Abstract
Cells in murine muscle have been reported to differentiate into hematopoietic stem and progenitor cells and thus repopulate the hematopoietic system of an irradiated animal. This activity was attributed to muscle stem cells. We used an in vitro and in vivo approach to identify the hematopoietic repopulating activity found in muscle tissue of mice by antibody staining and cell sorting. We confirmed existence of a hematopoietic repopulating cell in muscle tissue, but the data strongly suggest that repopulation is due not to muscle stem cells but to hematopoietic cells present in muscle tissue. Unexpectedly, the blood-forming cells were enriched in muscle relative to their frequency in peripheral blood.
Introduction
An emerging theme in stem cell biology is the flexibility or “plasticity” retained by adult stem cells such as hematopoietic and neural stem cells to express differentiation programs appropriate to specific tissue microenvironments.1-3Plasticity was also ascribed to skeletal muscle stem cells, which were reported to differentiate into hematopoietic stem cells when transplanted into lethally irradiated animals.4-6 To investigate the developmental potential and plasticity of cells in skeletal muscle, we sorted cells derived from murine muscle tissue on the basis of expression of surface markers found on hematopoietic cells, endothelial cells, and muscle stem and progenitor cells. The potential of stem and progenitor cells was analyzed in vitro by using a cobblestone area–forming cell (CAFC) assay originally described for studying hematopoietic cells7 and in vivo by using a transplantation assay.
Study design
Mice
C57BL/6J mice were from The Jackson Laboratory (Bar Harbor, ME). DBA/2CR, C57BL/6CR, and B6.SJL (Ptprca [Ly5.1]) mice were from Charles River Laboratories (Frederick, MD). For CAFC assays, muscle tissue was derived from DBA/2 mice; for transplantation experiments, C57BL/6 and C57BL/6 Ly5.1 mice were used.
Muscle cell preparation
Mice (5-8 weeks of age) were given a perfusion of 20 mL cold phosphate-buffered saline (PBS) at a flow rate of 4 mL/minute. Perfusate was pumped into the left ventricle of the heart and removed by means of the right atrium. The gastrocnemius, soleus, and plantaris muscles were excised and manipulated in further steps on ice in Iscoves modified Dulbecco medium (Life Technologies, Grand Island, NY), 15% fetal-calf serum (FCS; Hyclone Laboratories, Logan, UT), 0.5% chick-embryo extract (Sigma, St Louis, MO), 80 U/mL penicillin (Life Technologies), and 80 mg/mL streptomycin (Life Technologies). Muscle tissue was finely minced into pieces of about 1 mm3, washed, resuspended in collagenase and dispase (0.1 U/mL and 0.8 U/mL in PBS; Roche Molecular Biochemicals, Indianapolis, IN), and incubated for 1.5 to 2 hours at 37°C with gentle agitation. The tissue was then vigorously triturated and successively filtered through cell strainers with a pore size of 70 and 40 μm (BD Biosciences, San Jose, CA). Single cells were collected by centrifugation (400g at 4°C), resuspended, and counted. For flow cytometric analyses, cells were stained with antibodies in ice-cold Hanks medium containing 2% FCS, sorted, and either plated on culture trays or injected into mice.
CAFC assay
The CAFC assay was performed without modification as described previously for assessment of hematopoietic cells.7
Transplantation
Transplant-recipient female mice were given antibiotics in drinking water 1 week before irradiation. The mice received a dose of 9 Gy (11 Gy in 1 experiment) at least 4 hours before transplantation (11 Gy was split into 2 doses). In case of competitive transplantation, the competitor cells were of the same genotype as the recipient.
Flow cytometry
Cell suspensions were stained with antibodies according to standard procedures. The analyses were performed on either a FACS Vantage or FACScan device (BD Biosciences). The following antibodies (all from BD Biosciences) were used: CD5 (53-7.3), B220 (RA3-6B2), Mac-1 (M1/70), CD8a (53-6.7), Gr-1 (RB6-8C5), TER-119, c-Kit (2B8), Sca-1 (E13-161.7), Thy1.2 (30-H12), CD34 (RAM34), and CD31 (Mec13.1).
Results and discussion
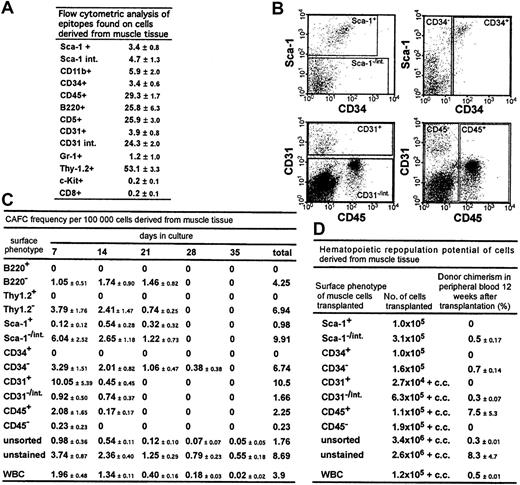
The expression patterns of hematopoietic epitopes on muscle-derived cells were analyzed by flow cytometry (Figure1A). Although the mice were perfused with 10 times their blood volume to remove contaminating peripheral blood, a high percentage of muscle-derived cells expressed hematopoietic cell markers, including CD45 (29%), in agreement with the findings of McKinney-Freeman et al8; and B220 (26%), in contrast to the results of Gussoni et al.4 The high percentage of cells positive for Thy-1.2 (53%), an epitope found mostly on T cells, is intriguing, because few CD8+ cells were detectable.
Stem and progenitor cell potential and the potential to differentiate into hematopoietic cells of cells derived from muscle tissue.
(A) Epitopes expressed on cells derived from muscle tissue. The percentages of total cells that stained positive for a given antibody (± SD) are shown. Summarized are results from at least 3 independent cell preparations and stainings per antibody; int. indicates intermediate. (B) Flow cytometry regions that were applied for sorting using antibodies to Sca-1, CD34, CD31, and CD45. (C) CAFC frequency in sorted cell populations derived from muscle tissue and in WBCs. The CAFC assay permits determination of frequencies of cells with proliferation potential at limiting dilution and provides temporal data indicating the hierarchy of stem and progenitor cells because later-developing colonies arise from more primitive cells. CAFC frequencies/100 000 cells (± SEM) from 3 independent analyses are shown. Muscle tissue for these experiments was derived from DBA/2 mice. WBCs (1 × 105) were, after lysis of erythrocytes, derived from approximately 45 μL of blood. (D) Donor chimerism in peripheral blood in recipient mice 12 weeks after transplantation. Preliminary analyses showed a stable engraftment pattern from 3 months to 8 months after transplantation. Mice were given transplants of the same sorted cell populations derived from muscle tissue and from WBCs reported for the CAFC analysis, allowing a direct comparison of the in vitro and in vivo potential of the cells. The percentage of donor-derived cells in peripheral blood (± SEM) is shown. Data are based on at least 2 independent cell sorts and transplantations for each cell population. In each experiment, each sorted cell population was transplanted into at least 2 mice; c.c. indicates 2 × 105 competitor cells.
Stem and progenitor cell potential and the potential to differentiate into hematopoietic cells of cells derived from muscle tissue.
(A) Epitopes expressed on cells derived from muscle tissue. The percentages of total cells that stained positive for a given antibody (± SD) are shown. Summarized are results from at least 3 independent cell preparations and stainings per antibody; int. indicates intermediate. (B) Flow cytometry regions that were applied for sorting using antibodies to Sca-1, CD34, CD31, and CD45. (C) CAFC frequency in sorted cell populations derived from muscle tissue and in WBCs. The CAFC assay permits determination of frequencies of cells with proliferation potential at limiting dilution and provides temporal data indicating the hierarchy of stem and progenitor cells because later-developing colonies arise from more primitive cells. CAFC frequencies/100 000 cells (± SEM) from 3 independent analyses are shown. Muscle tissue for these experiments was derived from DBA/2 mice. WBCs (1 × 105) were, after lysis of erythrocytes, derived from approximately 45 μL of blood. (D) Donor chimerism in peripheral blood in recipient mice 12 weeks after transplantation. Preliminary analyses showed a stable engraftment pattern from 3 months to 8 months after transplantation. Mice were given transplants of the same sorted cell populations derived from muscle tissue and from WBCs reported for the CAFC analysis, allowing a direct comparison of the in vitro and in vivo potential of the cells. The percentage of donor-derived cells in peripheral blood (± SEM) is shown. Data are based on at least 2 independent cell sorts and transplantations for each cell population. In each experiment, each sorted cell population was transplanted into at least 2 mice; c.c. indicates 2 × 105 competitor cells.
Next, using a CAFC assay, we tested the in vitro stem and progenitor cell potential of sorted muscle-derived cell populations and determined the frequencies of CAFCs in muscle cell populations positive (+) and negative (−) or intermediate (int) for Sca-1, CD34, CD31, and CD45 and in cells derived from peripheral blood (Figure 1B, C). In all cases, cobblestone areas derived from muscle cells looked similar to cobblestone areas derived from bone marrow (BM).
CD34+ cells, reported to be muscle stem and progenitor cells,9 did not generate cobblestone areas. Embryonic endothelial cells (CD31+) have the potential to differentiate into hematopoietic cells.10 We detected a high frequency of CAFCs in muscle cells positive for CD31. However, because of their low abundance, the CD31+ population accounted for only half of the total number of CAFCs in the muscle-derived cell suspension (data not shown). The remainder of the CAFC-forming activity was found in CD45+ cells. We also noted that the staining and sorting manipulations reduced CAFC activity to almost 25% that in unstained cells. Cells with in vitro myogenic activity were enriched in the CD45− and Thy1.2+ cell populations (data not shown). In summary, muscle-derived CAFCs were B220−, Thy1.2−, Sca1−/int, CD34−, CD31+ and CD31−/int, and CD45+, results indicating a hematopoietic, not a muscle, origin of the cells.
The ability of distinct muscle-derived cell populations to repopulate the hematopoietic system of irradiated mice was analyzed in a transplantation assay (Figure 1D). Cells were sorted according to regions used for the CAFC assays (Figure 1B) and subsequently transplanted into lethally irradiated Ly5.1-Ly5.2 mismatched mice, and in some cases, admixed with recipient-type BM competitor cells.5,11 Mice given transplants of unstained muscle-derived cells showed long-term (> 8 months) multilineage repopulation and chimerism in secondary BM transplants, confirming the presence of stem cell activity (data not shown). Spleen chimerism reflected the percentage found in peripheral blood (Figure 1D), whereas donor chimerism in the thymus and BM was typically 10% to 20% of the percentage observed in the periphery (data not shown). Interestingly, even in mice showing multilineage reconstitution, most donor-derived cells in peripheral blood were Thy-1+, a feature also reported, but not emphasized, by Kawada and Ogawa.12 We detected the same Thy-1 skewing when white blood cells (WBCs) were used as donor cells, in accordance with a previous study of mobilized peripheral blood cells.13
Muscle-derived cells with the ability to repopulate the hematopoietic compartment were Sca-1−/int, CD34−, CD31−/int, and CD45+ (Figure 1D). Thus, except for CD31, cells with in vitro and in vivo stem and progenitor cell activity showed the same pattern of epitope expression. Because staining with the CD31 antibody did not interfere with homing or engraftment of muscle-derived cells (data not shown), masking of functional protein domains by the antibody could not have accounted for the difference between the results of the 2 assays.
Taken together, the Thy-1 skewing characteristic of repopulation from peripheral blood progenitors and the surface phenotype of the repopulating cell indicate a peripheral hematopoietic stem cell as the repopulating unit. This conclusion is in agreement with observations by Kawada and Ogawa12 and McKinney-Freeman et al.8 In contrast to the findings of McKinney-Freeman et al, however, we detected the repopulating activity in the Sca-1−/int population and not in the Sca-1+population. Because their report does not show the precise position of the sorting region, a direct comparison is not possible.
As shown in Figure 1C and D, peripheral WBCs also contained CAFCs and repopulating activity. Transplantation of 2.5 × 106muscle-derived cells, the number we obtained on average from a C57BL/6 mouse, resulted in a chimerism of 8% (Figure 1D). This is 16-fold higher than the chimerism obtained with 1.2 × 105 WBCs (0.5%), which correspond to 55 μL of blood. Indeed, to achieve the same percentage of chimerism observed after transplantation of muscle cells derived from a single mouse would require WBCs derived from 900 μL of blood. This suggests that these repopulating cells are not contaminating stem cells found in peripheral blood. Given that we obtained on average 1.8 × 106 muscle-derived cells from a DBA/2 mouse, the CAFC activity in muscle tissue dissected from one animal would be contained in 1.8 mL of blood (Figure 1C).
Thus, we found that skeletal muscle tissue is enriched for hematopoietic stem cells. Further analyses will identify the location of these cells in muscle and determine whether they are sequestered in the wall of the vasculature or within the muscle tissue itself.
Since this manuscript was submitted for publication, McKinney-Freeman et al8 reported that muscle-derived hematopoietic stem cells are hematopoietic of origin, a finding supported by our analyses.
Supported by National Institutes of Health grant AG16653 (G.V.Z.) and a fellowship of the Deutsche Akademie der Naturforscher Leopoldina funded by the Bundesministerium für Bildung und Forschung (H.G.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hartmut Geiger, Division of Hematology/Oncology, Departments of Internal Medicine and Physiology, University of Kentucky Medical Center, 800 Rose Street, Lexington KY, 40536-0093; e-mail:ghart2@uky.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal