Abstract
Syndecan-1 (CD138) is a transmembrane heparan sulfate–bearing proteoglycan expressed by most myeloma plasma cells that regulates adhesion, migration, and growth factor activity. In patients with myeloma, shed syndecan-1 accumulates in the bone marrow, and high levels of syndecan-1 in the serum are an indicator of poor prognosis. To test the effect of soluble syndecan-1 on tumor cell growth and dissemination, ARH-77 B-lymphoid cells were engineered to produce a soluble form of syndecan-1. Controls included vector only (neo)–transfected cells and cells transfected with full-length syndecan-1 complementary DNA that codes for the cell surface form of syndecan-1. Assays reveal that all 3 transfectants have similar growth rates in vitro, but cells expressing soluble syndecan-1 are hyperinvasive in collagen gels relative to controls. When injected into the marrow of human bones that were implanted in severe combined immunodeficient mice, tumors formed by cells expressing soluble syndecan-1 grow faster than tumors formed by neo-transfected cells or by cells expressing cell surface syndecan-1. In addition, cells bearing cell surface syndecan-1 exhibit a diminished capacity to establish tumors within the mice as compared with both neo- and soluble syndecan-1–transfected cells. Tumor cell dissemination to a contralateral human bone is detected significantly more often in the tumors producing soluble syndecan-1 than in controls. Thus, high levels of soluble syndecan-1 present in patients with myeloma may contribute directly to the growth and dissemination of the malignant cells and thus to poor prognosis.
Introduction
The syndecans are a family of heparan sulfate–bearing proteoglycans that are expressed on the surfaces of many cell types.1,2 Their functions at the cell surface have been extensively characterized and include promotion of growth factor interaction and activity as well as cell–cell and cell–extracellular matrix adhesion. Expression of syndecans is often altered in cancer, and several studies suggest that loss of syndecan-1 expression in carcinomas accompanies the malignant phenotype.3,4 It has been suggested that this loss of syndecan-1 expression renders the tumor cells less adhesive, thereby increasing their potential to metastasize. However, in other cancers, such as adenocarcinoma of the pancreas and multiple myeloma, syndecan-1 expression on the cell surface remains high even though these tumors disseminate to sites distal from the primary tumor.5 6
In addition to being present at the cell surface, like some other membrane proteins, syndecans can be cleaved from the cell surface and released into the extracellular space by the action of secretases.7 The specific enzyme responsible for the shedding of syndecan-1 from the cell surface has not been identified, but its activity is inhibited by an inhibitor of metalloprotease-3.8 The proteolytically released extracellular domain (ectodomain) of syndecan-1 retains its biologically active heparan sulfate chains. For example, soluble syndecan-1 ectodomain from wound fluids binds to fibroblast growth factor-2 (FGF-2) and inhibits its mitogenicity, but subsequent degradation of the ectodomain heparan sulfate chains with platelet heparanase produces heparan sulfate fragments that activate FGF-2 mitogenicity.9 Thus, soluble syndecan-1 ectodomain can regulate the activity of growth factors.
To examine the role of soluble syndecans in cancer, initial studies were launched to determine if detectable levels of soluble syndecan-1 ectodomain are present in the serum of patients with multiple myeloma. We were first to report that some patients had relatively high levels of soluble syndecan-1 ectodomain in their serum and that these high levels correlated with high tumor burden and other markers of disease severity, including beta-2 microglobulin.10 Subsequent studies by others on a large population of patients with myeloma found that high levels of soluble syndecan-1 ectodomain in the serum correlated with a poor disease prognosis.11 Patients with high serum syndecan-1 had a median survival of 20 months, whereas those with low serum syndecan-1 had a median survival of 44 months. Very high levels of soluble syndecan-1 ectodomain (median concentration, 900 ng/mL) are also present in bone marrow aspirates obtained from patients with myeloma, where it binds to hepatocyte growth factor and regulates the biologic activity of this and possibly other cytokines.12
Although high levels of soluble syndecan-1 ectodomain correlate with poor prognosis in myeloma, it is not known if the ectodomain plays a direct role in promoting disease severity or if the high levels of syndecan-1 are simply a reflection of an aggressive tumor. For example, the high levels of soluble syndecan-1 ectodomain could be due to constitutive shedding by a large tumor mass. Alternatively, high levels of soluble ectodomain could result from tumors having high levels of protease activity. In contrast to in vivo findings raising the possibility that soluble syndecan-1 may promote tumor growth, in vitro studies have demonstrated that soluble syndecan-1 can inhibit growth of both carcinoma and myeloma cells.13 14 To clarify the role of soluble syndecan-1 and to determine if soluble syndecan-1 ectodomain has a direct effect on tumor growth and dissemination, we compared the behavior of cells that secrete high levels of soluble syndecan-1 with those that express syndecan-1 at their cell surface.
ARH-77 cells were established from a patient with a plasma cell leukemia and selected as a model for our studies because these cells lack syndecan-1 expression.14 Moreover, these cells have been previously used as a model for multiple myeloma and shown to grow within, and disseminate to, human fetal bones implanted in severe combined immunodeficient (SCID) mice (SCID-hu mice).15ARH-77 cells were transfected with a complementary DNA (cDNA) encoding the full-length syndecan-1 core protein or with a truncated construct lacking the coding region for the transmembrane and cytoplasmic domains of syndecan-1. Transfection with this truncated form of syndecan-1 results in cells that express and secrete high levels of soluble syndecan-1 ectodomain. We find that in vitro these cells grow at a similar rate to controls, including neo-transfected ARH-77 cells or to cells that express cell surface syndecan-1. However, in the SCID-hu mouse, tumors formed by cells producing high levels of the soluble syndecan-1 ectodomain grow significantly faster than control cells. In addition, both in vitro and in vivo results indicate that the soluble syndecan-1 ectodomain promotes dissemination of the tumor cells.
Materials and methods
Cells and cell culture
ARH-77 cells were obtained from the American Type Culture Collection (Rockville, MD). These cells were originally isolated from a patient with plasma cell leukemia, secrete human immunoglobulin kappa light chain, and are Epstein-Barr virus positive. They express little, if any, endogenous syndecan-1.14 Previous studies have described characterization of both neo- and syndecan-1–transfected ARH-77 cells.16 For the present study, ARH-77 cells were stably transfected with a cDNA containing the coding region for the extracellular portion (amino acids 1-252) of the murine syndecan-1 core protein in the pcDNA3 vector. Following selection in G418, cells were cloned by limiting dilution, grown on stromal cell feeder layers, and examined for levels of soluble syndecan-1 ectodomain by immunodot blotting by using monoclonal antibody 281.2.16Cells were cultured in RPMI 1640 medium supplemented with 5% fetal bovine serum, 2 mM L-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin sulfate.
Cell growth assay
Cells were plated on 96-well plates at a density of 2 × 104 cells in 100 μL complete RPMI 1640 medium per well, in triplicate. The plates were incubated at 37°C in 5% CO2 for 0, 24, 48, or 72 hours, then 50 μL of a 2 mg/mL solution of 3[4,5-dimethylthiazol-2-y]-2,5-diphenyltetrazolium (MTT) in complete media was added to each well.17 The plates were returned to 37°C in 5% CO2 incubator for 4 to 5 hours, then the medium from each well was carefully removed and 100 μL dimethyl sulfoxide was added. The plates were gently agitated until the color reaction was uniform, and OD540 was determined by using a microplate reader.
Cell invasion assay
The percentage of invasive cells was determined as previously described.16 Briefly, 5.0 × 104 cells were placed on the surface of a hydrated type I collagen gel (0.5 mg/mL; BD Biosciences, Bedford, MA) in complete media and returned to the cell culture incubator. Forty-eight hours later, a trypsin digestion followed by a limited collagenase digestion was used to release the attached “noninvasive” cells, and then complete collagenase digestion was used to release and recover the invasive cells. The number of noninvasive versus invasive cells was quantified by using a Coulter counter.
Affinity coelectrophoresis, Western blotting, and dot blotting
Purification of syndecan-1, affinity coelectrophoresis (ACE) gels, and Western blotting procedures were performed as previously described.18 Briefly, 35S-labeled syndecan-1 was purified from media conditioned by cells with the use of DEAE isolation followed by affinity purification with antibody 281.2. For ACE, the purified samples were analyzed on a 1% agarose gel having 9 parallel lanes of agarose containing decreasing amounts of type I collagen. Following imaging with the use of a PhosphorImager, an apparent Kd of the sample for collagen can be determined by the concentration range within which a shift in proteoglycan mobility occurs. For Western blots, affinity-purified syndecan-1 was run on 4% to 12% Tris-glycine gels, transferred to a cationic nylon filter, and detected by using 125I-labeled antibody 281.2. For immunodot blotting, samples were brought to pH 4.5, blotted onto a cationic nylon filter, and probed with125I-labeled antibody 281.2 as previously described.16
Growth of tumors in SCID-hu mice
Seven-week-old homozygous CB.17 scid/scid mice (SCID) were obtained from Harlan Sprague Dawley (Indianapolis, IN) and were housed and monitored in our animal facility. All experimental procedures and protocols were approved by the Institutional Animal Care and Use Committee. Implantation of human femur grafts into SCID mice to produce SCID-hu mice has been previously described.19 20 Briefly, the femora were cut into halves (approximately 5 × 5 × 10 mm) and implanted subcutaneously into both sides of SCID mice. Six weeks after implantation of bone, 1 × 105 cells were injected directly into the marrow cavity of one of the fetal bones implanted in the SCID-hu host. Murine sera were collected at biweekly intervals, and the levels of human immunoglobulin kappa light chain were assessed as an indicator of tumor growth. When the animals were killed, differences in mean tumor diameter, mean tumor weight, and mean sera kappa level among the experimental groups were compared by the Student ttest, and P values were reported (P ≤ .05 was considered statistically significant).
Determination of kappa light chain levels in SCID-hu serum
The levels of human immunoglobulin kappa light chain were determined by enzyme-linked immunosorbent assay (ELISA).20Plates were coated with 50 μL/well of primary antihuman kappa light chain (5 μg/mL; The Binding Site, San Diego, CA) and incubated overnight at 4°C. The plates were washed 3 times in phosphate-buffered saline (PBS) containing 1% (vol/vol) of Tween 20. A 50-μL sample diluted in PBS was added, and the plates were incubated at room temperature for 2 hours. After washing 3 times with PBS/Tween 20, 50 μL biotinylated antibody (affinity-purified antihuman kappa light chain at 0.5 μg/mL) was added to each well for 1 hour. The plates were then washed, and 50 μL streptavidinhorseradish peroxidase was added to each well and allowed to bind for 30 minutes. After a final wash, 50 μL ortho-phenylenediamine solution (2 tablets ortho-phenylenediamine in 6 mL 0.1 M citric acid/Phughyte; Dako, Carpinteria, CA) containing 3% H2O2 was added, and the color was allowed to develop for 3 to 5 minutes. The reaction was stopped by adding 100 μL 0.5M H2SO4, and the absorbance was read at 450 nm on an Auto-Reader II ELISA reader (Ortho Diagnostic Systems, Raritan, NJ). All samples were analyzed in duplicate simultaneously to preclude interassay variability. The standard curve was linear between 0.35 and 300 ng/mL, and samples were diluted to concentrations within this range.
Flow cytometry
For confirmation of the phenotype of the primary tumor and determination of tumor cell dissemination to other organs, fresh tissues, including the bone injected with tumor cells and the uninjected contralateral bone, spleen, liver, femora, and vertebrae, were excised after mice were killed. Organs were cut in halves, of which one was fixed in 10% formalin, and the other was separated into a single-cell suspension in PBS. For flow cytometry, cell suspensions were washed in PBS and stained with either antibodies to human syndecan-1 (CD138-fluorescein isothiocyanate [FITC]; Serotec), murine syndecan-1 (281.2-FITC),21 or human CD38-FITC/CD45-phycoerythrin (PE; BD Immunocytometry Systems, San Jose, CA). To identify nonviable cells, the cells were incubated with 0.4 mL PBS and 4 μL 2 mg/mL propidium iodide (for CD138 and 281.2 staining) or 20 μL 25 μg/mL 7-amino actinomycin D (7-AAD) (for CD38/45 staining). Samples were analyzed by using a FACScan flow cytometer (Becton Dickinson).
Histopathologic and immunocytochemical analysis
Bones were decalcified with 10% EDTA pH 7.0 prior to embedding, and all fixed tissues were embedded in paraffin, sectioned, and stained with hematoxylin and eosin. For immunochemical staining, sections were deparaffinized in xylene and rehydrated through graded concentrations of ethanol and distilled water. Slides were washed and incubated with 3% H2O2 for 5 minutes to block endogenous peroxidase, then incubated with blocking serum for 10 minutes. Rabbit antihuman kappa light chain (1:250) and biotinylated universal antibody and streptavidin/peroxidase complex (Universal Quick Kit; Vector Laboratories) were used following the manufacturer's instruction. Tissues were considered positive for metastasis of tumor if clear foci of kappa light chain–positive tumor cells were found in at least 2 nonoverlapping areas. Slides were analyzed independently by 2 investigators who did not know the identity of the mice. Staining of syndecan-1 within tissues used monoclonal antibody 281.2, biotinylated rabbit antirat, and the Vector Laboratories (Burlingame, CA) avidin-biotin complex kit. Between each step in all the immunohistochemical staining procedures, slides were rinsed 3 times with PBS. Peroxidase staining was visualized with DAB (3,3′-diaminobenzidine; Vector Laboratories) solution until desired stain intensity developed. Sections were rinsed in tap water and were lightly counterstained in Mayer hematoxylin.
Results
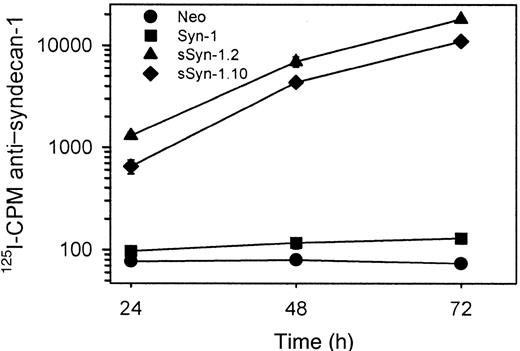
To determine the effects of soluble syndecan-1 ectodomain on tumor cell behavior, ARH-77 cells were transfected with a cDNA coding for the syndecan-1 extracellular domain, but lacking the transmembrane and cytoplasmic domains. This form of syndecan-1 does not anchor within the cell membrane and is secreted into the culture media. This action is in contrast to cells that produce wild-type syndecan-1 that is anchored to the cell surface and can be enzymatically shed, thereby releasing an intact ectodomain into the culture media. Following selection in G418, cells were cloned by limiting dilution and expanded, and 2 clones producing soluble syndecan-1 ectodomain were identified by immunodot blots of conditioned cell culture medium (not shown). Over a 72-hour period, high levels of syndecan-1 accumulate in the media from these 2 clonal cell lines as compared with vector-only transfected cells or cells transfected with full-length syndecan-1 cDNA that codes for the cell surface form of the proteoglycan (Figure 1).
High levels of syndecan-1 accumulate in the media of cells expressing soluble syndecan-1 ectodomain.
Immunodot assays using antibody 281.2 were performed on media isolated from cell cultures 24, 48, and 72 hours after introduction of cells. Soluble syndecan-1 (sSyn-1) is detected in high levels from both clones (sSyn-1.2 and sSyn-1.10). Cells transfected with full-length syndecan-1 (Syn-1) express syndecan-1 at the cell surface and produce low levels of soluble syndecan-1 ectodomain because of constitutive proteolytic shedding. The data represent means of triplicate samples ± SE.
High levels of syndecan-1 accumulate in the media of cells expressing soluble syndecan-1 ectodomain.
Immunodot assays using antibody 281.2 were performed on media isolated from cell cultures 24, 48, and 72 hours after introduction of cells. Soluble syndecan-1 (sSyn-1) is detected in high levels from both clones (sSyn-1.2 and sSyn-1.10). Cells transfected with full-length syndecan-1 (Syn-1) express syndecan-1 at the cell surface and produce low levels of soluble syndecan-1 ectodomain because of constitutive proteolytic shedding. The data represent means of triplicate samples ± SE.
Characterization of soluble syndecan-1 ectodomain
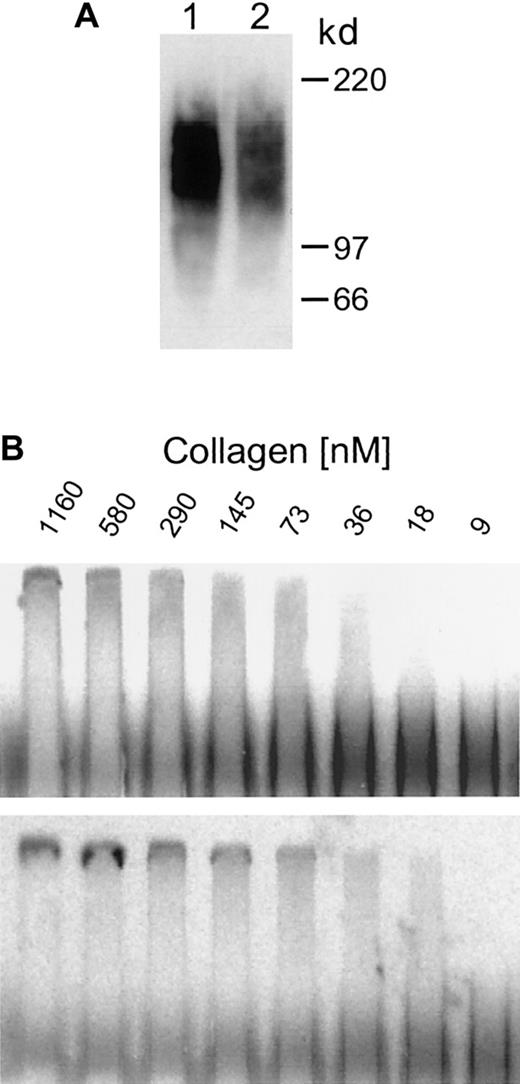
Western blotting of soluble syndecan-1 ectodomain secreted by these clones reveals that it is indistinguishable in size from the syndecan-1 ectodomain that is shed from the surface of cells expressing wild-type syndecan-1 (Figure 2A). In addition, ACE18 was used to compare the collagen-binding affinity of the soluble syndecan-1 with that of soluble syndecan-1 ectodomain shed from cells transfected with full-length syndecan-1. Gels reveal that a low- and high-affinity fraction are present in both samples, and the high-affinity fraction in both samples shows a shift in proteoglycan mobility that occurs at a collagen concentration between 9 and 36 nM, with the Kd falling within that range (Figure 2B). Together, these results indicate that the proteoglycans made by cells transfected with the truncated syndecan-1 are similar in structure and affinity for ligands to the wild-type syndecan-1 ectodomain.
Soluble syndecan-1 and shed syndecan-1 are identical in size and have similar affinities for type I collagen.
(A) Western blot. Purified syndecan-1 isolated from the media conditioned by cells expressing soluble syndecan-1 (clone sSyn-1.10, lane 1) and from Syn-1 cells that shed syndecan-1 from their cell surface (lane 2) was run on 4% to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to a cationic nylon filter, and probed with 125I-281.2 monoclonal antibody specific for syndecan-1. The 2 samples exhibit broad smears that are identical in size. (B) ACE analysis. Purified35S-labeled syndecan-1 isolated from the media conditioned by cells expressing soluble syndecan-1 (upper panel) and from cells shedding syndecan-1 from their cell surface (lower panel) was electrophoresed through varying concentrations of type I collagen and visualized by phosphorimaging. The Kd for both samples falls within the range of 9 to 36 nM.
Soluble syndecan-1 and shed syndecan-1 are identical in size and have similar affinities for type I collagen.
(A) Western blot. Purified syndecan-1 isolated from the media conditioned by cells expressing soluble syndecan-1 (clone sSyn-1.10, lane 1) and from Syn-1 cells that shed syndecan-1 from their cell surface (lane 2) was run on 4% to 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to a cationic nylon filter, and probed with 125I-281.2 monoclonal antibody specific for syndecan-1. The 2 samples exhibit broad smears that are identical in size. (B) ACE analysis. Purified35S-labeled syndecan-1 isolated from the media conditioned by cells expressing soluble syndecan-1 (upper panel) and from cells shedding syndecan-1 from their cell surface (lower panel) was electrophoresed through varying concentrations of type I collagen and visualized by phosphorimaging. The Kd for both samples falls within the range of 9 to 36 nM.
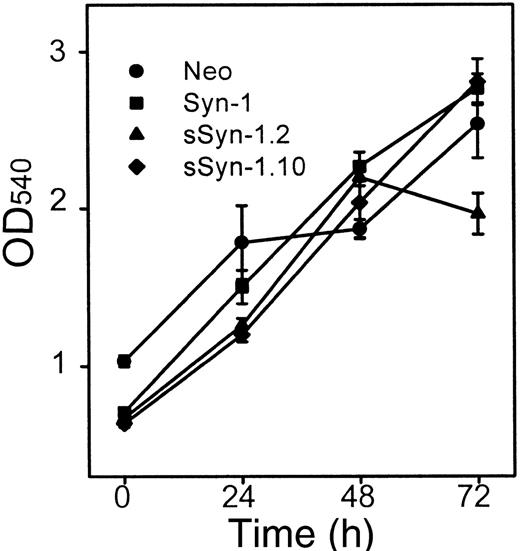
To determine if the soluble syndecan-1 ectodomain influences cell growth in vitro, MTT assays were performed. No significant differences in growth in vitro are detected between control cells and soluble syndecan-1 clone sSyn1.10, whereas growth of clone sSyn-1.2 is somewhat diminished by 72 hours (Figure 3). In addition, the level of secreted immunoglobulin kappa light chain, an indicator used to assess tumor burden in patients with myeloma, remains similar over time among the cell lines growing in vitro (data not shown).
Effect of soluble syndecan-1 ectodomain on cell growth.
Cells were plated at equal density, and the growth in cell numbers was assessed by MTT assay at 0, 24, 48, and 72 hours. Points represent means of triplicate samples ± SE from each of 2 experiments.
Effect of soluble syndecan-1 ectodomain on cell growth.
Cells were plated at equal density, and the growth in cell numbers was assessed by MTT assay at 0, 24, 48, and 72 hours. Points represent means of triplicate samples ± SE from each of 2 experiments.
Soluble syndecan-1–expressing cells are hyperinvasive in vitro
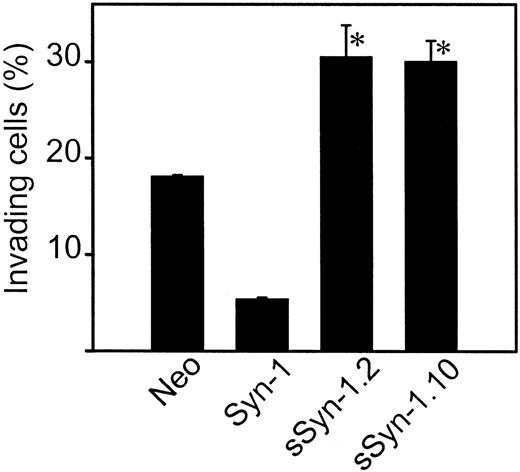
We have previously demonstrated that wild-type ARH-77 cells, which lack syndecan-1 expression, readily invade gels composed of type I collagen. Following transfection of these cells with the cDNA encoding the cell surface form of syndecan-1, the cells failed to invade.16 Therefore, we hypothesized that cells producing only soluble syndecan-1 ectodomain would not be inhibited from invading and would behave similarly to the syndecan-1–negative ARH-77 cells. However, surprisingly, cells producing soluble syndecan-1 ectodomain are hyperinvasive—the percentage of invading cells is significantly higher than cells lacking syndecan-1 expression and 6 times that of cells that express syndecan-1 on their cell surface (Figure 4).
Soluble syndecan-1 ectodomain promotes cell invasion in vitro.
Cells were placed on the surface of gels composed of type I collagen and allowed to invade for 48 hours. The percentage of invasive cells was calculated after determining the number of cells on the gel surface and the number of cells that had invaded the gel. Data are shown for the 2 soluble syndecan-1 clones (clones 2 and 10) and represent the means of quadruplicate wells ± SE. *P < .01 for neo versus sSyn-1.2 and neo versus sSyn-1.10.
Soluble syndecan-1 ectodomain promotes cell invasion in vitro.
Cells were placed on the surface of gels composed of type I collagen and allowed to invade for 48 hours. The percentage of invasive cells was calculated after determining the number of cells on the gel surface and the number of cells that had invaded the gel. Data are shown for the 2 soluble syndecan-1 clones (clones 2 and 10) and represent the means of quadruplicate wells ± SE. *P < .01 for neo versus sSyn-1.2 and neo versus sSyn-1.10.
Soluble syndecan-1 promotes growth of tumors in SCID-hu mice
Because a high concentration of soluble syndecan-1 ectodomain in the serum is an indicator of poor prognosis in patients with myeloma,10,11 we compared the in vivo growth and dissemination of ARH-77 cells that express either soluble syndecan-1 ectodomain or cell surface syndecan-1. An additional control included neo-transfected ARH-77 cells that lack syndecan-1 expression. These experiments were performed by using the SCID-hu model in which a fragment of human bone is implanted subcutaneously in each side of a SCID mouse. Although these experiments are very labor intensive, they provide an excellent model for studying the experimental growth of human tumors within a human marrow microenvironment. Several weeks after implantation of the bone, tumor cells were injected directly into the bone on the left side (primary tumor).20 At the conclusion of the experiment, the bone on the right side was analyzed for the presence of tumor cells as a measure of tumor dissemination (secondary tumor).15 Although injected directly into the bone, as the tumors enlarge, they often grow outside of the bone and surround the bone implant (Figure 5).
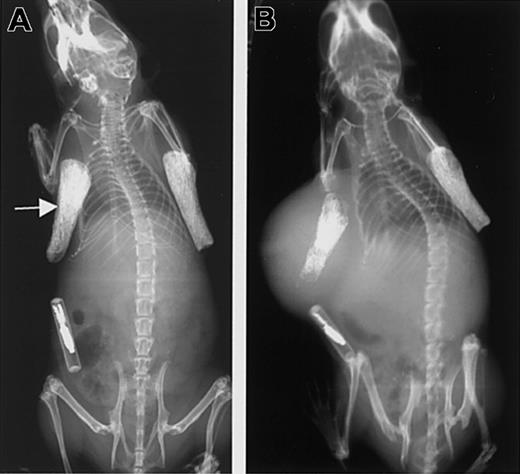
Growth of tumor in the SCID-hu mouse.
(A) X-ray showing the 2 bone implants just prior to injection of tumor cells into the left bone (arrow). (B) The same animal as in the left panel is shown 8 weeks after injection of cells expressing soluble syndecan-1 ectodomain. Note the injected bone shows evidence of osteolysis and that the primary tumor has enlarged and surrounds the bone.
Growth of tumor in the SCID-hu mouse.
(A) X-ray showing the 2 bone implants just prior to injection of tumor cells into the left bone (arrow). (B) The same animal as in the left panel is shown 8 weeks after injection of cells expressing soluble syndecan-1 ectodomain. Note the injected bone shows evidence of osteolysis and that the primary tumor has enlarged and surrounds the bone.
By manual palpation, tumors established with soluble syndecan-1 ectodomain-expressing cells were detected 4 to 5 weeks after injection of cells into the bones, whereas tumors established by using wild-type syndecan-1–expressing or neo-transfected cells were generally first detected 5 to 6 weeks after injection. In initial experiments, animals were killed 8 weeks after injection of tumor cells, and the level of human kappa light chain, mean tumor diameter, and mean wet weight were determined (Table 1). Results indicate that cells expressing cell surface syndecan-1 form tumors poorly, with only 1 in 5 animals having detectable levels of human light chain in its serum. This finding is in contrast to neo-transfected and soluble syndecan-1–expressing cells that are easily established within the SCID-hu host. Although not statistically significant, measurements of bone and associated tumor formed by soluble syndecan-1–expressing cells have a greater mean diameter than do controls. The lack of statistical significance may be due to the broad variability in the initial size of bones and the shape of the resulting tumors that usually overgrow the bone. Wet weight results are also somewhat difficult to interpret because of differences in initial bone weight and size between animals, and the extent to which bone is degraded by the tumor. However, the mean weight of the bone and associated tumor tissue from animals injected with soluble syndecan-1–expressing cells was significantly greater than that of tissue from animals injected with cells bearing cell surface syndecan-1.
Tumor growth in the SCID-hu host
| . | No. of animals injected* . | No. of animals with tumor . | Change in diameter, cm . | Wet weight, g . |
|---|---|---|---|---|
| Neo | 7 | 6 | 0.34 ± 0.09 | 1.72 ± 0.54 |
| Syn-1 | 5 | 1 | 0.22 ± 0.22 | 0.83 ± 0.53† |
| sSyn-1 | 6 | 6 | 0.72 ± 0.21 | 2.98 ± 0.68† |
| . | No. of animals injected* . | No. of animals with tumor . | Change in diameter, cm . | Wet weight, g . |
|---|---|---|---|---|
| Neo | 7 | 6 | 0.34 ± 0.09 | 1.72 ± 0.54 |
| Syn-1 | 5 | 1 | 0.22 ± 0.22 | 0.83 ± 0.53† |
| sSyn-1 | 6 | 6 | 0.72 ± 0.21 | 2.98 ± 0.68† |
No. of animals with tumor (at 8 weeks after injection) represents the number of animals with detectable levels of human kappa light chain, an indicator of the presence of active tumor. Change of diameter reflects the increase in mean tumor diameter during the 8-week growth period. Diameter of bones was measured prior to injection of tumor cells, and the diameter of tumor including bone was measured just prior to harvesting the tumor. At the time of harvest, 8 weeks after injection of tumor cells, the tumors usually have infiltrated and overgrown the bone. Wet weight is the mean weight of the remaining bone and any associated tumor. The data for diameter and weight represent the mean from all animals in each group ± SE. SCID-hu, human fetal bones implanted in severe combined immunodeficient mice; Neo, vector only; Syn-1, syndecan-1; sSyn-1, soluble syndecan-1.
Animal numbers are lower than shown in Figure 7 because in this initial experiment animals were killed after 8 weeks. Subsequent experiments were carried out for longer periods to assess tumor dissemination, making it impossible to obtain wet weights of the primary tumors at week 8.
P < .05.
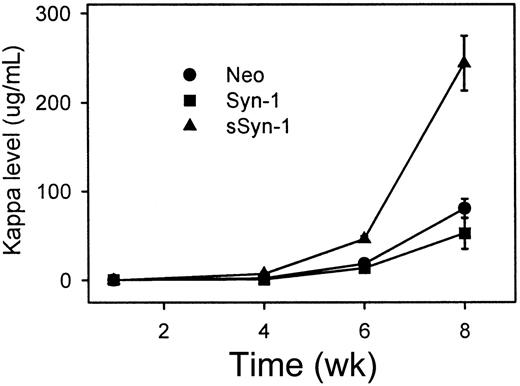
To quantitatively assess the rate of tumor growth within the marrow, blood was collected biweekly and analyzed for the level of human kappa light chain. Results reveal that the tumors producing soluble syndecan-1 ectodomain grow at a significantly faster rate than tumors lacking syndecan-1 expression (neo-transfectants) or tumors expressing syndecan-1 at the cell surface (Figure6). Results also indicate that cells expressing syndecan-1 at their cell surface form tumors poorly in SCID-hu mice, with 6 of the 14 animals having kappa levels below 1 μg/mL (Figure 7). However, when these cells expressing cell surface syndecan-1 do form tumors, some of these tumors grow significantly (4 of the 14 mice had kappa levels > 100 μg/mL). All animals injected with neo-transfected cells had detectable levels of kappa in their serum, but only 4 of 13 mice had kappa levels more than 100 μg/mL. In contrast, tumors formed in all of the mice injected with soluble syndecan-1–expressing cells, and 13 of 15 had kappa levels more than 100 μg/mL. The mean kappa levels in mice having soluble syndecan-1–expressing tumors are also significantly higher than mean kappa levels of controls even when mice that do not have detectable levels of kappa light chain are excluded from the analysis (Figure 8). Together these data indicate that within the SCID-hu host, syndecan-1 at the cell surface inhibits the establishment of the injected tumor cells, whereas expression of soluble syndecan-1 promotes growth of the tumor.
Human kappa light chain levels are significantly higher in animals bearing tumors expressing soluble syndecan-1.
Sera from animals were collected at 1, 4, 6, and 8 weeks after injection of tumor cells and analyzed for kappa light chain by ELISA. Data include all animals that were injected with tumor cells (13 neo, 14 Syn-1, and 15 sSyn-1) and shown are means from duplicate samples ± SD from a single ELISA assay. The sSyn-1 results reflect use of both the sSyn-1.10 and sSyn-1.2 clones that gave similar results in vivo. At week 8, P < .0001 for neo versus sSyn-1 and Syn-1 versus sSyn-1.
Human kappa light chain levels are significantly higher in animals bearing tumors expressing soluble syndecan-1.
Sera from animals were collected at 1, 4, 6, and 8 weeks after injection of tumor cells and analyzed for kappa light chain by ELISA. Data include all animals that were injected with tumor cells (13 neo, 14 Syn-1, and 15 sSyn-1) and shown are means from duplicate samples ± SD from a single ELISA assay. The sSyn-1 results reflect use of both the sSyn-1.10 and sSyn-1.2 clones that gave similar results in vivo. At week 8, P < .0001 for neo versus sSyn-1 and Syn-1 versus sSyn-1.
Values of human kappa light chain for each mouse.
Values were determined 8 weeks after injection of tumor. Note that tumors form poorly when cells expressing cell surface syndecan-1 (Syn-1) were injected. (P < .000 01 for neo versus sSyn-1.2 and Syn-1 versus sSyn-1.2, P < .002 for neo versus sSyn-1.10 and P < .0004 for Syn-1 versus sSyn1.10.)
Values of human kappa light chain for each mouse.
Values were determined 8 weeks after injection of tumor. Note that tumors form poorly when cells expressing cell surface syndecan-1 (Syn-1) were injected. (P < .000 01 for neo versus sSyn-1.2 and Syn-1 versus sSyn-1.2, P < .002 for neo versus sSyn-1.10 and P < .0004 for Syn-1 versus sSyn1.10.)
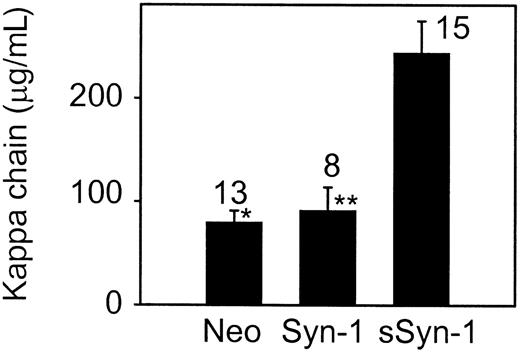
Among animals in which tumors become established, kappa light chain levels are significantly higher in those animals bearing tumors that express soluble syndecan-1 ectodomain.
Numbers represent the total number of animals having detectable levels of kappa light chain in their serum. Animals not having detectable levels of kappa light chain are excluded from this analysis. Data for sSyn-1 is from 2 distinct soluble syndecan-1–expressing clones (sSyn-1.2 and sSyn-1.10) that behaved similarly in vivo. Values represent mean ± SE. (Median values were similar to means: neo = 86, Syn-1 = 83, sSyn-1 = 243.) *P < .0001 for neo versus sSyn-1 and **P < .003 for Syn-1 versus sSyn-1.
Among animals in which tumors become established, kappa light chain levels are significantly higher in those animals bearing tumors that express soluble syndecan-1 ectodomain.
Numbers represent the total number of animals having detectable levels of kappa light chain in their serum. Animals not having detectable levels of kappa light chain are excluded from this analysis. Data for sSyn-1 is from 2 distinct soluble syndecan-1–expressing clones (sSyn-1.2 and sSyn-1.10) that behaved similarly in vivo. Values represent mean ± SE. (Median values were similar to means: neo = 86, Syn-1 = 83, sSyn-1 = 243.) *P < .0001 for neo versus sSyn-1 and **P < .003 for Syn-1 versus sSyn-1.
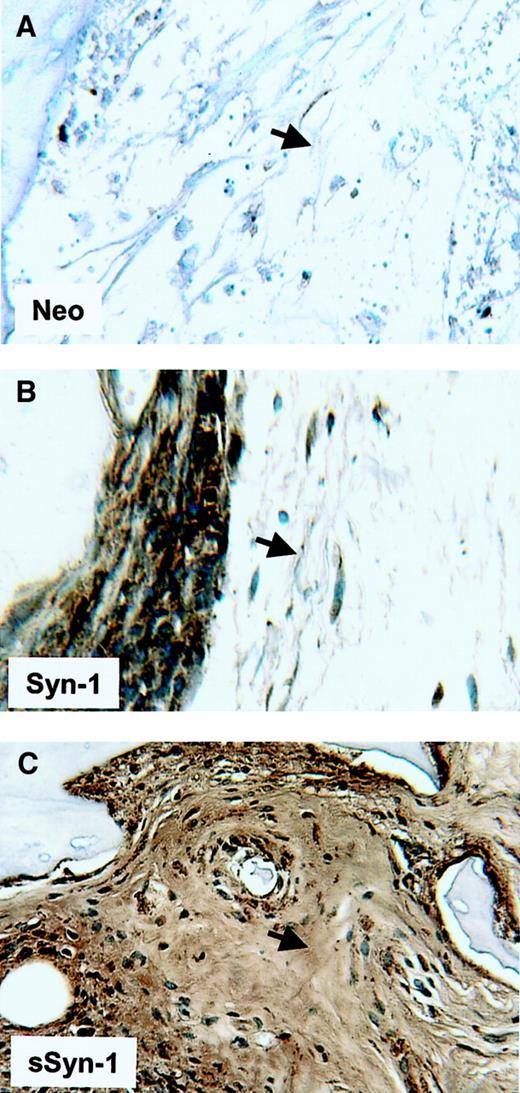
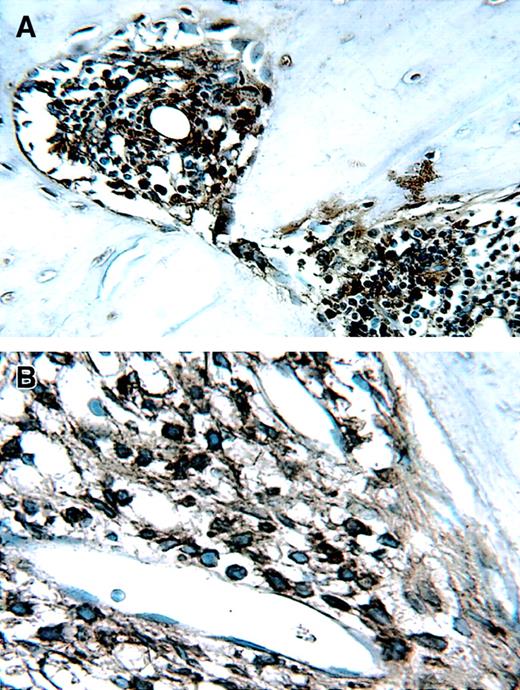
Soluble syndecan-1 accumulates within the bone marrow interstitial matrix
Recently, we have shown that soluble syndecan-1 accumulates within fibrotic regions of the bone marrow in some patients with myeloma.22 To determine if this accumulation occurs in the SCID-hu mice, we examined histologic sections of bone stained with antibody to syndecan-1. As expected, tumors established from neo-transfected cells show no staining for syndecan-1 within the interstitial matrix of the bone marrow (Figure9). Tumors established from cells bearing cell surface syndecan-1 show extensive staining on the tumor cell surface but only very weak staining within adjacent interstitial matrix, indicating low levels of shed syndecan-1 are accumulating in these matrices. In contrast, the bone marrow interstitial matrix stains heavily in animals bearing tumors composed of soluble syndecan-1–expressing cells. This finding suggests that the soluble syndecan-1 becomes trapped within interstitial matrix of the bone marrow and accumulates at high levels, similar to what is observed in patients with myeloma.
Soluble syndecan-1 accumulates in the bone marrow interstitial matrix.
Immunohistochemistry reveals strong syndecan-1 staining around cells expressing syndecan-1 on their cell surface (Syn-1, panel B), but only very weak staining in the adjacent interstitial matrix of the bone marrow (arrow). In contrast, tumors composed of cells expressing soluble syndecan-1 (sSyn-1, panel C) show extensive accumulation of syndecan-1 within the interstitial matrix (arrow). Animals bearing tumor composed of neo-transfected cells (neo, panel A) show no immunoreactivity for syndecan-1 within the bone marrow. Original magnification × 200.
Soluble syndecan-1 accumulates in the bone marrow interstitial matrix.
Immunohistochemistry reveals strong syndecan-1 staining around cells expressing syndecan-1 on their cell surface (Syn-1, panel B), but only very weak staining in the adjacent interstitial matrix of the bone marrow (arrow). In contrast, tumors composed of cells expressing soluble syndecan-1 (sSyn-1, panel C) show extensive accumulation of syndecan-1 within the interstitial matrix (arrow). Animals bearing tumor composed of neo-transfected cells (neo, panel A) show no immunoreactivity for syndecan-1 within the bone marrow. Original magnification × 200.
Tumor cells disseminate to secondary bones in the SCID-hu mouse
Finally, dissemination of the tumors to the secondary human bone and murine organs was assessed by flow cytometry or by kappa light chain immunohistochemistry of tissue sections (Figure10). Dissemination of tumor was not detected in the mouse organs examined except in the livers of 2 mice bearing soluble syndecan-1–expressing tumors. However, by immunohistochemistry, 11 weeks after injection of tumor cells into the primary bone, dissemination was detected within the contralateral human bone implants in 3 of 6 mice (50%) bearing neo tumors, 1 of 7 mice (14%) bearing syndecan-1 tumors, and 6 of 8 mice (75%) bearing soluble syndecan-1–expressing tumors (syndecan-1 versus soluble syndecan-1, P < .03).
Staining for kappa light chain identifies human tumor cells in contralateral bone fragments of a mouse injected with soluble syndecan-1–expressing cells.
To determine if tumor cells had disseminated from the bone that was injected with tumor, the noninjected contralateral bone was harvested at the time of animal killing and processed for immunohistochemistry by using anti-kappa light chain antibodies. Stained tumor cells are clearly localized within the bone at both (A) low (× 200) and (B) high (× 400) magnification.
Staining for kappa light chain identifies human tumor cells in contralateral bone fragments of a mouse injected with soluble syndecan-1–expressing cells.
To determine if tumor cells had disseminated from the bone that was injected with tumor, the noninjected contralateral bone was harvested at the time of animal killing and processed for immunohistochemistry by using anti-kappa light chain antibodies. Stained tumor cells are clearly localized within the bone at both (A) low (× 200) and (B) high (× 400) magnification.
Discussion
These results provide the first direct evidence that soluble syndecan-1 ectodomain promotes the growth of B-lymphoid tumors in vivo. Tumors formed by cells expressing soluble syndecan-1 ectodomain were detected earlier and grew more extensively over an 8-week period than did tumors expressing cell surface syndecan-1 or cells lacking expression of syndecan-1. The accelerated growth of soluble syndecan-1–expressing tumors was not due to selection of clones with an intrinsically faster rate of growth because in vitro studies demonstrate that these cells grow at a similar rate to control cells (Figure 3). Also, the presence of tumor cells in secondary bone was more prevalent in tumors formed by cells expressing soluble syndecan-1 ectodomain than in controls, suggesting that the soluble proteoglycan promotes the dissemination of tumor cells in addition to supporting their growth. Thus, we conclude that soluble syndecan-1 ectodomain may play an important role in regulating the behavior of B-lymphoid tumors in vivo.
The finding that soluble syndecan-1 ectodomain promotes tumor growth in vivo is surprising in light of the previous in vitro observation that addition of exogenous ectodomain induces apoptosis of myeloma cells and inhibits the growth of carcinoma cells.13,14 One explanation for this difference may be that the exogenous syndecan-1 ectodomain is in high enough concentration to inhibit factors necessary for maintenance of the tumor cells in culture. Alternatively, the difference in syndecan-1 ectodomain influence in vitro and in vivo may be due to the way the molecule is processed once it is in the extracellular compartment. Processing of heparan sulfate chains by degradative enzymes can regulate the biologic activity of the soluble syndecan-1 ectodomain. For example, heparanase, an endoglycosidase that cleaves heparan sulfate chains,23,24 can regulate the activity of syndecan heparan sulfate. The intact syndecan-1 ectodomain binds to soluble FGF-2 and inhibits its growth-promoting activity. However, in the presence of heparanase, highly sulfated fragments are generated from the syndecan-1 heparan sulfate chains, and these fragments act to enhance the growth-promoting activity of FGF-2.9 Interestingly, Western blots of soluble syndecan-1 ectodomain isolated from the sera of patients with myeloma show very extensive heterogeneity of the proteoglycan, suggesting that indeed the heparan sulfate chains may be degraded in vivo.10
Although the mechanism for promotion of tumor growth by soluble syndecan-1 in vivo is unknown, it may be acting to retain heparin-binding factors within the marrow microenvironment that act to stimulate tumor growth and/or support tumor cell survival. This possibility is supported by the finding that syndecan-1 expression is required for Wnt-1–induced mammary tumorigenesis.25 It is likely that the soluble syndecan-1 ectodomain binds to insoluble extracellular molecules within the marrow such as type I collagen, a known ligand of syndecan-1.26 In patients with myeloma, high levels of shed syndecan-1 are present within the bone marrow, particularly within regions of marrow fibrosis,22 and by the present study in which high levels of soluble syndecan-1 are found trapped in the marrow microenvironment (Figure 9). In patients, this reservoir of syndecan-1 with bound growth factors within the bone marrow may promote disease relapse by nurturing residual tumor cells that survive chemotherapy. Conversely, the syndecan-1 may act by some unknown mechanism to inhibit tumor cell apoptosis, thereby promoting the rapid growth of the tumor.
Another important finding of this work is that cells expressing cell surface syndecan-1 do not form tumors well in the SCID-hu host (Figure7). This finding is consistent with previous studies showing that cells expressing syndecan-1 on their surface grow poorly in SCID mice following their introduction via tail vein injection.14 It was not known previously if this result using tail vein injection was due to the inability of syndecan-1–expressing cells to extravasate from the blood into the marrow, or if the cells simply did not grow well once in the marrow. Results from the present study, in which cells are injected directly into the bone, suggest that the cells expressing cell surface syndecan-1 do not form tumors as well as cells lacking surface syndecan-1. In the present study we have used either cells that express cell surface, soluble, or no syndecan-1 to dissect the relative effects of this molecule on tumor behavior. However, in most myeloma tumors, both cell surface syndecan-1 and shed syndecan-1 are present.11,12,22,27 28 Thus, it is now important to examine the effects of high levels of soluble syndecan-1 on myeloma cells that also express the proteoglycan on their cell surface.
Analysis of tumor cell dissemination in vivo indicates that the cells expressing soluble syndecan-1 metastasize more extensively than cells bearing surface syndecan-1 or cells lacking syndecan-1 expression altogether. We cannot rule out the possibility that the higher dissemination rate seen with tumors formed from soluble syndecan-1–expressing cells is due to their faster growth. It is possible that high levels of syndecan-1 accumulating adjacent to the tumor cells concentrate factors that stimulate cell motility. Additionally, the high level of soluble syndecan-1 in the marrow matrix may coat other extracellular matrix molecules such as collagen and fibronectin, thereby blocking the binding of cells to these matrix ligands. The loosely attached cells could then more easily migrate away from the site of the primary tumor.
It is interesting to note that in general we find that the tumors in the SCID-hu do not disseminate readily to murine organs. In contrast to that seen in SCID mice lacking human bone in which the ARH-77 cells often metastasize to murine organs, in the SCID-hu they home preferentially to the human bone and in general do not grow within the murine organs. Thus, the presence of human bones plays a determining role in regulating dissemination of the tumor cells.
The finding that soluble syndecan-1 ectodomain promotes tumor growth in vivo is consistent with the observation that high levels of soluble syndecan-1 ectodomain in the serum of myeloma patients is an indicator of poor prognosis.11 Although it is not known if the soluble syndecan-1 in these patients plays an active role in promoting tumor growth or is simply a byproduct of a phenotypically aggressive tumor, our findings suggest that syndecan-1 does actually play an active role. Thus, therapies designed to block the shedding of syndecan-1 or to block the activity of shed syndecan-1 ectodomain may diminish tumor growth in patients with myeloma.
Supported by grants CA55819 and CA68494 from the National Cancer Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ralph D. Sanderson, Department of Pathology, Slot 517, University of Arkansas for Medical Sciences, 4301 West Markham, Little Rock, AR 72205; e-mail: sandersonralphd@uams.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal