Although common genetic variants in platelet collagen receptors influence platelet activation and thrombosis, the impact of polymorphisms in collagen genes on cardiovascular disease is unknown. To evaluate this, we genotyped a highly polymorphic intronic tandem repeat of the COL3A1 gene, encoding collagen type III, alpha 1. This revealed 4 common alleles (COL3A1-1, -2, -3, and -4). The 2 populations studied were as follows: (1) a cross-sectional study of 703 acute coronary syndrome (ACS) patients with myocardial infarction (MI) and unstable angina, and (2) a prospective study of 924 Caucasian patients from the OPUS (Orbofiban in Patients with Unstable coronary Syndromes)-TIMI-16 trial of the oral GPIIb/IIIa antagonist orbofiban. In addition, we studied 306 control subjects and 224 patients with stable angina. In the case-control population, COL3A1-4 carriers were protected against ACS (odds ratio [OR] = 0.57, 95% CI = 0.35-0.91, P = .02) and stable angina (OR = 0.35, 95% CI = 0.16-0.74, P = .006). In the OPUS population, allele 4 again appeared protective against composite end points (death, MI, stroke, recurrent ischemia, and urgent rehospitalization) (relative risk [RR] = 0.41, 95% CI = 0.17-1.00). There were significant interactions between COL3A1-1 and -3 variants and treatment. Allele COL3A1-3 was associated with an increased risk of the composite end point (RR = 1.65, 95% CI = 1.07-2.55) in patients randomized to orbofiban, but appeared protective in placebo patients (RR = 0.53, 95% CI = 0.28-0.98). We conclude that variants in the COL3A1 gene, the product of which is a vessel-wall protein and platelet ligand, modulate the risk of coronary artery disease and could also modulate the response to antithrombotic therapy. This is the first reported association between polymorphisms of extracellular matrix components and cardiovascular risk.
Introduction
Collagen fibers are involved in the adhesion and trapping of platelets to exposed subendothelium at sites of vascular damage.1 Collagen binds a number of receptors on platelets, principally glycoprotein (GP) Ia/IIa and GPVI.2It also indirectly binds to the platelet surface via von Willebrand factor (VWF). Under high-shear conditions, VWF attached to collagen binds to the platelet receptor complex GPIb-IX-V. Platelet velocity then decreases, permitting firm attachment of collagen to GPIa/IIa, establishing platelet adhesion. Collagen further interacts with the lower-affinity receptor GPVI, promoting platelet activation and thromboxane A2 production.3
To date, there are approximately 19 known, genetically distinct collagen types with specialized tissue distributions.4Collagen type III is the second most abundant collagen in human tissues, after collagen type I, and is expressed in blood vessels. It is encoded by the COL3A1 gene, which is 44 kb in length and contains 52 exons,5 and is closely linked with the COL5A2 gene.6
Type I and type III collagens appear together in the thickened intima of atherosclerotic lesions.7 This plaque collagen is produced mainly by smooth muscle cells, but can also be synthesized by endothelial cells. Fibrillar type I and type III collagens occupy approximately 80% of the section area in samples of restenotic lesions of the human coronary artery,7 contributing to plaque growth and arterial lumen narrowing. Degradation of collagens by matrix metalloproteinases may overcome synthesis, resulting in plaque rupture and thrombus formation.8
In this study, we examined a highly polymorphic region on intron 25 of the COL3A1 gene.9 In the absence of clear mechanistic models for the roles of alternative alleles, initial investigations of the role of genetic variability usually rely on linkage disequilibrium between disease-causing variants and the first variants genotyped. Such disequilibrium is typically very strong within a gene.10 We therefore chose to genotype a highly polymorphic marker within COL3A1 because that increased our power to detect a genetic association. The intron variant consists of between 3 and 13 copies of a 15–base pair variable number tandem repeat (VNTR).11 We compared allele frequencies in patients with an acute coronary syndrome (ACS), patients with stable angina, and controls. We then investigated whether the alleles that modulated outcome in a case-control population had an impact on recurrent events in patients with ACSs receiving an oral GPIIb/IIIa antagonist.
Materials and methods
Study populations
Studies were reviewed by institutional ethics committees, and written informed consent was obtained from each individual.
ACS population with stable angina and control comparison groups.
A total of 703 patients with a history of myocardial infarction (MI) or unstable angina were recruited from 13 Irish hospitals. The inclusion criterion for ACS patients was a history of either MI or unstable angina. MI was defined as chest pain of at least 20 minutes' duration, along with previous or current electrocardiogram or serum enzyme changes diagnostic of MI. Unstable angina was defined as chest pain typical of angina occurring at rest or lasting at least 20 minutes and requiring hospitalization in a patient with known coronary artery disease based on a positive stress test or a coronary angiogram. A comparison group of 224 patients with stable angina was recruited from the same sites. Inclusion criteria for stable angina were chest pain occurring with exercise typical of angina in a patient with known coronary artery disease based on a coronary angiogram or a positive treadmill test. A randomly selected group from the Irish population provided estimates of gene frequencies in a nondiseased population. These were a group of 306 sequentially recruited primigravid patients from 2 antenatal clinics.12
OPUS-TIMI-16 trial.
A total of 1014 patients were recruited for the genetics substudy from the OPUS (Orbofiban in Patients with Unstable coronary Syndromes)-TIMI-16 trial of the oral platelet glycoprotein IIb/IIIa antagonist orbofiban in patients with ACS.13 Patients were recruited from 888 hospitals in 29 countries around the world. Patients received aspirin and were randomized to receive the following: (1) 50 mg orbofiban daily, (2) 50 mg orbofiban twice daily for 30 days followed by 30 mg orbofiban twice daily, or (3) a placebo. The primary composite end point was MI, recurrent ischemia requiring rehospitalization, urgent revascularization, stroke, and/or death. Inclusion criteria for the OPUS-TIMI-16 trial included ACS defined as ischemic chest pain at rest within 72 hours of randomization, associated with positive cardiac markers, electrocardiographic changes, or prior cardiovascular disease. The genetic substudy is described in more detail elsewhere.14
Genotyping
Genomic DNA was extracted from whole blood using methods previously described.15 A 210-bp fragment, containing the 15-bp repeat on intron 25, was amplified using the following set of primers: forward primer, 5′-GGCAAGAGAATCACTAGAAC-3′; and reverse primer, 3′-CCTCAAAGGGCAATAAC-5′. Each polymerase chain reaction (PCR) contained 50 ng DNA, 10 pmol of both sense and antisense primer, 1 mM dNTPs, 1.5 mM MgCl2, and 1 U Bioline Taqpolymerase enzyme (Bioline, London, United Kingdom) in a final volume of 50 μL. The PCR conditions were as follows: 94°C for 5 minutes, 94°C for 1 minute, 62°C for 1 minute, and 72°C for 1 minute in a 30-cycle reaction. PCR product was verified by 2% agarose gel electrophoresis, stained with ethidium bromide, and visualized under ultraviolet light.
Statistical methods
The associations between genotype and presentation (comparisons among the Irish ACS, stable angina, and control groups) were investigated using a logistic regression. The 2 primary end points were ACS versus control and ACS versus stable angina. In the follow-up OPUS-TIMI-16 trial, the primary end point was the composite end point. Survival analysis considered the association between genotype and the time to first event for the composite end point, MI, and the first severe, major, or minor bleeding episode. Differences due to geographic and ethnic origin could create spurious genetic associations because genotypes vary markedly among races, and to a lesser extent among countries. Therefore, results are based on a Cox proportional hazards model of Caucasian patients only, adjusting for country of origin. Tests of interaction between genotype and treatment effect used the likelihood ratio test. Confidence intervals (CIs) presented are at the 95% level.
Results
Eleven different allelic forms of the COL3A1-VNTR were observed in the studies (Table 1). In all of the populations, alleles 1, 2, 3, and 4 were the most common.
Repeat number and PCR product length for alleles of COL3A1
| COL3A1 allele . | Number of repeats . | PCR product size . |
|---|---|---|
| 1 | 5 | 236 |
| 2 | 6 | 251 |
| 3 | 7 | 266 |
| 4 | 8 | 281 |
| 5 | 9 | 296 |
| 6 | 10 | 311 |
| 7 | 11 | 326 |
| 8 | 12 | 341 |
| 9 | 13 | 356 |
| 10 | 3 | 206 |
| 11 | 4 | 221 |
| COL3A1 allele . | Number of repeats . | PCR product size . |
|---|---|---|
| 1 | 5 | 236 |
| 2 | 6 | 251 |
| 3 | 7 | 266 |
| 4 | 8 | 281 |
| 5 | 9 | 296 |
| 6 | 10 | 311 |
| 7 | 11 | 326 |
| 8 | 12 | 341 |
| 9 | 13 | 356 |
| 10 | 3 | 206 |
| 11 | 4 | 221 |
Analysis in the ACS and stable angina populations
The genotype frequencies in the Irish groups are shown in Table2. COL3A1-4 carriers had a significantly lower risk of both ACS (odds ratio [OR] = 0.57, 95% CI = 0.35-0.91) and stable angina (OR = 0.35, 95% CI = 0.16-0.74) compared with controls (Table 3). The increased risk conferred by allele 3 was not significant for either the stable angina or the ACS group (Table 3). To correct for multiple statistical testing, we performed logistic regression treating ACS, stable angina, and controls as 3 classes. The effect of COL3A1-4 remained significant (P = .007) even after Bonferroni correction for the 4 major alleles (P = .028). Comparisons between stable and unstable disease should highlight variants that increase the risk of coronary thrombus or plaque rupture against a background of atherosclerotic disease. However, the frequency of the main COL3A1 alleles was similar in the stable angina and ACS groups (Table 3).
COL3A1 genotype frequencies in Irish patients with coronary artery disease and controls
| Genotype . | MI . | Unstable angina . | Stable angina . | Control . |
|---|---|---|---|---|
| 1/1 | 96 (18.1%) | 35 (20.3%) | 45 (20.1%) | 66 (21.6%) |
| 1/2 | 109 (20.5%) | 43 (25.0%) | 55 (24.6%) | 58 (19.0%) |
| 2/2 | 54 (10.2%) | 7 (4.1%) | 17 (7.6%) | 27 (8.8%) |
| 1/3 | 118 (22.2%) | 37 (21.5%) | 52 (23.2%) | 61 (19.9%) |
| 2/3 | 73 (13.7%) | 26 (15.1%) | 26 (11.6%) | 35 (11.4%) |
| 3/3 | 35 (6.6%) | 12 (7.0%) | 15 (6.7%) | 19 (6.2%) |
| 1/4 | 12 (2.3%) | 6 (3.5%) | 2 (0.9%) | 19 (6.2%) |
| 2/4 | 8 (1.5%) | 2 (1.2%) | 6 (2.7%) | 7 (2.3%) |
| 3/4 | 14 (2.6%) | 1 (0.6%) | 1 (0.4%) | 5 (1.6%) |
| 4/4 | 1 (0.2%) | 1 (0.6%) | 0 (0.0%) | 1 (0.3%) |
| 1/other | 7 (1.3%) | 2 (1.2%) | 0 (0.0%) | 4 (1.3%) |
| 2/other | 1 (0.2%) | 0 (0.0%) | 1 (0.4%) | 3 (1.0%) |
| 3/other | 2 (0.4%) | 0 (0.0%) | 3 (1.3%) | 0 (0.0%) |
| 4/other | 1 (0.2%) | 0 (0.0%) | 1 (0.4%) | 1 (0.3%) |
| Total | 531 | 172 | 224 | 306 |
| Genotype . | MI . | Unstable angina . | Stable angina . | Control . |
|---|---|---|---|---|
| 1/1 | 96 (18.1%) | 35 (20.3%) | 45 (20.1%) | 66 (21.6%) |
| 1/2 | 109 (20.5%) | 43 (25.0%) | 55 (24.6%) | 58 (19.0%) |
| 2/2 | 54 (10.2%) | 7 (4.1%) | 17 (7.6%) | 27 (8.8%) |
| 1/3 | 118 (22.2%) | 37 (21.5%) | 52 (23.2%) | 61 (19.9%) |
| 2/3 | 73 (13.7%) | 26 (15.1%) | 26 (11.6%) | 35 (11.4%) |
| 3/3 | 35 (6.6%) | 12 (7.0%) | 15 (6.7%) | 19 (6.2%) |
| 1/4 | 12 (2.3%) | 6 (3.5%) | 2 (0.9%) | 19 (6.2%) |
| 2/4 | 8 (1.5%) | 2 (1.2%) | 6 (2.7%) | 7 (2.3%) |
| 3/4 | 14 (2.6%) | 1 (0.6%) | 1 (0.4%) | 5 (1.6%) |
| 4/4 | 1 (0.2%) | 1 (0.6%) | 0 (0.0%) | 1 (0.3%) |
| 1/other | 7 (1.3%) | 2 (1.2%) | 0 (0.0%) | 4 (1.3%) |
| 2/other | 1 (0.2%) | 0 (0.0%) | 1 (0.4%) | 3 (1.0%) |
| 3/other | 2 (0.4%) | 0 (0.0%) | 3 (1.3%) | 0 (0.0%) |
| 4/other | 1 (0.2%) | 0 (0.0%) | 1 (0.4%) | 1 (0.3%) |
| Total | 531 | 172 | 224 | 306 |
COL3A1 allele carrier status: comparisons among cross-sectional study groups
| Allele . | ACS versus control . | Stable angina versus control . | ACS versus stable angina . |
|---|---|---|---|
| COL3A1-1 | 0.92 (0.69-1.23) | 1.04 (0.71-1.50) | 0.89 (0.64-1.23) |
| COL3A1-2 | 1.15 (0.88-1.51) | 1.19 (0.84-1.69) | 0.96 (0.71-1.30) |
| COL3A1-3 | 1.29 (0.98-1.69) | 1.21 (0.85-1.71) | 1.07 (0.79-1.45) |
| COL3A1-4 | 0.57 (0.35-0.91)3-150 | 0.35 (0.16-0.74)3-151 | 1.63 (0.79-3.40) |
| Allele . | ACS versus control . | Stable angina versus control . | ACS versus stable angina . |
|---|---|---|---|
| COL3A1-1 | 0.92 (0.69-1.23) | 1.04 (0.71-1.50) | 0.89 (0.64-1.23) |
| COL3A1-2 | 1.15 (0.88-1.51) | 1.19 (0.84-1.69) | 0.96 (0.71-1.30) |
| COL3A1-3 | 1.29 (0.98-1.69) | 1.21 (0.85-1.71) | 1.07 (0.79-1.45) |
| COL3A1-4 | 0.57 (0.35-0.91)3-150 | 0.35 (0.16-0.74)3-151 | 1.63 (0.79-3.40) |
Data are presented as odds ratios (95% confidence intervals).
P = .02.
P = .006.
Influence of baseline covariates on ischemic end points and bleeding in the OPUS-TIMI-16 trial
In the OPUS-TIMI-16 study with analysis of 924 Caucasian subjects, no significant association was found for age, sex, or geographic origin and the risk of composite events (or the risk of MI when considered alone). Severe, major, or minor bleeding occurred in 18.4% of patients during follow-up. Genetic samples were taken from patients in the Americas (n = 742), Europe (n = 172), and South Africa (n = 10). There was a significant variation between countries and with increasing age in the risk of bleeding. Comparing those older than 60 years with those younger than 60, the relative risk (RR) of bleeding was 1.44 (95% CI = 1.06-1.97). The risk of bleeding relative to placebo was 1.38 (95% CI = 0.92-2.08) for the low-dose and 2.08 (95% CI = 1.38-3.00) for the high-dose orbofiban groups, respectively. Subsequent analysis adjusted for the confounding effects of age, sex, treatment, and country.
Influence of genotype on ischemic end points and bleeding in the OPUS-TIMI-16 study and the interaction with GPIIb/IIIa antagonists
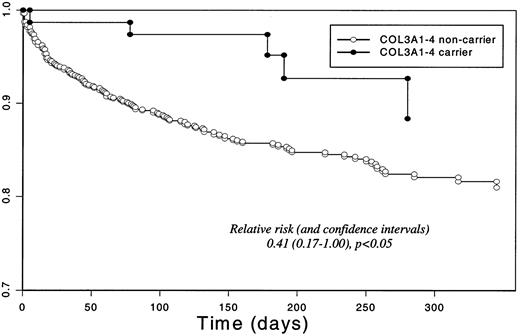
Table 4 describes the risk associated with the COL3A1 genotype by comparing carriers with noncarriers for alleles 1 to 4. As in the case-control population, carriers of allele 4 had a significantly reduced risk of composite events (RR = 0.41, 95% CI = 0.17-1.00). For each of alleles 1, 2, and 3, carrier status was not associated with the overall risk of composite events. Figure 1 illustrates the survival curve for COL3A1-4 carriers in the OPUS-TIMI-16 population.
Impact of genotype on the composite end point, MI alone, and bleeding outcomes in the OPUS study (placebo and treated groups combined)
| Allele . | Composite end point . | MI . | Bleeding . |
|---|---|---|---|
| COL3A1-1 | 1.08 (0.74-1.57) | 1.51 (0.61-3.74) | 0.82 (0.59-1.15) |
| COL3A1-2 | 1.13 (0.81-1.58) | 1.28 (0.60-2.74) | 0.83 (0.60-1.14) |
| COL3A1-3 | 1.07 (0.76-1.50) | 0.79 (0.35-1.76) | 1.44 (1.06-1.97)4-150 |
| COL3A1-4 | 0.41 (0.17-1.00)4-151 | 0.90 (0.21-3.82) | 1.37 (0.82-2.27) |
| Allele . | Composite end point . | MI . | Bleeding . |
|---|---|---|---|
| COL3A1-1 | 1.08 (0.74-1.57) | 1.51 (0.61-3.74) | 0.82 (0.59-1.15) |
| COL3A1-2 | 1.13 (0.81-1.58) | 1.28 (0.60-2.74) | 0.83 (0.60-1.14) |
| COL3A1-3 | 1.07 (0.76-1.50) | 0.79 (0.35-1.76) | 1.44 (1.06-1.97)4-150 |
| COL3A1-4 | 0.41 (0.17-1.00)4-151 | 0.90 (0.21-3.82) | 1.37 (0.82-2.27) |
Data are presented as relative risk (95% confidence interval) for carriers of the allele shown relative to noncarriers, obtained from a Cox proportional hazards model that adjusted for age, sex, country, and treatment.
P = .02.
P = .05.
Survival for the composite end point in the OPUS-TIMI-16 study population by COL3A1 allele-4 carrier status.
Survival for the composite end point in the OPUS-TIMI-16 study population by COL3A1 allele-4 carrier status.
Carriers of allele 3 (versus noncarriers) had a significantly increased risk of bleeding (Table 4) (RR = 1.44, 95% CI = 1.06-1.97). Carriers of allele 4, who were protected against composite end points, also showed a slight, albeit nonsignificant, increase in bleeding risk (RR = 1.37, 95% CI = 0.82-2.27).
Interaction between genotype and treatment
When outcomes were compared among the treated and placebo groups (Table 5), treatment appeared to alter the impact of the COL3A1 genotype on the relative risk of the composite end point. The increased risk of COL3A1 allele 3 (versus noncarrier) was evident in those receiving the GPIIb/IIIa antagonist, but not among those receiving placebo. There was also a significant interaction between treatment and the COL3A1 allele 1, which conferred an increased risk in patients receiving the placebo but not in those taking orbofiban.
Relative risks of the composite end point for carriers versus noncarriers
| Allele . | Placebo . | Orbofiban treated . | P for interaction . |
|---|---|---|---|
| COL3A1-1 | 2.14 (1.04-4.39) | 0.73 (0.46-1.15) | .006 |
| COL3A1-2 | 1.45 (0.85-2.47) | 0.94 (0.61-1.47) | .29 |
| COL3A1-3 | 0.53 (0.28-0.98) | 1.65 (1.07-2.55) | .002 |
| COL3A1-4 | 0.56 (0.14-2.30) | 0.34 (0.11-1.07) | .56 |
| Allele . | Placebo . | Orbofiban treated . | P for interaction . |
|---|---|---|---|
| COL3A1-1 | 2.14 (1.04-4.39) | 0.73 (0.46-1.15) | .006 |
| COL3A1-2 | 1.45 (0.85-2.47) | 0.94 (0.61-1.47) | .29 |
| COL3A1-3 | 0.53 (0.28-0.98) | 1.65 (1.07-2.55) | .002 |
| COL3A1-4 | 0.56 (0.14-2.30) | 0.34 (0.11-1.07) | .56 |
Data are presented as relative risk (95% confidence interval) based on a Cox proportional hazards model that adjusted for age, sex, and country, comparing risk in carriers with that in noncarriers.
There was no interaction between COL3A1 genotype and treatment for bleeding risk; that is, the increase in bleeding among COL3A1-3 carriers was not significantly different between placebo (RR = 1.40, 95% CI = 0.75-2.60) and treated patients (RR = 1.44, 95% CI = 1.01-2.07).
Covariate adjustment
Covariate adjustment helps indicate whether a variant confers its risk through known factors or by an independent pathway. We chose not to adjust for major risk factors because genetic effects are causative rather than a byproduct. In the OPUS study, covariate adjustment altered the results only slightly. Models were fitted including adjustment for age, sex, country, race, diabetes, smoking, hypertension requiring drug treatment, high cholesterol requiring drug treatment, and family history for the effects of the following: COL3A1-4 on the composite end point (RR = 0.41, 95% CI = 0.17-0.99), COL3A1-3 on bleeding (RR = 1.47, 95% CI = 1.07-2.01), COL3A1-3 on the composite end point among the orbofiban-treated patients (RR = 1.60, 95% CI = 1.03-2.46), and COL3A1-3 on the composite end point among placebo patients (RR = 0.53, 95% CI = 0.28-0.99).
Discussion
Common genetic variants in platelet collagen receptors GPIa and GPVI have been identified as potentially important risk factors in coronary disease.16-19 Our findings indicate that variants in the collagen genes themselves may influence coronary disease. In contrast to most variants linked to coronary artery disease, a variant that we have identified appears to protect against the disease. Thus, an intronic variant, COL3A1-4, protected against presentation with ACSs and stable angina. As in all case-control studies, there is the possibility that the conclusions are biased by an impact of the genotype on mortality. However, in a prospective study, we found that the same variant, COL3A1-4, protected against the recurrence of coronary events in the OPUS-TIMI-16 trial population. This reduces the likelihood that the original observation was merely a chance finding.
Although the observation regarding allele 4 appears straightforward, we found more complex apparent associations with allele 3 in the OPUS prospective study. This allele protected against events in the placebo group, but conferred increased risk among those treated with IIb/IIIa antagonists. In addition, allele 3 appeared to increase the risk of bleeding, although in a treatment-independent fashion. These results are difficult to interpret without proposing a complex model for the role of COL3A1 variation in thrombosis and hemostasis, and require further replication in other studies. We have performed genetic analysis of COL3A1 in 539 subjects in the Evaluation of oral Xemilofiban In Controlling Thrombotic Events (EXCITE) study population,20 who were randomized to another oral IIb/IIIa antagonist, xemilofiban. The numbers of events were too small to shed clear light on the findings of the OPUS study. However, the results were again suggestive of an increased risk with allele 3 among patients taking IIb/IIIa antagonists (6 of the 7 patients with MIs were allele-3 carriers taking Xemilofiban treatment; C.M. and D.C.S., unpublished observations, December 2001). Clearly, a larger analysis in another IIb/IIIa antagonist study is required to clarify the allele-3 associations in the OPUS study and to determine which of the 3 formally significant associations for allele 3 are true positive findings.
What are the implications of the findings for our understanding of how collagen synthesis and platelet signaling at diseased sites contribute to disease? It is unlikely that the intronic VNTR polymorphism is itself causal because it does not modify the protein sequence and is not close to the promoter of the gene. Therefore, it is likely to be in strong disequilibrium10 with (ie, very strongly associated with) unidentified causative genetic factor(s) in either the promoter or the protein-coding sequences. Further elucidation will require analyses in both the promoter and protein-coding regions. The formation of the fibrous cap on an atherosclerotic plaque reflects a balance between the rate of synthesis of collagens and other connective tissue proteins, and the rate of synthesis of collagen-degrading enzymes such as metalloproteinases.21 In addition to a difference in the propensity to lay down collagen (perhaps through the influence of a promoter variant), gene variants may influence the propensity for degradation (perhaps through a structural variant). Alternatively, the risk may be mediated through platelet signaling because our data suggest that the COL3A1-3 variant may influence the response to an antiplatelet agent.
Regardless of the mechanism, this genetic study highlights the importance of vessel-wall components in determining the risk of coronary events. It will be of interest to determine whether genetic variations in other vascular-wall components represent an important element of cardiovascular risk.
We thank the Irish Cardiology genetics network (ACS study), and the OPUS investigators for contributing samples to this study. This is a publication from the Biopharmaceutical Sciences Network funded by the Irish Higher Education Authority.
Prepublished online as Blood First Edition Paper, April 30, 2002; DOI 10.1182/blood-2002-01-0283.
Supported by grants from the Higher Education Authority (Ireland), and from Searle.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Denis C. Shields, Department of Clinical Pharmacology, Royal College of Surgeons in Ireland, 123 St Stephen's Green, Dublin 2, Ireland; e-mail: dshields@rcsi.ie.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal