Abstract
During acute infection, latent and lytic Epstein-Barr virus (EBV) epitope-specific CD8+ T cells have a CD45RO+CD45RA− phenotype. However, after resolution of the infection, a large proportion of these cells, particularly those specific for lytic viral epitopes, re-express the CD45RA molecule. The role of CD8+ CD45RA+ T cells in ongoing immunity to EBV and other viruses is unknown. We now demonstrate that, relative to their CD45RO+ counterparts, the EBV-specific CD8+ T cells that revert to CD45RA expression after acute infectious mononucleosis are not in cell cycle, have longer telomeres, and are more resistant to apoptosis partly because of increased Bcl-2 expression. However, the EBV-specific CD8+CD45RA+ T cells have shorter telomeres than the total CD8+ CD45RA+ T-cell pool and predominantly express low levels of the CCR7 chemokine receptor, indicating that they are not naive cells. In addition, EBV-specific CD8+CD45RA+ T cells can be induced to proliferate and exhibit potent cytotoxic activity against target cells loaded with specific peptide. Our results strongly suggest, therefore, that EBV-specific CD8+ CD45RA+ T cells represent a stabilized virus-specific memory pool and not terminally differentiated effector cells. The identification of mechanisms that enable stable virus-specific CD8+ T cells to persist after acute infection may lead to the enhancement of antiviral immunity in immunocompromised and elderly persons.
Introduction
The central goal of this study was to identify the constraints that regulate the persistence of Epstein-Barr virus (EBV)–specific CD8+ T cells after the resolution of acute infectious mononucleosis (AIM) to identify how the memory pool specific for this virus may be maintained. Primary encounter with EBV induces specific naive CD8+ T cells to proliferate and express phenotypic markers of priming, such as CD45RO.1,2Most of this expanded population succumbs to apoptosis after the acute infection resolves.3,4 The EBV-specific memory pool that escapes apoptosis consists of a mixture of cells specific for lytic and latent viral proteins, which are differentially expressed during early and late stages of the infection, respectively.2 5-7 It is unclear whether apoptosis remains a constraint on the persistence of EBV-specific CD8+ T cells after the acute infection resolves and whether the cells that are specific for different viral epitopes are equally susceptible to death.
A second limit to the maintenance of memory CD8+ T cells after the initial infection may be telomere erosion, resulting from excessive proliferation of specific clones.8,9 This may lead to the development of terminally differentiated end-stage effector cells that are unable to proliferate on restimulation.10It has been shown, however, that despite the considerable expansion of EBV-specific CD8+ T cells, telomere loss does not occur during AIM because of the induction of the enzyme telomerase in these cells.11,12 Telomere erosion does occur in the EBV-specific CD8 pool during persistent infection,12possibly because of frequent restimulation by virus, leading to progressively reduced telomerase induction that is insufficient to maintain telomere length in these cells.9 The loss of some highly expanded clones of EBV-specific CD8+ T cells during acute infection that are later replaced by other less expanded populations during chronic infection,56 suggests indirectly that telomere loss may limit the persistence of virus-specific CD8+ T cells. However, the critical point at which telomere loss may lead to the development of a terminally differentiated senescent population of EBV-specific CD8+ T cells remains to be determined.
Overlapping markers such as CD45RA, CD45RO, CD28, CD27, and CCR7 have been used to identify the differentiation state of EBV13-16 and other virus-specific CD8+ T cells17-20 that persist after acute infection. A consistent observation is that during acute infection, cells that are specific for different viruses are CD45RO+.1,17 On the resolution of acute infection, however, a proportion of the specific CD8+ T cells re-express the CD45RA molecule, and this has been found after EBV14-16 and cytomegalovirus (CMV)17,20 but not after influenza virus infections.19 Virus-specific CD45RA+ and CD45RO+ T-cell compartments found after the resolution of AIM are derived from the same clones and thus represent the same cells at different stages of differentiation or maturation.21 22
It has been suggested that previously primed CD8+ T cells that re-express CD45RA and down-regulate CCR7 expression may be approaching end-stage differentiation18,23 and that this process may be defective in human immunodeficiency-1 (HIV-1)–infected persons.18,24 We showed previously that EBV lytic epitope-specific CD8+ T cells that re-express CD45RA are predominantly CCR7low.14,15 It is not clear whether these cells represent an end-stage population in EBV-infected persons or if they can be re-activated to proliferate in response to specific peptides. The loss of the CD27 marker has also been associated with terminal differentiation of CD8+ T cells.25 Although the EBV-specific CD8+CD45RA+ T-cell pool expresses high levels of CD27,14,15 the CMV-specific CD8+CD45RA+ T-cell population,20 shows low expression of this marker.18,20 This suggests that the EBV-specific CD8+ CD45RA+ T cells may be less differentiated than the equivalent subset of CMV-specific T cells.18 However, it is not known whether either or both of these populations of virus-specific CD45RA+ T cells have reached end-stage differentiation.
To clarify the residual replicative potential of EBV-specific CD8+ T cells after resolution of AIM, we investigated the characteristics of EBV epitope-specific CD8+ T cells that re-express CD45RA during and after AIM in the same patients and in healthy carriers of the virus. Our results indicate that EBV-specific CD8+ T cells that re-express CD45RA are not a terminally differentiated end-stage effector population. On the contrary, these cells have the characteristics of a stable apoptosis-resistant memory pool that retains functional and substantial replicative capacity.
Materials and methods
Sample collection and preparation
Heparinized peripheral blood was collected from patients with acute infectious mononucleosis, and from the same patients at least 12 months following resolution of acute infection (chronic phase). Peripheral blood mononuclear cells (PBMCs) were isolated as described and were cryopreserved in liquid nitrogen, and the phenotype of the EBV-specific CD8+ T cells was analyzed simultaneously in acute and follow-up samples after thawing.12 PBMC populations from healthy laboratory staff were used as controls. Purified CD8+ T-cell subsets were separated from PBMCs by negative selection using the VARIO MACS system (Miltenyi Biotec, Surrey, United Kingdom) as described in detail elsewhere.12 The purity of isolated subsets was greater than 95% as determined by flow cytometry.
Peptide-HLA class 1 tetramers
Soluble phycoerythrin (PE)–labeled peptide-HLA class 1 tetramers were constructed as described15 using peptide5 epitopes derived from lytic and latent cycle proteins of EBV.2 15 Lytic epitopes used were the HLA-A2–restricted peptide ligands GLCTLVAML (from the EBV lytic protein BMLF1) and YVLDHLIVV (from the lytic cycle protein BRLF1) and the HLA-B8–restricted peptide ligand RAKFKQLL (from the lytic protein BZLF1). Latent epitopes used were the HLA-A2–restricted peptide ligand CLGGLLTMV (derived from LMP-2) and the HLA-B8–restricted peptide ligands FLRGRAYGL and QAKWRLQTL (both derived from EBNA3A). The description of each tetramer used has been abbreviated to the first 3 amino acids of the peptide sequence (eg, GLC, YVL, RAK).
Tetramers used in this study were highly specific, as demonstrated by the labeling of EBV peptide-specific CD8+ T-cell clones with the appropriate tetramer but not with others of the same HLA-type but directed to other EBV, influenza, or HIV peptide epitopes.5,16 In addition, EBV-seronegative subjects bearing the appropriate HLA type did not react with EBV-specific tetramers, whereas seropositive subjects did not react with HLA-mismatched EBV tetramers.5,15 16 All the batches of EBV-specific tetramers used in this study were checked for specificity as above before use.
Flow cytometric analysis
Paired acute- and chronic-phase PBMC samples from AIM patients were analyzed by 4-color flow cytometry using PE-conjugated HLA class 1–peptide tetrameric complexes. Specific antibodies directed to other surface markers used included CD8 (Beckman-Coulter, High Wycombe, United Kingdom), CCR7 (Millennium Pharmaceuticals, Cambridge, MA), CD45RA and CD45RO (Serotec, Oxford, United Kingdom), Bcl-2 (DAKO, Cambridge, United Kingdom), and perforin (PharMingen, Oxford, United Kingdom). These antibodies were directly conjugated to fluorescein isothiocyanate (FITC), PE-Cy5, PerCP, allophycocyanin (APC), or PE-Texas Red (ECD). Indirect labeling with the CCR7 antibody was achieved with an anti-mouse IgG FITC-conjugated antibody (Southern Biotechnology Associates, Birmingham, AL). For intracellular proteins, PBMCs were labeled initially with tetramer-PE and anti-CD8–ECD for 30 minutes. Cells were then resuspended in Permeafix (Ortho Diagnostic Systems, Amersham, United Kingdom) for 40 minutes at room temperature, before labeling with anti-CD45RA PE-Cy5 and either an FITC-conjugated isotype control antibody or anti–Bcl-2 FITC. Samples were analyzed on an Epics XL (Beckman-Coulter) or a FACSCalibur (Becton Dickinson) flow cytometer. We also analyzed TCR Vβ usage by EBV-specific CD8+ T cells in patients who had recovered from AIM using the same panel of 22 anti-human Vβ chain–specific antibodies described in detail previously.5
Cell cycle analysis
PBMCs were labeled with tetramer-PE, anti–CD8-ECD, and anti-CD45RA–PE-Cy5 antibodies before fixing in 70% ethanol for at least 4 hours at −20°C. Cells were then stained with FITC-labeled isotype control or anti-Ki-67–FITC antibody for 30 minutes before analysis on the Epics XL flow cytometer. This antibody detects all cells in cycle.25
Assessment of telomere length in specific populations using 2-color flow fluorescence in situ hybridization
Cells were first stained with biotin-labeled anti-CD4 or anti-CD8 (Beckman-Coulter) followed by streptavidin-Cy5 (Southern Biotech Associates, supplied by Euro-Path, Cornwall, United Kingdom) or with tetramers conjugated to Cy5 as described previously.12 Telomere length was determined using the flow–fluorescence in situ hybridization (FISH) technique.12,26,27 Briefly, PBMCs were washed in PBSA (0.2% BSA) followed by permeabilization using PermeaFix for 30 minutes. Samples were washed twice in PBSA followed by a wash in 1 mL hybridization buffer as described.12 Cells were then resuspended in 200 μL hybridization buffer and incubated with 0.3 μg/mL PNA telomeric (C3TA2)3 or PNA control (alphoid sequences of the X-chromosome, CCCATAACTAAACAC) probe conjugated to FITC as described previously.26-29 PNA probes were obtained from Perseptive Biosystems. Samples were incubated for 20 minutes in the dark, followed by heating at 80°C for 10 minutes and rapid cooling on ice, and then were allowed to hybridize for 2 hours at room temperature in the dark. After washing twice in posthybridization buffer as described previously,12 the samples were analyzed by flow cytometry using a FACSCalibur flow cytometer and Cell Quest Software (Becton Dickinson). Polyfluorescent beads (DAKO FluoroSpheres) were used at the beginning of each experiment to standardize the cytometer.
Measurement of virus-specific cell survival in culture
PBMCs were isolated from patients who had recovered from AIM up to 20 years earlier and were cultured in 24-well plates at 1 × 106 cells/mL in RPMI 1640 medium (Life Technologies) supplemented with antibiotics and 10% fetal calf serum. Viable cell recovery was assessed at different times by trypan blue dye exclusion. The percentage of CD8+, tetramer-positive cells was determined before and after culture, and this was then multiplied by the viable cell recovery to give the absolute number of tetramer-positive cells that were present. Percentage cell survival was calculated as a fraction of this number relative to the original number of tetramer-positive cells on day 0. In some experiments, the CD8+, tetramer-bearing cells were also examined for CD45RA or Bcl-2 expression before and after culture to determine the relative survival of the CD45RA+ or CD45RA− subsets of these cells.
Restimulation of CD45RA+ and CD45RO+virus-specific CD8+ T cells with peptide in vitro
PBMCs from patients who had recovered from AIM at least 20 years earlier were stimulated with autologous EBV lytic (RAK or GLC) epitope peptide-pulsed PBMCs (0.5 μg/mL/106 cells). Lytic peptide-pulsed PBMCs were incubated with this peptide for 2 hours at 37°C, after which they were irradiated with 120 Gy for 7 minutes using a γ-irradiator. Responder and peptide-loaded stimulator cells were cultured in a 1:1 ratio in the presence of 50 U recombinant IL-2 (R & D Systems, Abingdon, United Kingdom). EBV lytic epitope-specific activated CD8+ T cells were expanded in culture by the further addition of IL-2 and fresh culture medium at 3-day intervals. These cells were reactivated with peptide-pulsed PBMCs at 3-week intervals. The percentage of lytic epitope-specific CD8+ T cells and their CD45RA or CD45RO reactivity was determined before and after restimulation. Cytotoxic activity of expanded virus-specific CD8+ T-cell populations was assayed in a standard 5-hour chromium release assay using EBV peptide or dimethyl sulfoxide–pulsed Na251CrO4-labeled autologous PHA blasts as target cells.
Statistics
The Student t test was used to determine the significance of the results.
Results
Changes in EBV epitope-specific CD8+ T-cell populations before and after resolution of AIM
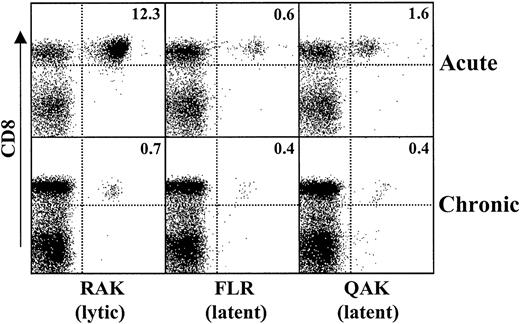
During AIM, there is a large expansion of CD8+ T cells specific for lytic viral epitopes and a lesser expansion of latent epitope-specific cells (Figure 1). This is followed by a dramatic reduction in lytic epitope-specific cells 1 year after recovery from AIM, though latent epitope-specific CD8+ T-cell populations are not reduced as extensively. Such relative change in lytic versus latent epitope-specific CD8+ T cells is a consistent observation in 12 patients examined during and after the resolution of AIM.
Relative expansion and contraction of EBV lytic and latent epitope-specific CD8+ T-cell populations after resolution of AIM.
PBMCs were isolated from patients with AIM (acute) and 12 months after the resolution of symptoms (chronic). Cells were stained for CD8 and HLA class 1 tetramers specific for lytic (RAK) and latent (FLR, QAK) epitopes of EBV. Histograms show the 2-color staining characteristics of cells that were first gated on forward and side scatter. The percentage of cells in each quadrant is indicated. Results are representative of those from at least 10 different persons investigated.
Relative expansion and contraction of EBV lytic and latent epitope-specific CD8+ T-cell populations after resolution of AIM.
PBMCs were isolated from patients with AIM (acute) and 12 months after the resolution of symptoms (chronic). Cells were stained for CD8 and HLA class 1 tetramers specific for lytic (RAK) and latent (FLR, QAK) epitopes of EBV. Histograms show the 2-color staining characteristics of cells that were first gated on forward and side scatter. The percentage of cells in each quadrant is indicated. Results are representative of those from at least 10 different persons investigated.
We next investigated the CD45RA and CD45RO expression of lytic and latent EBV epitope-specific CD8+ T cells before and after the resolution of AIM. During AIM, almost all virus-specific CD8+ T cells have been shown to have a CD45RA−(CD45RO+) phenotype.14-16 We investigated the extent of CD45RA and CD45RO expression by EBV-specific CD8+T cells specific for either lytic or latent virus epitopes in patients at least 12 months after recovery from AIM (Figure2A-B). After the resolution of AIM, greater proportions of lytic epitope-specific CD8+ T cells expressed CD45RA than those specific for latent virus epitopes (mean, 21.7% vs 5.9%, respectively; P < .002). There is a corresponding reduction in the number of lytic epitope-specific CD8+ T cells that are CD45RO+compared to latent epitope-specific (mean, 75.7% vs 93.7%, respectively; P < .001). At least 3 different lytic or latent epitope-specific populations are included in Figure 2A-B, and all show similar differences as described above. These results indicate that there are greater numbers of CD45RA+ lytic epitope-specific CD8+ T cells than latent-specific populations after the resolution of AIM.
Relative re-expression of CD45RA by epitope-specific CD8+ T cells before and after resolution of AIM.
We investigated the CD45RA (A) and CD45RO (B) expression of EBV lytic and latent epitope-specific CD8+ T cells in 14 patients 12 months after the resolution of AIM. The results in panels A and B show the CD45RA and CD45RO expression, respectively, of lytic and latent epitope-specific CD8+ T cells that are directed to at least 3 different EBV epitopes within each group. Dotted lines in panels A and B represent the median proportion of tetramer staining cells that are positive for either CD45RA or CD45RO. Vβ usage of GLC tetramer-positive cells present in a patient after recovery from AIM was investigated next. First, PBMCs from this patient were stained with GLC-tetramer PE, CD8-tricolor, and 22 different anti-human Vβ antibodies coupled to FITC. The Vβ staining profile of the GLC-specific cells was determined on CD8-gated populations (C). Two major expansions were noted for Vβ 1 and Vβ 22. A 4-color staining analysis was then performed on the cells from this patient using CD8-tricolor, tetramer-PE, Vβ-(1 or 22) FITC, and CD45RA-cy5. Cells were gated on CD8 to show the GLC and Vβ 1 (D) and GLC and Vβ 22 (E) profiles, respectively. Cells were then gated on CD8 and GLC to determine the CD45RA expression of either Vβ 1 (F) or Vβ 22 (G) expanded populations of cells. Similar observations were obtained for 5 different patients tested.
Relative re-expression of CD45RA by epitope-specific CD8+ T cells before and after resolution of AIM.
We investigated the CD45RA (A) and CD45RO (B) expression of EBV lytic and latent epitope-specific CD8+ T cells in 14 patients 12 months after the resolution of AIM. The results in panels A and B show the CD45RA and CD45RO expression, respectively, of lytic and latent epitope-specific CD8+ T cells that are directed to at least 3 different EBV epitopes within each group. Dotted lines in panels A and B represent the median proportion of tetramer staining cells that are positive for either CD45RA or CD45RO. Vβ usage of GLC tetramer-positive cells present in a patient after recovery from AIM was investigated next. First, PBMCs from this patient were stained with GLC-tetramer PE, CD8-tricolor, and 22 different anti-human Vβ antibodies coupled to FITC. The Vβ staining profile of the GLC-specific cells was determined on CD8-gated populations (C). Two major expansions were noted for Vβ 1 and Vβ 22. A 4-color staining analysis was then performed on the cells from this patient using CD8-tricolor, tetramer-PE, Vβ-(1 or 22) FITC, and CD45RA-cy5. Cells were gated on CD8 to show the GLC and Vβ 1 (D) and GLC and Vβ 22 (E) profiles, respectively. Cells were then gated on CD8 and GLC to determine the CD45RA expression of either Vβ 1 (F) or Vβ 22 (G) expanded populations of cells. Similar observations were obtained for 5 different patients tested.
To investigate whether the GLC-specific CD8+ T cells that are CD45RA+ represent the same expanded populations as their GLC-specific CD45RO+ counterparts, we examined the Vβ distribution in GLC-tetramer specific cells from a patient who had recovered from AIM. This patient had 2 major expansions of Vβ 1- and Vβ 22-positive cells (Figure 2C) that constituted 51% and 15% of the GLC-tetramer specific population, respectively (Figure 2D-E). We found that 22% of the Vβ 1- and 34% of the Vβ 22–expanded GLC-specific cells expressed CD45RA. These results, together with previous observations that heteroduplex analysis-defined clonal expansions of CD8+ CD45RO+ T cells in patients with AIM are found in CD45RA+ and CD45RO+populations after recovery from AIM,21 suggest that EBV-specific CD45RA and CD45RO populations are derived from the same cells. This indicates that re-expression of CD45RA occurs in EBV-specific CD8+ T cells after recovery from AIM.
Proliferation in virus-specific CD8+ T cells before and after resolution of AIM
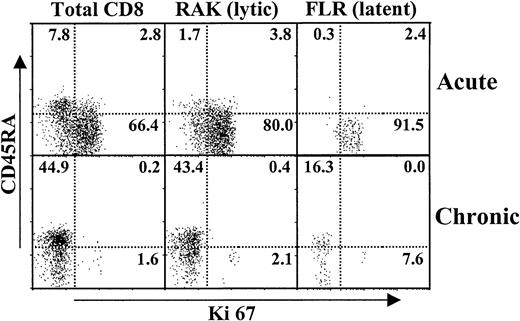
We next investigated the extent of proliferation of virus-specific cells before and after AIM, as determined by Ki67 antibody reactivity.14 25 Although there were greater numbers of EBV lytic epitope-specific CD8+ T cells during AIM, there were extremely high levels of cell cycling in lytic and latent epitope-specific populations (Figure 3). This proliferative activity was confined to the CD45RA−(CD45RO+) subset (Figure 3). After the resolution of AIM, there was a dramatic reduction of cell cycling in total CD8+ and of latent and lytic virus-specific T cells. The CD45RA re-expressing populations were totally quiescent, whereas the small number of cycling tetramer-positive cells that remained were in the CD45RA− populations (Figure 3). Thus, though they were antigen-experienced, virus-specific CD8+ T cells, especially those that re-expressed CD45RA after AIM, returned to a resting state.
Extent of cell cycling in virus-specific CD8+ T cells during and after resolution of AIM.
PBMCs were isolated from patients before (acute) and after (chronic) the resolution of AIM. These cells were stained by 4-color flow cytometry for CD8, latent or lytic HLA class 1 tetramers, CD45RA, and the cell cycle marker Ki67. Representative results from 1 of 3 similar experiments are shown.
Extent of cell cycling in virus-specific CD8+ T cells during and after resolution of AIM.
PBMCs were isolated from patients before (acute) and after (chronic) the resolution of AIM. These cells were stained by 4-color flow cytometry for CD8, latent or lytic HLA class 1 tetramers, CD45RA, and the cell cycle marker Ki67. Representative results from 1 of 3 similar experiments are shown.
Susceptibility to apoptosis of virus-specific CD8+ T cells before and after resolution of AIM
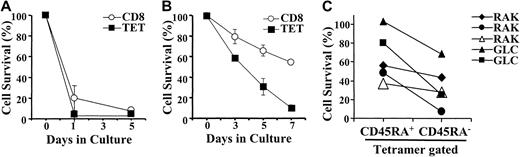
Previous studies have shown that the expanded CD8+T-cell pool present during AIM is susceptible to growth factor deprivation-induced apoptosis.1 3 We now investigated whether this was also true for EBV-specific CD8+ T cells within the total CD8+ pool of patients with AIM (Figure4). When cultured in the absence of exogenous growth factors, the total CD8+ T cells from patients with AIM perished rapidly by apoptosis, resulting in less than 20% and less than 5% survival after 1 and 5 days of culture, respectively (Figure 4A). During AIM, lytic and latent epitope-specific CD8+ T cells were equally susceptible to rapid apoptosis (only data for lytic epitope-specific cells is shown; Figure 4A). When cells from patients who recovered from AIM were cultured under identical conditions, we found that the total CD8+ T-cell population was now relatively resistant to apoptosis after 5 days of culture (Figure 4B; 65% survival after 5 days of culture). EBV epitope-specific CD8+ T cells present after AIM were more susceptible to death than the total CD8+ population (35% vs 65% survival after 5 days of culture) but were more resistant to death when compared with lytic epitope-specific CD8+ T cells found during AIM (35% vs 5% survival after 5 days;P < .01). Enhanced survival of total CD8+ T cells after recovery from AIM relative to virus-specific populations was probably attributable in part to the presence of apoptosis-resistant naive CD8+ T cells in the former but not in the latter population. Similar observations as to the extent of apoptosis in total CD8+ T cells compared to EBV-specific populations were obtained when healthy EBV carriers who had never had AIM were investigated (data not shown).
Susceptibility of virus-specific CD8+ T cells to apoptosis.
PBMCs were isolated from donors with AIM and were cultured in the absence of exogenous mediators (A). Total viable cell recovery was measured at various times after culture. In addition, the percentage of total CD8+ T cells or virus-specific (RAK lytic epitope) CD8+ T cells was also determined in the cultured PBMC populations. This enabled the absolute number of total CD8+T cells and EBV-specific CD8+ T cells to be determined after culture. Survival of the cells after culture was expressed as a percentage of the original number of cells at day 0. Survival of total CD8+ and EBV-specific CD8+ T cells in culture was also determined in patients who had recovered from AIM (B). We also measured the absolute number of CD45RA+ or CD45RA− virus-specific CD8+ T cells at different times after culture by multiplying the percentages of these cells that were present at different times by the cell recovery (C). The percentage survival of CD45RA+ or CD45RA−EBV-specific CD8+ T cells was determined relative to the initial input at day 0. Results shown in panels A and B are the mean and SEM of triplicate determinations for the survival of cells from 1 individual each and are representative of at least 4 separate experiments that have been performed in each case. In panel C, the relative survival of EBV-specific CD8+ T cells that do or do not re-express CD45RA in 5 patients tested are shown.
Susceptibility of virus-specific CD8+ T cells to apoptosis.
PBMCs were isolated from donors with AIM and were cultured in the absence of exogenous mediators (A). Total viable cell recovery was measured at various times after culture. In addition, the percentage of total CD8+ T cells or virus-specific (RAK lytic epitope) CD8+ T cells was also determined in the cultured PBMC populations. This enabled the absolute number of total CD8+T cells and EBV-specific CD8+ T cells to be determined after culture. Survival of the cells after culture was expressed as a percentage of the original number of cells at day 0. Survival of total CD8+ and EBV-specific CD8+ T cells in culture was also determined in patients who had recovered from AIM (B). We also measured the absolute number of CD45RA+ or CD45RA− virus-specific CD8+ T cells at different times after culture by multiplying the percentages of these cells that were present at different times by the cell recovery (C). The percentage survival of CD45RA+ or CD45RA−EBV-specific CD8+ T cells was determined relative to the initial input at day 0. Results shown in panels A and B are the mean and SEM of triplicate determinations for the survival of cells from 1 individual each and are representative of at least 4 separate experiments that have been performed in each case. In panel C, the relative survival of EBV-specific CD8+ T cells that do or do not re-express CD45RA in 5 patients tested are shown.
We determined the relative survival in culture of EBV-specific CD8+ T cells that do or do not re-express CD45RA (Figure4C). In these experiments we only investigated lytic epitope-specific populations because there were generally too few CD45RA re-expressing latent virus epitope-specific cells to investigate accurately (Figure2). We found that in each of 5 experiments performed, EBV-specific cells that re-express CD45RA show better survival than the cells that do not express this molecule (60.8 ± 15.3 vs 30.2 ± 11.7, respectively; P < .012).
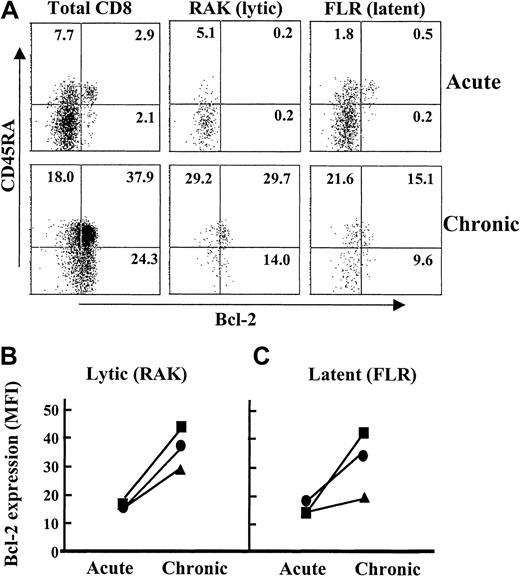
We investigated why virus-specific CD8+ T cells re-expressing CD45RA were more resistant to death. Previous studies using unselected CD8+ T cells during AIM showed that their susceptibility to apoptosis was linked to reduced expression of the anti-apoptotic molecule Bcl-2.1 We now demonstrate that lytic and latent virus epitope-specific CD8+ T cells also have low Bcl-2 expression during AIM (Figure5A; acute). However, 1 year after the resolution of AIM, lytic and latent EBV epitope-specific CD8+ T cells up-regulate their Bcl-2 expression (Figure 5A; chronic). The up-regulation of Bcl-2 in virus-specific CD8+T cells after the resolution of acute viral infection confirms previous observations that were made in animal models.30 A small population of CD8+ CD45RA+ T cells has high Bcl-2 expression during AIM (Figure 5A). These cells are CCR7+ (data not shown) and probably represent naive cells that are not EBV-specific and have not been stimulated during the infection.
Expression of Bcl-2 by virus-specific CD8+ T cells before and after the resolution of AIM.
PBMC samples were isolated from 1 patient during (acute) and 1 year after the resolution of AIM (chronic). These samples were analyzed by 4-color flow cytometry for CD8, latent or lytic EBV specificity, CD45RA, and the anti-apoptotic molecule Bcl-2. The expression of Bcl-2 by CD45RA+ and CD45RA− T cells within the total CD8+, lytic, and latent epitope-specific cells is shown (A). The relative level of Bcl-2 expression (MFI) before and after resolution of AIM in the total CD8+ population of the same patient as above (squares), the CD45RA+tetramer-positive cells (circles), or CD45RA−tetramer-positive cells (triangles) were determined in cells specific for lytic (B) and latent (C) EBV epitopes. Results are representative of 5 separate experiments.
Expression of Bcl-2 by virus-specific CD8+ T cells before and after the resolution of AIM.
PBMC samples were isolated from 1 patient during (acute) and 1 year after the resolution of AIM (chronic). These samples were analyzed by 4-color flow cytometry for CD8, latent or lytic EBV specificity, CD45RA, and the anti-apoptotic molecule Bcl-2. The expression of Bcl-2 by CD45RA+ and CD45RA− T cells within the total CD8+, lytic, and latent epitope-specific cells is shown (A). The relative level of Bcl-2 expression (MFI) before and after resolution of AIM in the total CD8+ population of the same patient as above (squares), the CD45RA+tetramer-positive cells (circles), or CD45RA−tetramer-positive cells (triangles) were determined in cells specific for lytic (B) and latent (C) EBV epitopes. Results are representative of 5 separate experiments.
We next compared the extent to which Bcl-2 increases in CD45RA+ and CD45RA− virus-specific CD8+ T cells before and after recovery from AIM (Figure 5B-C). Virus-specific CD8+ T cells that re-express CD45RA up-regulate Bcl-2 to a greater extent than the cells that remain CD45RA−, and this was found for lytic and latent epitope-specific cells in the same patient (Figure 5B-C). The higher Bcl-2 expression by CD45RA re-expressing virus-specific CD8+ T cells was observed in each of 5 different patients investigated (not shown). We did not investigate CD95-mediated activation-induced death in virus-specific CD8+ T cells after the resolution of AIM because these cells were not in cycle (Figure 3) and were thus not susceptible to this apoptotic pathway.31 An additional observation was that EBV-specific CD8+ T cells that re-expressed CD45RA also consistently had higher levels of the antiapoptotic molecule Bcl-xL than their CD45RO counterparts (not shown).
Telomere length in virus-specific CD8+CD45RA+ T cells
Our observations suggest that the virus-specific cells that re-express CD45RA are a noncycling apoptosis-resistant CD8+T-cell memory population. One prediction from this would be that the extent of cell cycling over time would be lower in the virus-specific cells that re-express CD45RA than in those that remain CD45RA−, which may be reflected in differences in their relative telomere lengths.
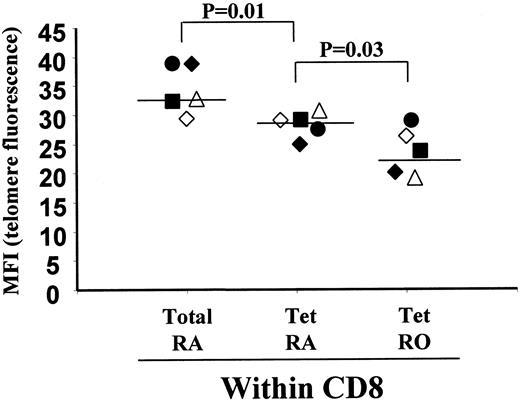
To investigate this, we first purified CD8+CD45RA+ and CD8+ CD45R0+ T cells from healthy persons who recovered from AIM and then analyzed the telomere length of EBV-lytic epitope-specific cells by 2-color flow-FISH, as described previously (Figure6).12 In 5 persons tested, the lytic epitope-specific CD8+ T cells within the CD45RA population had shorter telomeres than the total CD8+CD45RA+ T cells, indicating that they had been expanded in vivo (Figure 6; P < .01). However, in 4 of the 5 persons tested, the CD45RA-expressing cells within the virus-specific CD8+ T-cell populations had longer telomeres than CD45RA− CD45RO+ populations, suggesting that they have retained more replicative potential than these cells (Figure6; P < .03). Healthy carriers who had EBV-specific CD8+ T cells in their PBMC populations but had not had the AIM syndrome showed similar differences in telomere lengths between their CD45RA+ and CD45RA− populations of EBV lytic epitope-specific CD8+ T-cell populations (data not shown). The low number of EBV latent epitope-specific cells that re-express CD45RA after resolution of acute infection (Figure 2A-B) did not allow similar investigations of relative telomere length to be performed. These results, together with those outlined above, suggest that CD8+ CD45RA+ T cells may constitute a stable memory population with preserved replicative potential.
Assessment of telomere length in CD45RA+ and CD45RO+ EBV-specific CD8+ T cells.
CD8+, CD45RA+ and CD8+, CD45RO+ T cells were first isolated by magnetic bead depletion from PBMCs of healthy persons who recovered from AIM. These cells were then stained with EBV lytic epitope-specific tetramers coupled to Cy5 and the PNA telomeric probe coupled to FITC as described in “Materials and methods.” We gated on tetramer-positive populations, and the telomeric staining (median fluorescence intensity) of total CD8+CD45RA+, lytic epitope-specific CD8+ T cells that express CD45RA+ (Tet RA) or CD45RO+ (Tet RO) in 5 different persons who had recovered from AIM were investigated. Each person is represented by one symbol, and the telomere staining of the 3 different subsets for that subject is shown. The significance of the results was determined by the Student t test.
Assessment of telomere length in CD45RA+ and CD45RO+ EBV-specific CD8+ T cells.
CD8+, CD45RA+ and CD8+, CD45RO+ T cells were first isolated by magnetic bead depletion from PBMCs of healthy persons who recovered from AIM. These cells were then stained with EBV lytic epitope-specific tetramers coupled to Cy5 and the PNA telomeric probe coupled to FITC as described in “Materials and methods.” We gated on tetramer-positive populations, and the telomeric staining (median fluorescence intensity) of total CD8+CD45RA+, lytic epitope-specific CD8+ T cells that express CD45RA+ (Tet RA) or CD45RO+ (Tet RO) in 5 different persons who had recovered from AIM were investigated. Each person is represented by one symbol, and the telomere staining of the 3 different subsets for that subject is shown. The significance of the results was determined by the Student t test.
Reactivation of virus-specific CD8+ CD45RA+T cells by specific peptide
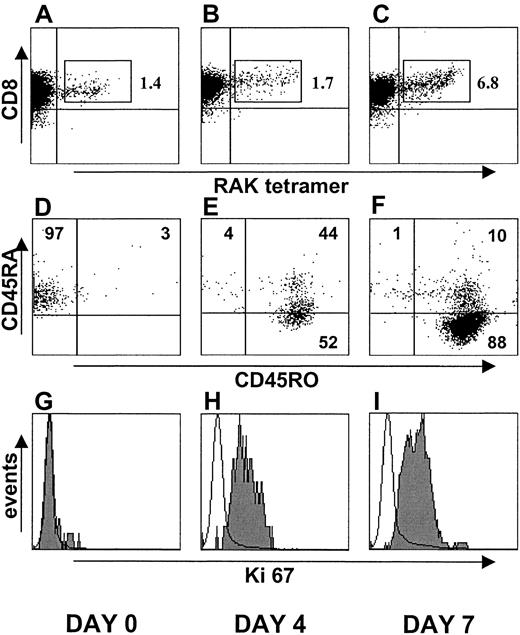
To directly test whether EBV-specific CD45RA+ T cells could still replicate, we isolated the CD45RA+ T-cell subset from a person who recovered from AIM 20 years previously. We focused on the lytic epitope (RAK)–specific CD8+ T cells within these CD45RA+ cells before (Figure7A), 4 days after (Figure 7B), and 7 days after (Figure 7C) stimulation with peptide-pulsed APC. By combining the viable cell recovery and the percentage of virus-specific cells present, we found that there was an 8-fold increase in total virus-specific CD8+ T-cell numbers after 7 days of culture. We also investigated the extent of cycling after peptide stimulation directly in the virus-specific CD8+ T cells by Ki67 antibody staining, before and after culture (Figure 7G-I). Most virus-specific CD8+ T cells were induced to enter cell cycle after specific stimulation. Although 98% of the virus-specific CD8+ T cells expressed CD45RA before culture (Figure 7D), most of these cells coexpressed CD45RO after 4 days of stimulation (Figure 7E). After 7 days of stimulation, 98% of the virus-specific cells expressed CD45RO, and 88% were CD45RA−CD45RO+ (Figure 7F). Activated virus-specific cells that were originally CD45RA+ also expressed perforin after 4 and 7 days of stimulation, showing that they expressed cytotoxic effector molecules (not shown). These results collectively demonstrate that virus-specific CD8+ T cells that re-express CD45RA can be reactivated to proliferate.
Re-activation of EBV lytic epitope (RAK)–specific CD45RA+ T cells with RAK peptide.
CD8+, CD45RA+ T cells (97% purity) were isolated from the peripheral blood of a person who recovered from AIM 20 years earlier. Cells were stimulated with RAK peptide-pulsed irradiated APC. On days 0 (A, D, G), 4 (B, E, H), and 7 (C, F, I), the cells were stained with anti-CD8 mAb and B8-RAK tetramer (A-C, respectively). CD45 isoform expression was determined on the RAK-specific cells on the same days (D, E, F, respectively). The extent of cell cycling was determined at the same time by reactivity with Ki67 antibody (G-I, respectively). Similar results were obtained in a second identical experiment using blood from a different donor.
Re-activation of EBV lytic epitope (RAK)–specific CD45RA+ T cells with RAK peptide.
CD8+, CD45RA+ T cells (97% purity) were isolated from the peripheral blood of a person who recovered from AIM 20 years earlier. Cells were stimulated with RAK peptide-pulsed irradiated APC. On days 0 (A, D, G), 4 (B, E, H), and 7 (C, F, I), the cells were stained with anti-CD8 mAb and B8-RAK tetramer (A-C, respectively). CD45 isoform expression was determined on the RAK-specific cells on the same days (D, E, F, respectively). The extent of cell cycling was determined at the same time by reactivity with Ki67 antibody (G-I, respectively). Similar results were obtained in a second identical experiment using blood from a different donor.
We investigated whether the EBV-specific CD8+ T cells could be expanded in longer-term culture and whether the perforin expression observed above was directly linked to the cytotoxic activity of the cells. In the experiment shown (Figure8), GLC lytic epitope-specific CD45RA and CD45RO populations were first isolated then stimulated with specific peptide for 3 weeks. There was a 73- and an 84-fold increase of CD45RA-enriched and CD45RO-enriched GLC/A2 tetramer staining cells after 3 weeks of stimulation, respectively (not shown), indicating that both these populations of epitope-specific cells were capable of considerable expansion in vitro. On expansion, cells that originally were CD45RA+ switched to CD45RO expression, whereas cells that originally were CD45RO retained this phenotype (Figure 8). The GLC-specific CD8+ T cells that were expanded from the CD45RA+ and CD45RO+ subsets were highly cytotoxic. Some of the tetramer-negative cells that originally were CD45RA+ lost this marker after culture with specific peptide (Figure 8). This may reflect bystander proliferation or response to other endogenous peptides that might have been present on the antigen-presenting cells used. Nevertheless, it is clear that though most tetramer-positive cells that were expanded from the CD45RA+ virus-specific subset were CD45RA−after specific stimulation, only 30% of the non–tetramer-positive population lost the expression of this marker. These results collectively indicate that EBV lytic epitope-specific CD8+CD45RA+ T cells found after the resolution of acute infection are capable of considerable expansion and cytotoxic function on restimulation by specific viral epitopes.
Long-term expansion and cytotoxic activity of re-stimulated EBV lytic epitope-specific CD45RA+ and CD45RO+CD8+ T cells.
CD8+, CD45RA+ and CD8+, CD45RO+ T-cell subsets were enriched from PBMCs of an HLA-A2 donor by negative selection. CD45 isoform expression of these specific cells was determined before and after 3 weeks of stimulation with irradiated APC pulsed with the GLC lytic peptide epitope. Activated cells were supplemented with IL-2 every 3 to 4 days. Cytotoxic activity of the expanded populations was tested against51Cr-labeled GLC peptide-pulsed PHA blast target cells. Similar results were obtained in a second identical experiment.
Long-term expansion and cytotoxic activity of re-stimulated EBV lytic epitope-specific CD45RA+ and CD45RO+CD8+ T cells.
CD8+, CD45RA+ and CD8+, CD45RO+ T-cell subsets were enriched from PBMCs of an HLA-A2 donor by negative selection. CD45 isoform expression of these specific cells was determined before and after 3 weeks of stimulation with irradiated APC pulsed with the GLC lytic peptide epitope. Activated cells were supplemented with IL-2 every 3 to 4 days. Cytotoxic activity of the expanded populations was tested against51Cr-labeled GLC peptide-pulsed PHA blast target cells. Similar results were obtained in a second identical experiment.
Discussion
In the present study, we identified 2 fundamental changes that occur in EBV lytic and latent epitope-specific CD8+ T cells selected for survival after the resolution of AIM. First, the cells are induced to exit the cell cycle and become quiescent; second, the persisting cells up-regulate Bcl-2 and become relatively resistant to apoptosis. Both these processes are particularly apparent in the CD45RA re-expressing populations, and this may help stabilize the memory CD8+ T-cell pool to this infection. We found that CD45RA+ EBV-specific CD8+ T cells had longer telomeres relative to their CD45RO+counterparts. However, the EBV-specific CD8+CD45RA+ T cells had consistently shorter telomeres than the total CD8+ CD45RA+ T-cell population, indicating that they had been expanded in vivo. We have also shown previously that most CD45RA-expressing, EBV-specific CD8+ T cells found after the resolution of AIM do not express the CCR7 chemokine receptor, which further supports the possibility that they are antigen experienced14,15 and not naive T cells. Although some EBV-specific CD8+ T-cell clones found during AIM may be lost and replaced by others in memory,5,6 the appearance of CD45RA+EBV-specific CD8+ T cells is not due to the selection of less expanded populations because the specific clones of cells that do persist after AIM are found in CD45RA and CD45RO compartments.21 Nevertheless, the relative extent of proliferation that has actually occurred in the EBV-specific CD45RA+ and CD45RO+ T cells cannot be determined because the enzyme telomerase can replenish telomeres in proliferating cells.32 Further investigations are in progress to determine whether virus-specific CD8+ cells in CD45RA+ and CD45RO+ compartments up-regulate this enzyme equally after reactivation.
The stabilization of lymphocyte populations, by inducing quiescence and resistance to death after episodes of extensive proliferation, is not unique to virus-specific CD8+ T cells after acute infection. During thymic differentiation there is considerable proliferation of CD4+/CD8+ CD45RO+thymocytes, which occurs in parallel with low Bcl-2 expression and susceptibility of these cells to apoptosis.33,34 However, the selected single-positive CD4+ or CD8+ naive T cells are quiescent, up-regulate Bcl-2, and re-express CD45RA.33,35 Another example is in the B-cell system, where considerable proliferation of germinal center B cells occurs during immune responses.36 These cells have low Bcl-2 expression and are susceptible to death.37 However, when memory cells are selected from these proliferating populations, they are not in cycle, they up-regulate Bcl-2, and they are resistant to death.37 Although the signals associated with stabilizing selected thymocytes and virus-specific CD8+ T cells are not well characterized, there is some evidence that the ligation of certain costimulatory molecules, such as CD40 on germinal center B cells, may be involved.38 39 The functional significance of CD45RA re-expression per se is unclear; however, it may be one of a set of genes induced when cells are triggered by putative stability-inducing signals. Identification of these signals may enable antiviral immunity to be manipulated in immunocompromised persons and in the elderly.
Our data indicate that EBV lytic epitope-specific CD45RA+ T cells found after the resolution of AIM can be activated specifically to proliferate and to express perforin and cytotoxic activity and CD45RO. This, together with the observations that they are resistant to apoptosis and have longer telomeres than their CD45RO counterparts, suggests that they are a stable, noncycling, memory pool of cells and not end-stage effector cells as has been reported.18Discrepancies in the ability to reactivate virus-specific CD8+ T cells that re-express CD45RA between our study and previous reports may be attributed to the fact that we stimulated EBV-specific CD8+ T cells with peptide-pulsed APCs, which exhibit a wide array of costimulatory molecules.13-15 In contrast, anti-CD3 together with anti-CD28 was previously used for the stimulation of virus-specific CD45RA+ CD8+ T cells,18 which might have been suboptimal because a large proportion of these cells have lost CD28 expression.13Another possibility is that CD45RA re-expressing virus-specific CD8+ cells found during EBV infection may not be as terminally differentiated as other populations found during CMV infection. This is supported by the fact that though the CD45RA re-expressing EBV-specific cells we studied are largely CD27+,14,15 large numbers of these virus-specific cells are CD27− in CMV-seropositive patients,20,40 and loss of this marker has previously been associated with terminally differentiated effector cells.41 Nevertheless, it has been shown recently that CD45RA re-expressing CMV-specific CD8+ T cells that are CD27− can also be induced to proliferate on stimulation with specific peptide in vitro (M. Wills, P. Sissons, A. Carmichael, personal communication).
The observation that there is less re-expression of CD45RA by EBV-latent epitope-specific cells suggests that they may not be as stable as those directed to lytic epitopes. The same applies to influenza virus-specific cells that are exclusively CD45RO+19 and to HIV-specific cells that may have a deficient capacity to differentiate into a CD45RA re-expressing population.18,24 The finding that more CD45RA re-expression occurs in CMV- than in EBV-specific CD8+ T cells and that CMV induces higher levels of CD8 immunity than EBV has led to the hypothesis that the higher the virus load in vivo, the greater the CD45RA re-expression by virus-specific CD8+ T cells.15 20 If correct, this would suggest that a stable memory CD8+ CD45RA+ T-cell pool would only be established for viral epitopes that are present at high enough levels to induce sufficient specific CD8+ T-cell differentiation.
Because EBV-specific CD8+ CD45RA+ T cells are not a terminally differentiated end-stage effector population, it suggests that there must be a dynamic balance between the virus-specific CD45RA and CD45RO compartments of cells during chronic infection. This is supported by the observation that they have the same clonality.21 The factors that determine whether the virus-specific cells persist in a CD45RA or CD45RO state include stimulatory signals, such as antigen and cytokines, that will preferentially induce CD45RO expression and cycling of these cells. However, access to putative signals that confer resistance to apoptosis and exit from cell cycle will promote the survival of these cells in a quiescent/stable CD45RA state. Two crucial questions that must be answered are, first, what exactly is the nature of the signals involved and, second, do repeated cycles of activation of virus-specific CD8+ T cells—followed by induction of quiescence, stabilization, and CD45RA expression—eventually lead to end-stage terminal differentiation of these cells over time? However, even if virus-specific CD8+ T cells reach a state of nonproliferative replicative senescence, the relative resistance of those that express CD45RA+ to apoptosis may be one reason these cells accumulate during aging.20 22 We are investigating the characteristics of EBV-specific CD8+T-cell populations between old and young persons to further address this question.
We would like to thank Professors G. Janossy and P. C. L. Beverley for discussions.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2002-01-0160.
Supported by grants from Aventis (P.J.D.) and the Medical Research Council (P.J.D.), BBSRC SAGE initiative 77/SAG1002 (F.J.P.), the Arthritis Research Campaign (J.M. Faint), and Programa PRAXIS XXI grant BD/9254/96 (M.V.D.S.). Supported also by a grant from the European Union (Bio-4 Cat CT 98-0214).
P.J.D. and J.M. Faint contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Arne N. Akbar, Department of Clinical Immunology, Royal Free and University College Medical School, Pond St, Hampstead, London NW3 2QG, United Kingdom; e-mail: aakbar@rfc.ucl.ac.uk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal