) | 12 (20%) | 24 (20%) |
| M2 | 23 (38%) | 22 (37%) | 45 (38%) |
| M4 | 11 (18%) | 9 (15%) | 20 (17%) |
| M5 | 3 (5%) | 6 (10%) | 9 (8%) |
| M6 | 4 (7%) | 3 (5%) | 7 (6%) |
| M7 | 1 (2%) | 0 (0%) | 1 (1%) |
| AML, not specified | 2 (3%) | 2 (3%) | 4 (3%) |
| Other acute leukemia | 3 (5%) | 3 (5%) | 6 (5%) |
| Cytogenetic risk | |||
| Low | 3 (5%) | 3 (5%) | 6 (5%) |
| Intermediate | 29 (48%) | 30 (51%) | 59 (49%) |
| High | 7 (11%) | 8 (14%) | 15 (13%) |
| No risk assignment | 7 (11%) | 4 (7%) | 11 (9%) |
| Not evaluable | 7 (11%) | 6 (10%) | 13 (11%) |
| Not available | 8 (13%) | 8 (14%) | 16 (13%) |
| DiOC2(3) efflux ± PSC-8333-150 | |||
| Median | 0.29 | 0.25 | 0.26 |
| Range | 0.04-0.89 | 0.00-0.93 | 0.00-0.93 |
| D 0.2 or more | 22 (67%) | 21 (64%) | 43 (65%) |
| MRK16 staining3-150 | |||
| Median | 0.13 | 0.10 | 0.12 |
| Range | 0.02-0.69 | 0.03-0.53 | 0.02-0.69 |
| D 0.1 or more | 25 (76%) | 18 (55%) | 43 (65%) |
KS D value; n = 33 in each arm.
All but 3 patients received the treatment to which they were randomly assigned. Two patients were randomized to ADE but not treated; their outcomes were included as nonresponses (NR) based on the intent-to-treat principle. The third patient was randomized to ADEP, but the PSC-833 infusion was stopped immediately because of allergic manifestations; this patient achieved a CR with chemotherapy without PSC-833, and the outcome was counted as a CR to ADEP, again based on intent-to-treat.
The outcome of induction therapy in patients treated with ADE and ADEP is shown in Table 4. Rates of CR, NR, and induction death were 46%, 34%, and 20% for ADE and 39%, 17%, and 44% for ADEP (P = .008). Although the CR rates were similar (P = .47), the causes of treatment failure were different, with an excess of early deaths (< 30 days) with the ADEP regimen. The induction death rate with ADEP (44%) was more than double that with ADE (20%), and the excessive mortality on the ADEP arm of the study led to early closure of that arm.
Outcome of remission induction therapy
| . | ADE (n = 61) . | ADEP (n = 59) . | Total (n = 120) . | P4-150 . |
|---|---|---|---|---|
| Outcome for all patients | ||||
| CR | 28 (46%) | 23 (39%) | 51 (43%) | .008 (3 × 2) |
| NR | 21 (34%) | 10 (17%) | 31 (26%) | .47 (2 × 2) |
| Death | 12 (20%) | 26 (44%) | 38 (32%) | |
| Death within 30 d | 7 | 19 | 26 | |
| Death within 31-67 d4-151 | 5 | 7 | 12 | |
| No. of inductions | ||||
| None‡ | 2 | 0 | 2 | .48 |
| 1 | 50 | 52 | 102 | |
| 2 | 9 | 7 | 16 | |
| Outcome for patients receiving 1 induction course | ||||
| CR | 27 (54%) | 23 (44%) | 50 (49%) | .79 (3 × 2) |
| NR | 14 (28%) | 7 (13%) | 21 (21%) | 1.00 (2 × 2) |
| Death | 9 (18%) | 22 (42%) | 31 (30%) | |
| Death within 30 d | 7 | 17 | 24 | |
| Death within 31-67 d | 2 | 5 | 7 | |
| Outcome for patients receiving 2 induction courses | ||||
| CR | 1 (11%) | 0 (0%) | 1 (6%) | .02 (3 × 2) |
| NR | 5 (56%) | 3 (43%) | 8 (50%) | .22 (2 × 2) |
| Death4-153 | 3 (33%) | 4 (57%) | 7 (44%) | |
| Death within 30 d | 0 | 2 | 2 | |
| Death within 31-67 d4-151 | 3 | 2 | 5 |
| . | ADE (n = 61) . | ADEP (n = 59) . | Total (n = 120) . | P4-150 . |
|---|---|---|---|---|
| Outcome for all patients | ||||
| CR | 28 (46%) | 23 (39%) | 51 (43%) | .008 (3 × 2) |
| NR | 21 (34%) | 10 (17%) | 31 (26%) | .47 (2 × 2) |
| Death | 12 (20%) | 26 (44%) | 38 (32%) | |
| Death within 30 d | 7 | 19 | 26 | |
| Death within 31-67 d4-151 | 5 | 7 | 12 | |
| No. of inductions | ||||
| None‡ | 2 | 0 | 2 | .48 |
| 1 | 50 | 52 | 102 | |
| 2 | 9 | 7 | 16 | |
| Outcome for patients receiving 1 induction course | ||||
| CR | 27 (54%) | 23 (44%) | 50 (49%) | .79 (3 × 2) |
| NR | 14 (28%) | 7 (13%) | 21 (21%) | 1.00 (2 × 2) |
| Death | 9 (18%) | 22 (42%) | 31 (30%) | |
| Death within 30 d | 7 | 17 | 24 | |
| Death within 31-67 d | 2 | 5 | 7 | |
| Outcome for patients receiving 2 induction courses | ||||
| CR | 1 (11%) | 0 (0%) | 1 (6%) | .02 (3 × 2) |
| NR | 5 (56%) | 3 (43%) | 8 (50%) | .22 (2 × 2) |
| Death4-153 | 3 (33%) | 4 (57%) | 7 (44%) | |
| Death within 30 d | 0 | 2 | 2 | |
| Death within 31-67 d4-151 | 3 | 2 | 5 |
The 3 × 2 analysis compares CR, NR, and death, while the 2 × 2 analysis compares CR and no CR (NR plus death).
The last induction death occurred on day 67.
Two patients never started treatment with ADE and are included as nonresponders in the overall comparison based on intent-to-treat.
Deaths occurred within 30 (or 31-67) days from the first day of the initial induction course.
The distribution of patients receiving 1 or 2 courses of induction therapy was similar on both arms of the study. Only 1 of the 16 patients who received a second induction course on either arm of the study achieved a CR. Thus, 50 of the 51 patients who achieved CR with either ADE or ADEP did so following a single course of therapy.
The 30 patients classified as NR following induction included 25 with leukemic marrows following therapy and 5 with cytopenias without leukemia (4 with thrombocytopenia and 1 with both thrombocytopenia and neutropenia).20 The patients with cytopenias were all on the ADE arm.
Table 5 shows induction outcome by patient and disease characteristics for all patients on the study. The high-risk cytogenetic subgroup had a significantly lower proportion of patients achieving a CR than the other risk groups (P = .04). However, outcome did not differ significantly between the 2 treatment arms for patients in the low- (P = .41), intermediate- (P = .85), and high- (P = .92) risk cytogenetic groups. Moreover, outcome did not differ significantly between the 2 treatment arms for patients with AML de novo (P = .65), with prior MDS (P = .47), or with prior cytotoxic therapy (P = .78). After adjusting for treatment arm in a logistic regression model, the type of AML (de novo vs prior MDS vs prior cytotoxic therapy) did not have prognostic significance (P = .41).
Induction outcome by patients characteristics
| Characteristic . | CR (n = 51) . | NR (n = 31) . | Death (n = 38) . | Total (n = 120) . | P5-150 . |
|---|---|---|---|---|---|
| Age, y | |||||
| <70 | 26 (48%) | 10 (19%) | 18 (33%) | 54 | .28 |
| ≥70 | 25 (38%) | 20 (30%) | 21 (32%) | 66 | |
| Sex | |||||
| Male | 26 (39%) | 14 (21%) | 27 (40%) | 67 | .11 |
| Female | 25 (47%) | 16 (30%) | 12 (23%) | 53 | |
| Race | |||||
| White | 45 (42%) | 28 (26%) | 34 (32%) | 107 | .81 |
| Other | 6 (46%) | 2 (15%) | 5 (38%) | 13 | |
| Creatinine, mg/dL (μM/L) | |||||
| <1.0 (88.5) | 26 (45%) | 16 (28%) | 16 (28%) | 58 | .54 |
| ≥1.0 (88.5) | 25 (40%) | 14 (23%) | 23 (37%) | 62 | |
| Bilirubin, mg/dL (μM/L) | |||||
| <0.6 (10.27) | 22 (45%) | 15 (31%) | 12 (24%) | 49 | .21 |
| ≥0.6 (10.27) | 28 (41%) | 14 (20%) | 27 (39%) | 69 | |
| Left ventricular ejection fraction | |||||
| <60% | 23 (43%) | 12 (22%) | 19 (35%) | 54 | .76 |
| ≥60% | 21 (46%) | 12 (26%) | 13 (28%) | 46 | |
| Disease | |||||
| De novo | 35 (43%) | 21 (26%) | 26 (32%) | 82 | .44 |
| Prior MDS | 10 (36%) | 6 (21%) | 12 (43%) | 28 | |
| Prior chemotherapy/radiation therapy | 6 (60%) | 3 (30%) | 1 (10%) | 10 | |
| FAB | |||||
| M1 | 12 (50%) | 5 (21%) | 7 (29%) | 24 | .09 |
| M2 | 22 (49%) | 15 (33%) | 8 (18%) | 45 | |
| M4 | 7 (35%) | 5 (25%) | 8 (40%) | 20 | |
| Others | 10 (32%) | 5 (16%) | 16 (52%) | 31 | |
| Cytogenetic risk | |||||
| Low | 3 (50%) | 1 (17%) | 2 (33%) | 6 | .04 |
| Intermediate | 32 (54%) | 11 (19%) | 16 (27%) | 59 | |
| High | 2 (13%) | 6 (40%) | 7 (47%) | 15 | |
| MRK16 staining5-151 | |||||
| <0.10 | 13 (57%) | 6 (26%) | 4 (17%) | 23 | .66 |
| ≥0.10 | 22 (51%) | 9 (21%) | 12 (28%) | 43 | |
| DiOC2(3) efflux5-151 | |||||
| <0.20 | 16 (70%) | 3 (13%) | 4 (17%) | 23 | .17 |
| ≥0.20 | 19 (44%) | 12 (28%) | 12 (28%) | 43 |
| Characteristic . | CR (n = 51) . | NR (n = 31) . | Death (n = 38) . | Total (n = 120) . | P5-150 . |
|---|---|---|---|---|---|
| Age, y | |||||
| <70 | 26 (48%) | 10 (19%) | 18 (33%) | 54 | .28 |
| ≥70 | 25 (38%) | 20 (30%) | 21 (32%) | 66 | |
| Sex | |||||
| Male | 26 (39%) | 14 (21%) | 27 (40%) | 67 | .11 |
| Female | 25 (47%) | 16 (30%) | 12 (23%) | 53 | |
| Race | |||||
| White | 45 (42%) | 28 (26%) | 34 (32%) | 107 | .81 |
| Other | 6 (46%) | 2 (15%) | 5 (38%) | 13 | |
| Creatinine, mg/dL (μM/L) | |||||
| <1.0 (88.5) | 26 (45%) | 16 (28%) | 16 (28%) | 58 | .54 |
| ≥1.0 (88.5) | 25 (40%) | 14 (23%) | 23 (37%) | 62 | |
| Bilirubin, mg/dL (μM/L) | |||||
| <0.6 (10.27) | 22 (45%) | 15 (31%) | 12 (24%) | 49 | .21 |
| ≥0.6 (10.27) | 28 (41%) | 14 (20%) | 27 (39%) | 69 | |
| Left ventricular ejection fraction | |||||
| <60% | 23 (43%) | 12 (22%) | 19 (35%) | 54 | .76 |
| ≥60% | 21 (46%) | 12 (26%) | 13 (28%) | 46 | |
| Disease | |||||
| De novo | 35 (43%) | 21 (26%) | 26 (32%) | 82 | .44 |
| Prior MDS | 10 (36%) | 6 (21%) | 12 (43%) | 28 | |
| Prior chemotherapy/radiation therapy | 6 (60%) | 3 (30%) | 1 (10%) | 10 | |
| FAB | |||||
| M1 | 12 (50%) | 5 (21%) | 7 (29%) | 24 | .09 |
| M2 | 22 (49%) | 15 (33%) | 8 (18%) | 45 | |
| M4 | 7 (35%) | 5 (25%) | 8 (40%) | 20 | |
| Others | 10 (32%) | 5 (16%) | 16 (52%) | 31 | |
| Cytogenetic risk | |||||
| Low | 3 (50%) | 1 (17%) | 2 (33%) | 6 | .04 |
| Intermediate | 32 (54%) | 11 (19%) | 16 (27%) | 59 | |
| High | 2 (13%) | 6 (40%) | 7 (47%) | 15 | |
| MRK16 staining5-151 | |||||
| <0.10 | 13 (57%) | 6 (26%) | 4 (17%) | 23 | .66 |
| ≥0.10 | 22 (51%) | 9 (21%) | 12 (28%) | 43 | |
| DiOC2(3) efflux5-151 | |||||
| <0.20 | 16 (70%) | 3 (13%) | 4 (17%) | 23 | .17 |
| ≥0.20 | 19 (44%) | 12 (28%) | 12 (28%) | 43 |
NR indicates alive, with no response.
Refers to overall comparison.
KS D value.
Table 6 presents the induction outcomes for 66 of the 120 patients (33 on each arm of the study) who had pretreatment samples submitted and evaluable for Pgp function and expression. With ADE, rates of CR, NR, and induction death were 41%, 41%, and 18% for patients whose pretreatment AML cells exhibited dye efflux that was inhibited by PSC-833, compared with 91%, 9%, and 0% for cases without efflux (P = .03). In contrast, outcomes to ADEP did not differ by presence or absence of efflux, with virtually identical proportions in each response category.
Induction outcome as a function of treatment presence or absence of DiOC2(3) efflux modulated by PSC-833 in vitro in patients with pretreatment samples studied for MDR
| . | Efflux positive (n = 43) . | Efflux negative (n = 23) . | Total (n = 66) . | P . |
|---|---|---|---|---|
| ADE | ||||
| CR | 9 (41%) | 10 (91%) | 19 (58%) | .03 |
| NR | 9 (41%) | 1 (9%) | 10 (30%) | |
| Death | 4 (18%) | 0 (0%) | 4 (12%) | |
| Total | 22 | 11 | 33 | |
| ADEP | ||||
| CR | 10 (48%) | 6 (50%) | 16 (48%) | 1.0 |
| NR | 4 (19%) | 2 (17%) | 6 (18%) | |
| Death | 7 (33%) | 4 (33%) | 11 (33%) | |
| Total | 21 | 12 | 33 |
| . | Efflux positive (n = 43) . | Efflux negative (n = 23) . | Total (n = 66) . | P . |
|---|---|---|---|---|
| ADE | ||||
| CR | 9 (41%) | 10 (91%) | 19 (58%) | .03 |
| NR | 9 (41%) | 1 (9%) | 10 (30%) | |
| Death | 4 (18%) | 0 (0%) | 4 (12%) | |
| Total | 22 | 11 | 33 | |
| ADEP | ||||
| CR | 10 (48%) | 6 (50%) | 16 (48%) | 1.0 |
| NR | 4 (19%) | 2 (17%) | 6 (18%) | |
| Death | 7 (33%) | 4 (33%) | 11 (33%) | |
| Total | 21 | 12 | 33 |
NR indicates alive, with no response.
Hematologic toxicity was similar on the 2 arms of the study. Median number of days with less than 0.5 × 109neutrophils was 26 (range, 12 days-68 days) for ADE and 25 (range, 8 days-48 days) for ADEP. Median number of days with less than 1.0 × 109 neutrophils was 28 (range, 12 days-68 days) for ADE and 27 (range, 10 days-52 days) for ADEP.
Nonhematologic toxicities of induction therapy on the 2 arms are shown in Table 7. Grades 3 to 5 toxicities occurring more frequently with ADEP than with ADE included hyperbilirubinemia (53% vs 25%; P = .004), stomatitis (25% vs 7%; P = .01), anorexia (24% vs 7%;P = .02), esophagitis (17% vs 3%; P = .03), and diarrhea (24% vs 8%; P = .04). Hyperbilirubinemia generally occurred transiently during the PSC-833 infusion in patients treated with ADEP. Grade 5 toxicities included 3 infections on the ADE arm and 7 infections and 1 hepatic toxicity with ADEP. The cause of death in most other patients was multiorgan failure occurring in the setting of fever but without documented infection.
Grade 3 or higher nonhematologic induction toxicities occurring in more than 10% of all treated patients
| Toxicity . | ADE (n = 59) . | ADEP (n = 59) . | Total (n = 118) . | P . |
|---|---|---|---|---|
| Hyperbilirubinemia | 15 (25%) | 31 (53%) | 46 (39%) | .004 |
| Stomatitis | 4 (7%) | 15 (25%) | 19 (16%) | .01 |
| Anorexia | 4 (7%) | 14 (24%) | 18 (15%) | .02 |
| Esophagitis | 2 (3%) | 10 (17%) | 12 (10%) | .03 |
| Diarrhea | 5 (8%) | 14 (24%) | 19 (16%) | .04 |
| Skin | 4 (7%) | 12 (20%) | 16 (13%) | .06 |
| Malaise/fatigue | 8 (14%) | 17 (29%) | 25 (21%) | .07 |
| Blood urea nitrogen | 9 (15%) | 18 (31%) | 27 (23%) | .08 |
| Dysrhythmia | 5 (8%) | 11 (19%) | 16 (13%) | .18 |
| Hyperglycemia | 5 (8%) | 10 (17%) | 15 (13%) | .27 |
| Central nervous system | 5 (8%) | 10 (17%) | 15 (13%) | .27 |
| Dyspnea | 6 (10%) | 10 (17%) | 16 (13%) | .42 |
| Infection | 27 (46%) | 24 (41%) | 51 (43%) | .71 |
| Pulmonary edema | 8 (14%) | 10 (17%) | 18 (15%) | .80 |
| No. of patients with at least 1 toxicity grade 3 or higher | 52 (88%) | 54 (92%) | 106 (90%) | .76 |
| Toxicity . | ADE (n = 59) . | ADEP (n = 59) . | Total (n = 118) . | P . |
|---|---|---|---|---|
| Hyperbilirubinemia | 15 (25%) | 31 (53%) | 46 (39%) | .004 |
| Stomatitis | 4 (7%) | 15 (25%) | 19 (16%) | .01 |
| Anorexia | 4 (7%) | 14 (24%) | 18 (15%) | .02 |
| Esophagitis | 2 (3%) | 10 (17%) | 12 (10%) | .03 |
| Diarrhea | 5 (8%) | 14 (24%) | 19 (16%) | .04 |
| Skin | 4 (7%) | 12 (20%) | 16 (13%) | .06 |
| Malaise/fatigue | 8 (14%) | 17 (29%) | 25 (21%) | .07 |
| Blood urea nitrogen | 9 (15%) | 18 (31%) | 27 (23%) | .08 |
| Dysrhythmia | 5 (8%) | 11 (19%) | 16 (13%) | .18 |
| Hyperglycemia | 5 (8%) | 10 (17%) | 15 (13%) | .27 |
| Central nervous system | 5 (8%) | 10 (17%) | 15 (13%) | .27 |
| Dyspnea | 6 (10%) | 10 (17%) | 16 (13%) | .42 |
| Infection | 27 (46%) | 24 (41%) | 51 (43%) | .71 |
| Pulmonary edema | 8 (14%) | 10 (17%) | 18 (15%) | .80 |
| No. of patients with at least 1 toxicity grade 3 or higher | 52 (88%) | 54 (92%) | 106 (90%) | .76 |
Grade 5 events for the toxicities included 3 infections on the ADE arm and 7 infections and 1 hepatic toxicity on the ADEP arm.
Extensive analyses were performed in an attempt to explain the unexpectedly high induction death rate in patients treated with ADEP in the phase 3 study. Patient age, antecedent MDS, left ventricular ejection fraction, and serum creatinine did not correlate with induction death on either arm. Pretreatment serum bilirubin correlated with induction death in patients treated with ADE, with a Pvalue of .02, but not with induction death in patients treated with ADEP.
Twenty-four of 28 patients who achieved CR with ADE and 19 of 23 patients who achieved CR with ADEP received postremission therapy. Reasons for not administering postremission therapy to ADE patients were renal insufficiency (2 patients), fungal infection (1 patient), and patient preference (1 patient), whereas ADEP patients did not receive postremission therapy because of patient preference (2 patients), abnormal liver function (1 patient), and poor pulmonary function (1 patient).
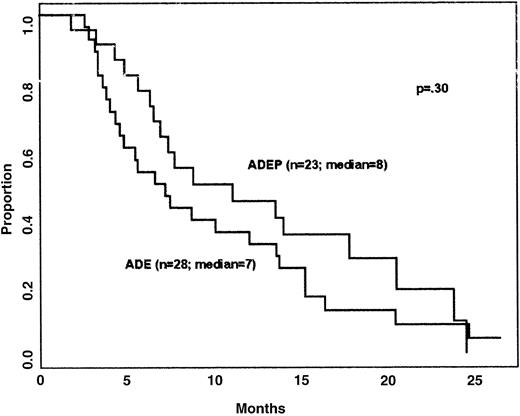
Survival and DFS of patients on the 2 arms of the study are shown in Figures 1 and2, respectively. The long-term results are similar to previous studies in patients over 60 years of age. Despite the higher numbers of induction deaths on the ADEP arm, overall survival did not differ on the 2 arms (P = .48); for example, the percentage of patients surviving for 1 year was quite similar on the 2 regimens (approximately 33%). DFS did not differ in patients treated with ADE and ADEP (medians 7 and 8 months, respectively; P = .38 by the stratified log-rank test). The power to detect differences in CR rates, DFS, and survival was low due to small patient numbers resulting from premature termination of randomization.
For patients with PSC-833–inhibitable efflux, median DFS was 5 months with ADE and 14 months with ADEP (P = .07); survival did not differ. It should be emphasized that the small number of patients whose cells were studied for PSC-833–inhibitable efflux and Pgp expression and who attained CR and were evaluable for DFS greatly limited the power to evaluate differences in DFS based on efflux, Pgp expression, and treatment arm.
Discussion
We report a phase 3 randomized trial of the MDR modulator PSC-833 in conjunction with chemotherapy in AML patients 60 years of age and older. Although the regimens that were compared in the phase 3 trial were chosen based on their equitoxicity in a prior phase 1 study, the observed early mortality on this study was excessive on the PSC-833–containing arm, and that arm was closed early. However, further follow-up demonstrates no overall survival difference: The percentage of 1-year survivors (33%) was similar on the 2 regimens and similar to historical results. Notably, the power to detect differences in DFS and survival was low due to the small number of patients. The efflux-positive patients (those whose pretreatment AML cells exhibited dye efflux modulated in vitro by PSC-833) had a lower CR rate than patients who were efflux-negative when treated with chemotherapy alone but not when treated with chemotherapy plus PSC-833. The difference in outcome on the 2 arms of the study as a function of PSC-833–modulated efflux lends support to the concept that pharmacologic reversal of efflux may improve outcome. However, this is a subgroup analysis based on a small number of patients, and the small number of patients whose cells were studied for efflux and Pgp expression limited the power to detect differences in outcome. Further testing of modulation in older AML patients must await the design of less toxic regimens.
The daunorubicin and etoposide dose reductions in the PSC-833–containing arm of the trial, derived from the prior phase 1 study, were within the range of those derived from other phase 1 studies. In our study, the daunorubicin dose was reduced from 60 mg/m2 to 40 mg/m2 (33% reduction) and the etoposide dose from 100 mg/m2 to 60 mg/m2 (40% reduction) in the PSC-833–containing regimen. In phase 1 trials of doxorubicin with PSC-833, doxorubicin clearance decreased by 30% to 50% and the area under the curve approximately doubled.34-36 In phase 1 trials of etoposide with PSC-833, etoposide clearance decreased by approximately 50% and the area under the curve approximately doubled.37-39 Dose reductions of 25% to 70% were found to be necessary when these drugs were administered with PSC-833.25,34-41 Of note, in a phase 1/II study of daunorubicin administered by continuous infusion with PSC-833, reduction of the daunorubicin dose was not found to be required.42
The unanticipated higher induction death rate on the PSC-833–containing arm of the phase 3 trial, in relation to that seen in the phase 1 study, is not readily explained. The eligibility criteria for the phase 3 trial did not include numeric cutoffs for hepatic, renal, or cardiac function, as the phase 1 trial had, but fewer than 5% of patients on either arm of the phase 3 trial would have been excluded from the phase 1 study based on these cutoffs, and their exclusion would not change the results. Additionally, patients with prior MDS and prior cytotoxic therapy were eligible for the phase 3 trial despite exclusion from the prior phase 1 study, but outcome did not differ between AML patients with prior MDS or prior cytotoxic therapy treated on the 2 arms of the phase 3 trial; nor did patients with prior MDS or prior cytotoxic therapy respond differently overall than those with de novo AML. The pharmacokinetic effects of modulators such as PSC-833 on chemotherapeutic agents that are MDR substrates mandate that phase 1 studies be performed to establish equitoxic regimens with and without modulator for comparison in phase 3 trials. This was done in this case, but for unclear reasons the phase 1 study did not accurately predict toxicities on the subsequent phase 3 trial.
Despite encouraging results of phase 2 studies of PSC-833 in AML,40-42 results of both of the phase 3 studies reported to date have been disappointing. In addition to this study, an Eastern Cooperative Oncology Group phase 3 trial comparing mitoxantrone, etoposide, and Ara-C with and without PSC-833 in refractory and relapsed AML closed because the CR rates did not differ on the 2 arms43; patients are being followed for DFS.
Results of CSA modulation studies have been more favorable, albeit not universally. A phase 1/2 evaluation of CSA with high- dose Ara-C and infusional daunorubicin in poor-risk AML patients produced a 62% CR rate, and induction outcome did not correlate with Pgp expression.44 A subsequent Southwest Oncology Group phase 3 randomized trial comparing high-dose Ara-C and infusional daunorubicin induction with and without CSA in poor-risk AML demonstrated significant improvement in DFS and overall survival with the CSA-containing regimen.45 In contrast, 3 other studies, with bolus administration of anthracyclines, did not show superiority of CSA-containing regimens.46-48
An understanding of the disparate results of modulation trials to date would be helpful in guiding the design of future studies. Despite similar effects on Pgp-mediated drug efflux, CSA and PSC-833 might have differential effects on apoptosis49 or on resistance mediated by other MDR proteins,50 and CSA likely has unique immunologic effects. Alternatively, the CSA trials of List et al44,45 may have succeeded because continuous-infusion administration of daunorubicin averted the toxic pharmacokinetic effects seen with bolus injection of anthracyclines in modulator-containing regimens.42 Another approach to avoiding these toxicities will be the use of new modulators that have fewer pharmacokinetic interactions, particularly with anthracyclines; biricodar (VX-710)51 and LY335979 52 are representatives of this new generation of MDR modulators.
The use of ADE induction therapy represents a new approach for CALGB because of both the administration of a higher daunorubicin dose and the incorporation of etoposide. The 60 mg/m2daunorubicin dose used in ADE is higher than the 30 to 45 mg/m2 doses used in previous CALGB trials in older patients.1,2 The rationale for incorporating etoposide into induction therapy was to develop a regimen that included 2 Pgp substrates for use in testing the therapeutic benefits of Pgp modulation. The addition of etoposide to induction therapy was previously shown to improve DFS in patients younger than 55 years old but not in older patients.53 The 46% CR rate to ADE in the present study is comparable to the 47% and 53% CR rates achieved in older patients in previous CALGB trials.1,2 Of note, previous CALGB trials were restricted to patients with AML de novo, whereas CALGB 9720 included patients with prior MDS and with therapy-related AML, and these patients have generally had lower CR rates than de novo AML patients.6 Moreover, the induction death rate of 20% in the 59 patients on the ADE arm of this study did not compare unfavorably with the induction death rates of 31% and 25% in patients 60 years of age and older in the 2 previous CALGB studies.1 2
Based on the results reported here, with this more intensive ADE induction regimen incorporating both a higher daunorubicin dose and etoposide, administration of a second induction course based on the results of day 14 bone marrow studies may be associated with little additional therapeutic benefit and/or excessive toxicity. If the data are confirmed in the ongoing larger series of patients treated with ADE, an alternative approach to be considered in future studies is to allow clinical recovery after the initial course of therapy and then proceed to alternative therapy for primary refractory disease.
Ten (91%) of the 11 patients whose pretreatment AML cells did not exhibit functional dye efflux modulated in vitro by PSC-833 had a complete response. Other studies in older patients have also shown more favorable responses in patients whose cells did not exhibit Pgp-mediated MDR.6 Because the current study is ongoing with all patients receiving ADE induction, we will be able to follow up on the result of this study of a small number of patients in a much larger series to see if dose intensification (here, addition of etoposide and escalation of daunorubicin dose) is beneficial in older patients with drug-sensitive cells.
In patients whose pretreatment AML cells did not exhibit functional dye efflux modulated in vitro by PSC-833, there was a suggested inferior response to ADEP than to ADE. Again, the small numbers of patients involved preclude any definite conclusion. However, the apparently lower response to ADEP may be attributable to the lower doses of daunorubicin and etoposide administered in the ADEP regimen as well as to the increased toxicity of the regimen. If the inferior response to ADEP in patients whose cells did not exhibit dye efflux was due at least in part to the lower doses of daunorubicin and etoposide, this would suggest that future trials of modulators requiring dose reductions in substrate chemotherapy drugs might test the strategy of assigning chemotherapy together with a Pgp modulator only to patients whose cells exhibit dye efflux that can be modulated.
The outcomes of patients whose AML cells exhibited PSC-833–modulated dye efflux were not better on the PSC-833–containing arm of this study. This lack of improvement in response, albeit in a small series of patients, was likely due to increased toxicity but may also have been due to the presence of additional mechanisms of resistance not modulated by PSC-833. CALGB is currently studying PSC-833 modulation in younger patients with untreated de novo AML,54 a group in which toxicities are better tolerated, and multiple mechanisms of resistance may be less likely to be present. Further Pgp modulation trials in older patients must await the design of less toxic regimens.
The authors thank Dr Cheryl L. Willman and Dr I-Ming Chen for their very gracious collaboration in standardizing CALGB MDR assays with those of the Southwest Oncology Group.
The following institutions and principal investigators participated in the study:
| Institution . | Principal investigator . | Grant no. . |
|---|---|---|
| CALGB Statistical Office, Durham, NC | Stephen George | CA33601 |
| Dana Farber Cancer Institute, Boston, MA | George P. Canellos | CA32291 |
| Dartmouth Medical School-Norris Cotton Cancer Center, Lebanon, NH | Marc Ernstoff | CA04326 |
| Duke University Medical Center, Durham, NC | Jeffrey Crawford | CA47577 |
| Georgetown University Medical Center, Washington, DC | Edward P. Gelman | CA77597 |
| Massachusetts General Hospital, Boston, MA | Michael L. Grossbard | CA12449 |
| Medical University of South Carolina, Charleston, SC | Mark Green | CA03927 |
| Mount Sinai School of Medicine, New York, NY | Lewis Silverman | CA04457 |
| North Shore-Long Island Jewish Medical Center, Manhasset, NY | Daniel R. Budman | CA35279 |
| Rhode Island Hospital, Providence, RI | William Sikov | CA08025 |
| Roswell Park Cancer Institute, Buffalo, NY | Ellis Levine | CA02599 |
| SUNY Upstate Medical University, Syracuse, NY | Stephen L. Graziano | CA21060 |
| The Ohio State University, Columbus, OH | Clara D. Bloomfield | CA77658 |
| University of California at San Diego, CA | Stephen Seagren | CA11789 |
| University of Chicago Medical Center, IL | Gini Fleming | CA41287 |
| University of Illinois at Chicago, IL | Jeffrey A. Sosman | CA74811 |
| University of Iowa, Iowa City, IA | Gerald Clamon | CA47642 |
| University of Maryland Cancer Center, Baltimore, MD | David Van Echo | CA31983 |
| University of Massachusetts Medical Center, Worcester, MA | Mary Ellen Taplin | CA37135 |
| University of Missouri/Ellis Fischel Cancer Center, Columbia, MO | Michael C. Perry | CA12046 |
| University of Nebraska Medical Center, Omaha, NE | Anne Kessinger | CA77298 |
| University of North Carolina at Chapel Hill, NC | Thomas C. Shea | CA47559 |
| University of Tennessee Memphis, TN | Harvey B. Niell | CA47555 |
| Vermont Cancer Center, Burlington, VT | Hyman B. Muss | CA77406 |
| Wake Forest University School of Medicine, Winston-Salem, NC | David D. Hurd | CA03927 |
| Weill Medical College of Cornell University, New York, NY | Michael Schuster | CA07968 |
| Institution . | Principal investigator . | Grant no. . |
|---|---|---|
| CALGB Statistical Office, Durham, NC | Stephen George | CA33601 |
| Dana Farber Cancer Institute, Boston, MA | George P. Canellos | CA32291 |
| Dartmouth Medical School-Norris Cotton Cancer Center, Lebanon, NH | Marc Ernstoff | CA04326 |
| Duke University Medical Center, Durham, NC | Jeffrey Crawford | CA47577 |
| Georgetown University Medical Center, Washington, DC | Edward P. Gelman | CA77597 |
| Massachusetts General Hospital, Boston, MA | Michael L. Grossbard | CA12449 |
| Medical University of South Carolina, Charleston, SC | Mark Green | CA03927 |
| Mount Sinai School of Medicine, New York, NY | Lewis Silverman | CA04457 |
| North Shore-Long Island Jewish Medical Center, Manhasset, NY | Daniel R. Budman | CA35279 |
| Rhode Island Hospital, Providence, RI | William Sikov | CA08025 |
| Roswell Park Cancer Institute, Buffalo, NY | Ellis Levine | CA02599 |
| SUNY Upstate Medical University, Syracuse, NY | Stephen L. Graziano | CA21060 |
| The Ohio State University, Columbus, OH | Clara D. Bloomfield | CA77658 |
| University of California at San Diego, CA | Stephen Seagren | CA11789 |
| University of Chicago Medical Center, IL | Gini Fleming | CA41287 |
| University of Illinois at Chicago, IL | Jeffrey A. Sosman | CA74811 |
| University of Iowa, Iowa City, IA | Gerald Clamon | CA47642 |
| University of Maryland Cancer Center, Baltimore, MD | David Van Echo | CA31983 |
| University of Massachusetts Medical Center, Worcester, MA | Mary Ellen Taplin | CA37135 |
| University of Missouri/Ellis Fischel Cancer Center, Columbia, MO | Michael C. Perry | CA12046 |
| University of Nebraska Medical Center, Omaha, NE | Anne Kessinger | CA77298 |
| University of North Carolina at Chapel Hill, NC | Thomas C. Shea | CA47559 |
| University of Tennessee Memphis, TN | Harvey B. Niell | CA47555 |
| Vermont Cancer Center, Burlington, VT | Hyman B. Muss | CA77406 |
| Wake Forest University School of Medicine, Winston-Salem, NC | David D. Hurd | CA03927 |
| Weill Medical College of Cornell University, New York, NY | Michael Schuster | CA07968 |
The research for CALGB 9720 was supported in part by grants from the National Cancer Institute to the Cancer and Leukemia Group B (CA31946) and to the CALGB Statistical Center (CA33601).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Maria R. Baer, Dept of Medicine, Roswell Park Cancer Institute, Elm and Carlton Sts, Buffalo, NY 14263; e-mail:maria.baer@roswellpark.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal