Abstract
We describe a new B220+ subpopulation of immaturelike dendritic cells (B220+ DCs) with low levels of expression of major histocompatibility complex (MHC) and costimulatory molecules and markedly reduced T-cell stimulatory potential, located in the thymus, bone marrow, spleen, and lymph nodes. B220+ DCs display ultrastructural characteristics resembling those of human plasmacytoid cells and accordingly produce interferon-α after virus stimulation. B220+ DCs acquired a strong antigen-presenting cell capacity on incubation with CpG oligodeoxynucleotides, concomitant with a remarkable up-regulation of MHC and costimulatory molecules and the production of interleukin-12 (IL-12) and IL-10. Importantly, our data suggest that nonstimulated B220+ DCs represent a subset of physiological tolerogenic DCs endowed with the capacity to induce a nonanergic state of T-cell unresponsiveness, involving the differentiation of T regulatory cells capable of suppressing antigen-specific T-cell proliferation. In conclusion, our data support the hypothesis that B220+ DCs represent a lymphoid organ subset of immature DCs with a dual role in the immune system—exerting a tolerogenic function in steady state but differentiating on microbial stimulation into potent antigen-presenting cells with type 1 interferon production capacity.
Introduction
Maintenance of immunologic self-tolerance is an essential process directed at preventing harmful autoimmune diseases caused by autoreactive T cells capable of responding to self-antigens. Avoidance of pathologic reactivity of self-reactive T cells may occur as a consequence of T-cell deletion, T-cell unresponsiveness, or, in some instances, T helper cell type 2 (TH2) skewing (reviewed in Hackstein et al1). Deletion of autoreactive T-cell clones, resulting in T-cell–negative selection, takes place essentially in the thymus under the control of thymic dendritic cells (DCs) and epithelial cells (reviewed in Ardavı́n2). In contrast, the molecular mechanisms controlling T-cell unresponsiveness or anergy, which is the basis of peripheral tolerance, are not fully understood. However, increasing evidence supports that T regulatory (Treg) cells play an essential role in the control of autoreactive T-cell clones and, therefore, in the maintenance of T-cell peripheral tolerance because of their capacity to suppress antigen-specific T-cell responses (reviewed in Roncarolo and Levings3). Interestingly immature DCs have been demonstrated to participate in the differentiation of Tregcells (reviewed in Jonuleit et al4). In this sense, human and mouse interleukin-10 (IL-10)–treated immature DCs have been reported to induce antigen-specific T-cell anergy.5-9 In addition, in vitro–generated human immature DCs have been demonstrated to induce the differentiation of Treg cells in vitro and in vivo.9,10 Therefore, on the basis of these data, the tolerogenic potential of DCs has been proposed to be correlated with an immature DC state.1 On the other hand, DC-mediated induction of murine T-cell tolerance has also been reported to be exerted by specialized DCs, obtained from nonlymphoid organs such as the liver11 or from mucosal locations such as the Peyer patches12 and the lung.13
With regard to the characterization of physiological tolerogenic DCs, splenic CD8α+ DCs have been claimed to be involved in tolerance induction, though data dealing with this issue remain controversial (reviewed in Banchereau et al14). On the other hand, it has been recently reported that mesenteric lymph node DCs could exert a tolerogenic function by inducing the differentiation of T-helper type 3 regulatory T cells.13 Therefore, though experimental evidence supports the existence of tolerogenic DCs in mouse lymphoid organs (reviewed in Jonuleit et al4), their nature is still largely unknown.
Murine DCs can be subdivided into 3 main subpopulations, based on CD8α expression: CD8α− and CD8α+ DCs distributed in lymphoid and interstitial tissues and Langerhans cells (LCs), which, on migration and maturation, generate the CD8int lymph node DC subset.15 Although endowed with differential functional capacity, all 3 DC subtypes have a strong stimulatory potential; therefore, interaction of DCs with T cells triggers their activation, proliferation, and subsequent effector functions.14 In contrast, here we describe a new B220+ subpopulation of immaturelike DCs (B220+DCs), with low levels of expression of major histocompatibility complex (MHC) and costimulatory molecules and markedly reduced T cell stimulatory potential, located in the thymus, bone marrow, spleen, and lymph nodes. Our data suggest that B220+ DCs induce a nonanergic state of T-cell unresponsiveness involving the differentiation of Treg cells with the capacity to inhibit antigen-specific T-cell proliferation and could represent a physiological subset of tolerogenic DCs.
Materials and methods
Mice
C57BL/6 and BALB/c mice were purchased from IFFA Credo (L'Arbresle, France). B-cell–deficient (μKO) C57BL/6 mice16 were obtained from the CDTA (CNRS, Orléans, France). DO.11.10 mice17 expressing an ovalbumin 323-339 peptide (OVA)-specific transgenic T-cell receptor (TCR), on a BALB/c background, were from D. Y. Loh (Washington University, St Louis, MO).
Isolation of B220+ DCs and B220−DCs
Organs were cut into small fragments and then digested with collagenase A (0.5 mg/mL; Boehringer-Mannheim, Germany) and DNase I (40 μg/mL; Boehringer-Mannheim) in RPMI 1640 medium supplemented with 5% fetal calf serum (FCS) for 10 minutes at 37°C, with continuous agitation. Digested fragments were filtered through a stainless-steel sieve, and cell suspensions were washed twice in phosphate-buffered saline solution supplemented with 5% FCS and 5 mM EDTA containing 5 μg/mL DNase I. Enzymatic digestion was avoided when isolating DCs from lymph node or bone marrow samples. The cells were then resuspended in cold iso-osmotic Optiprep solution (Nyegaard Diagnostics, Oslo, Norway), pH 7.2, density 1.061 g/cm3, containing 5 mM EDTA, and a low-density cell fraction, representing 1% to 2% of the starting cell suspension, was obtained by centrifugation at 1700g for 10 minutes. DC-enriched cell fractions were then obtained after immunomagnetic depletion of B cells with antimouse immunoglobulin–coated magnetic beads (Dynabeads; Dynal, Oslo, Norway) and T cells with anti-CD3 (clone KT3-1.1, rat IgG) followed by antirat immunoglobulin–coated magnetic beads. Magnetic bead depletion was performed as previously described18 using a 7:1 bead-to-cell ratio. For the isolation of thymic B220+ DCs, B220− DCs were previously depleted with magnetic beads after incubation with anti-DEC-205 (clone NLDC-145), and then B220+ DCs were purified by magnetic cell sorting (MACS) with MACS separation columns (Miltenyi Biotec, Bergisch, Germany) after incubation with anti-CD11c–conjugated MACS microbeads (Miltenyi Biotec). For the isolation of thymic B220− DCs, B220+ DCs were previously depleted with magnetic beads after incubation with anti-Ly-6C (clone ER-MP20), and then B220− DCs were purified by MACS after incubation with anti-CD11c–conjugated MACS microbeads. For reverse transcription–polymerase chain reaction (RT-PCR) assays, B220+ DCs and B220− DCs were sorted by fluorescence-activated cell sorting (FACS) from thymic DC-enriched cell fractions after double immunofluorescence staining with fluorescein (FITC)–conjugated anti-CD11c (clone N418) and phycoerythrin (PE)–conjugated anti-B220 (clone RA3-6B2; PharMingen, San Diego, CA), using a FACSort flow cytometer (Becton Dickinson, Mountain View, CA). Sorted cell populations had greater than 98% purity.
Flow cytometry
Analysis of B220+ DCs in low-density cell fractions from C57BL/6 or BALB/c mouse thymus, spleen, lymph node, and bone marrow was performed after double staining with FITC-conjugated anti-CD11c (clone N418) and PE-conjugated anti-B220. Analysis of B220+ DCs from B-cell–deficient mice was performed after double staining with FITC-conjugated anti-B220 (clone RA3-6B2; Caltag, San Francisco, CA) and PE-conjugated anti–MHC class II (MHC class II; clone M5/114; PharMingen). Phenotypic analysis of B220+ DCs and B220− DCs was performed on DC-enriched cell fractions after triple staining with FITC-conjugated anti-CD11c (clone N418), PE-conjugated anti-B220, and biotin-conjugated anti–MHC class II (clone FD11-54.3), anti–DEC-205 (clone NLDC-145), anti-CD24 (heat-stable antigen [HSA], clone M1/69), anti-CD11b (Mac-1, clone M1/70), anti–macrophage antigen F4/80 (clone 31-A3-1), anti-CD4 (clone GK1.5), anti-CD62L (clone Mel-14), anti–Ly-6C (clone ER-MP20), anti–Ly-6G (Gr-1; clone RB6-8C5), anti-CD40 (clone FGK45), anti-CD86 (B7-2, clone GL1; PharMingen), anti–IL-3Rα (CD123, clone 5B11; PharMingen), anti-CD19 (clone 1D3; PharMingen), or anti-CD8α (clone 53-6.72) followed by streptavidin-tricolor (Caltag).
Electron microscopy
MACS-sorted thymic B220+ DCs were fixed with 1% glutaraldehyde and 1% paraformaldehyde in 0.1 M, pH 7.6, Sørensen phosphate buffer for 1 hour at 4°C, postfixed with 1% OsO4 in the same buffer for 1 hour at 4°C, dehydrated in graded acetone solutions, and embedded in Embed-812 (Electron Microscopy Sciences, Washington, PA). Ultrathin sections (70-80 nm) were counterstained with uranyl acetate and lead citrate and were examined with a JEOL 1010 electron microscope (JEOL, Tokyo, Japan).
Treatment with CpG oligodeoxynucleotides
MACS-sorted (or FACS-sorted for cytokine detection and RT-PCR analysis) thymic B220+ DCs, thymic B220− DCs, and, in some experiments, peritoneal macrophages were cultured for 12 hours at 37°C in control conditions or in the presence of 6 μg/mL CpG oligodeoxynucleotide-1826, TCC ATG ACG TTC CTG ACG TT19(CpG). Culture medium was RPMI 1640 supplemented with 10% FCS, 10 mM HEPES, 50 μM 2-mercaptoethanol, 100 U/mL penicillin-streptomycin. CpG-treated or untreated cells were subsequently assayed for the expression of DC maturation-related cell surface markers, for T-cell stimulatory potential in mixed leukocyte reaction (MLR), for the production of IL-10, IL-12, and interferon α (IFN-α), and for the expression of the chemokine receptors CCR5, CCR6, and CCR7. The effect of CpG on the expression of DC maturation-related markers was compared with that of bacterial lipopolysaccharide (LPS) after 12-hour culture in the presence of 1 μg/mL LPS from Salmonella typhimurium (Sigma, St Louis, MO).
MLR assay
MACS-sorted CpG-treated or -untreated thymic B220+DCs and B220− DCs from C57BL/6 (H-2b) mice were cultured with T cells purified from BALB/c (H-2d) mesenteric lymph nodes in flat-bottomed, 96-well plates (1 × 105 cells per well), at different antigen-presenting cell (APC)/T cell ratios (1:10 to 1:100). T-cell proliferation was assessed after 4 days by [3H] thymidine (1 μCi/well) uptake in a 4-hour pulse.
Enzyme-linked immunosorbent assay for IL-10, IL-12, and IFN-α
Cryopreserved supernatants from FACS-sorted thymic B220+ DCs, thymic B220− DCs, and peritoneal macrophages, cultured (at 105 cells in 200 μL in 96-well plates) in control medium or in the presence of CpG for 12 hours, were tested for the presence of IL-10 and IL-12, using mouse IL-10 and IL-12 (p70) enzyme-linked immunosorbent assay (ELISA) kits (PharMingen), respectively.
Cryopreserved supernatants from FACS-sorted thymic B220+DCs, cultured (at 105 cells in 200 μL in 96-well plates) in control medium or in the presence of CpG for 12 hours or Sendai virus for 18 hours, were tested for the presence of IFN-α using a mouse IFN-α ELISA kit (Performance Biomedical Laboratories, New Brunswick, NJ).
RT-PCR
mRNA was purified from FACS-sorted cells with magnetic beads (mRNA direct micro kit; Dynal), reverse transcribed, and subjected to PCR amplification using the following primers: CCR5-forward, ACT TGG GTG GTG GCT GTG TTT; CCR5-reverse, TTG TCT TGC TGG AAA ATT GAA20; CCR6-forward, CTG CAG TTC GAA GTC ATC; CCR6-reverse, GTC ATC ACC ACC ATA ATG TTG; CCR7-forward, AGC ACC ATG GAC CCA GGG AAA CC; CCR7-reverse, CAG CAT CCA GAT GCC CAC A; IFN-α-forward, TGT CTG ATG CAG CAG GTG G; IFN-α-reverse, AAG ACA GGG CTC TCC AGA C. PCR conditions were: CCR5, 15 seconds at 94°C, 30 seconds at 57°C, 60 seconds at 72°C; CCR6, 15 seconds at 94°C, 15 seconds at 59°C, 45 seconds at 72°C; CCR7, 15 seconds at 94°C, 90 seconds at 68°C; IFN-α, 40 seconds at 94°C, 40 seconds at 62°C, 60 seconds at 72°C. PCR products were: CCR5, 539 base pair (bp); CCR6, 320 bp; CCR7, 561bp; IFN-α, 166 bp. PCR was performed on a GeneAmp PCR System 9700, using 1.25 U AmpliTaq Gold polymerase per PCR reaction (Perkin-Elmer, Foster City, CA). PCR products were analyzed on agarose gels stained with ethidium bromide and photographed with a Nikon Coolpix 950 digital camera (Nikon, Tokyo, Japan).
Induction of Treg cell differentiation by B220+ DCs
As summarized in Figure 7, this assay included 3 independent culture phases. During the first phase, OVA-specific TCR transgenic T cells (OVA-TCR TCs) were cultured for 72 hours with either thymic B220+ DCs or B220−DCs isolated from BALB/c mice, at 3:1 or 6:1 T cell–APC ratios, at 105 cells per well, in 96-well plates, in the presence or absence of 10 μg/mL OVA. Cell proliferation was assessed by [3H] thymidine (1 μCi/well) uptake in a 16-hour pulse. In the second phase, OVA-TCR TCs, which were precultured during the first phase with either thymic B220+ DCs (OVA-TCs[+B220+ DCs]) or B220− DCs (OVA-TCs[+DCs]), at a 3:1 T cell–APC ratio, were then washed twice to remove remaining OVA, transferred to 24-well plates, and cultured at 5 × 105 cells per well for an additional 72 hours, without OVA, in the presence of 1 ng/mL mouse IL-2 (Peprotech, London, United Kingdom). After this second phase, precultured OVA-TCR TCs were used to assess whether OVA-TCs[+B220+ DCs] were anergic T cells or Treg cells. For the anergy reversal assay, OVA-TCs[+B220+ DCs] or OVA-TCs[+DCs] (105per well) were cultured with BALB/c splenocytes (5 × 104per well) in 96-well plates, with 10 μg/mL OVA, in the presence or absence of 5 ng/mL mouse IL-2. Cell proliferation was assessed after 48 hours by [3H] thymidine (1 μCi/well) uptake in a 16-hour pulse. The Treg cell assay was performed according to a protocol modified from Thorstenson and Khoruts21 by culturing OVA-TCR TCs (105 per well) with BALB/c splenocytes (105 per well) in 96-well plates, with 10 μg/mL OVA, in the presence or absence of OVA-TCs[+B220+DCs] or OVA-TCs[+DCs] (105 per well). Cell proliferation was assessed after 36 hours by [3H] thymidine (1 μCi/well) uptake in a 16-hour pulse.
Results
Identification of B220+ DCs
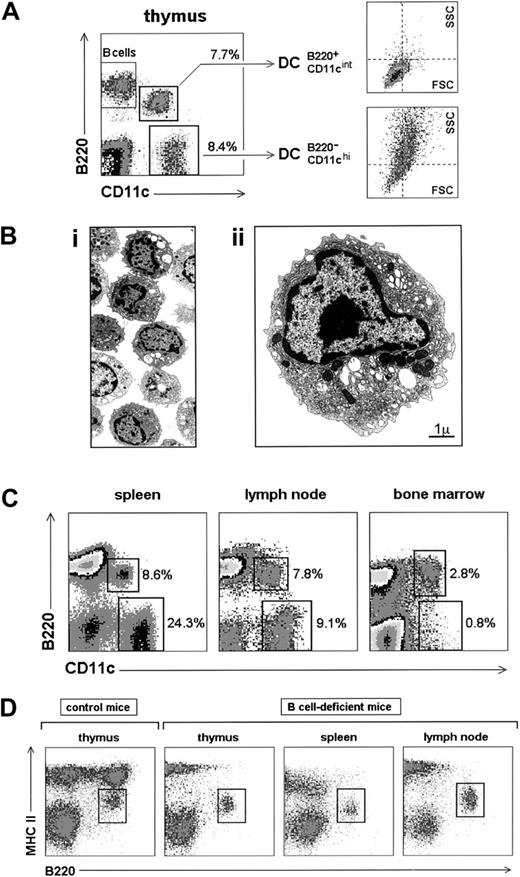
Preliminary studies of thymic DC CD11c expression (not shown) revealed the existence of CD11cint and CD11chisubpopulations. This differential CD11c expression level in fact corresponded to 2 discrete cell subsets that could be precisely defined by correlating CD11c with the expression of the B-cell marker B220 (Figure 1A). B220− DCs with high levels of CD11c corresponded to the cell population considered conventional DCs, whereas CD11cint cells expressed B220 and will be named hereafter B220+ DCs, for B220+regulatory DCs. CD11c− B220+ cells corresponded to thymic B cells.22 B220− DCs and B220+ DCs were present in the thymus at approximately a 1:1 ratio. As a consequence, in numerous reports B220+ DCs were inadvertently included in thymic DC preparations, unless the isolation method used involved the elimination of cells expressing CD4, Ly-6C, Ly-6G (Gr-1) (Figure 2A) or nonadherent cells because B220+ DCs had a low adherence potential (not shown). Thymic B220− DCs had a slightly higher forward-scatter profile than thymic B220+ DCs; the latter displayed significantly lower side scatter, reflecting less complex cytoplasmic and cell surface characteristics, in agreement with electron microscopic studies. As shown in Figure 1B, thymic B220+ DCs displayed an oval or irregularly shaped nucleus, short microvilli at the cell surface, numerous rough endoplasmic reticulum cisternae, and a clearly defined perinuclear area containing a well-developed Golgi apparatus and numerous mitochondria. Thymic B220+ DCs had lower viability on culture than B220− DCs (approximately 20% versus 60% viable cells, after 12-hour incubation in RPMI medium with 5% FCS, in the absence of exogenous cytokines; data not shown). Although this result could reflect that B220+ DCs represented a final differentiation stage of a B220− DC subset, this appeared to be unlikely because B220+ DC and B220− DC subpopulations were generated simultaneously after the reconstitution of irradiated mice with bone marrow precursors (C.A., unpublished data).
Identification of B220+ DCs.
(A) CD11c versus B220 profile of thymus low-density cell fractions. The percentages represented by B220+ CD11cint DCs and B220− CD11chi DCs and their forward scatter (FSC) versus side scatter (SSC) profiles are indicated. (B) Electron microscopic analysis of B220+ DCs. Bi: low magnification (original magnification × 2200) of MACS-purified thymic B220+ DCs. Bii: ultrastructural characteristics of B220+ DCs. B220+ DC size was 7.3 ± 0.6 μm (n = 8). (C) CD11c versus B220 profiles of spleen, lymph node, and bone marrow low-density cell fractions, showing the relative proportions of B220+ CD11cint DCs and B220− CD11chi DCs. (D) Characterization of B220+ DCs in MHC class II (MHCII) versus B220 profiles of B-cell–deficient mouse low-density cell fractions. Data are representative of 5 experiments with similar results.
Identification of B220+ DCs.
(A) CD11c versus B220 profile of thymus low-density cell fractions. The percentages represented by B220+ CD11cint DCs and B220− CD11chi DCs and their forward scatter (FSC) versus side scatter (SSC) profiles are indicated. (B) Electron microscopic analysis of B220+ DCs. Bi: low magnification (original magnification × 2200) of MACS-purified thymic B220+ DCs. Bii: ultrastructural characteristics of B220+ DCs. B220+ DC size was 7.3 ± 0.6 μm (n = 8). (C) CD11c versus B220 profiles of spleen, lymph node, and bone marrow low-density cell fractions, showing the relative proportions of B220+ CD11cint DCs and B220− CD11chi DCs. (D) Characterization of B220+ DCs in MHC class II (MHCII) versus B220 profiles of B-cell–deficient mouse low-density cell fractions. Data are representative of 5 experiments with similar results.
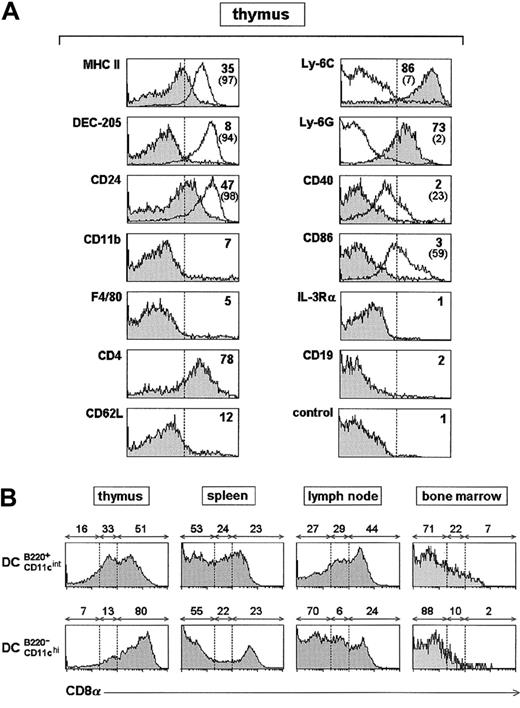
Phenotypic analysis of B220+ DCs.
(A) Phenotype of thymic B220+ DCs performed on DC-enriched cell fractions. Percentage of cells with fluorescence intensity over the dashed vertical lines, corresponding to the upper limit of control background staining, is indicated. White profiles correspond to the expression of the indicated marker for thymic B220−CD11chi DCs, for which the percentage of cells with fluorescence intensity over the dashed vertical lines is indicated in brackets. Data are representative of 4 experiments with similar results. (B) CD8α expression by B220+CD11cint DCs and B220− CD11chiDCs, in different organs. Percentages of CD8α−, CD8αint, and CD8αhi cells are indicated. Data are representative of 3 experiments with similar results.
Phenotypic analysis of B220+ DCs.
(A) Phenotype of thymic B220+ DCs performed on DC-enriched cell fractions. Percentage of cells with fluorescence intensity over the dashed vertical lines, corresponding to the upper limit of control background staining, is indicated. White profiles correspond to the expression of the indicated marker for thymic B220−CD11chi DCs, for which the percentage of cells with fluorescence intensity over the dashed vertical lines is indicated in brackets. Data are representative of 4 experiments with similar results. (B) CD8α expression by B220+CD11cint DCs and B220− CD11chiDCs, in different organs. Percentages of CD8α−, CD8αint, and CD8αhi cells are indicated. Data are representative of 3 experiments with similar results.
B220+ DCs were also found in the spleen, peripheral and mesenteric lymph nodes, and bone marrow (Figure 1C), but not in the blood. The B220− DC/B220+ DC ratio was different in these locations—approximately 3:1 in spleen, 1:1 in lymph nodes, and 1:3 in bone marrow—though individual variations in these proportions have been observed in nonstimulated control mice, particularly in the lymph nodes. Those changes in B220+ DC number could reflect their involvement in ongoing immune responses; in this sense, a strong increase in B220− DCs23and B220+ DCs (C.A., unpublished data, June 2001) occurred in the popliteal lymph nodes during experimental infection by the mouse mammary tumor virus. The non–B-cell nature of B220+ DCs was confirmed by assessing their existence in B-cell–deficient mouse lymphoid organs (Figure 1D) and their negativity for CD19 (Figure 2A).
Phenotypic analysis of B220+ DCs
The phenotype of thymic B220+ DCs performed in DC-enriched cell fractions is presented in Figure 2A. B220+DCs displayed lower CD11c and MHC class II expression levels than B220− DCs. In addition B220+ DCs shared CD8α and CD24 (HSA) expression and nonexpression of CD11b, F4/80, and CD62L with thymic B220− DCs, but, in contrast to the latter, they were negative for DEC-205 and for the costimulatory molecules CD40 and CD86. Interestingly, thymic B220+ DCs, but not B220− DCs, expressed Ly-6C and Ly-6G (Gr-1). The phenotype of B220+ DCs located in the spleen, lymph nodes, and bone marrow did not differ significantly from that of thymic B220+ DCs except for the expression of CD8α. This molecule has allowed the definition of CD8α−, CD8αint, and CD8α+ DC subsets (not expressing B220), with differential distribution among lymphoid and hemopoietic organs.15 Regarding B220+ DCs, their CD8α expression differed depending on their location (Figure2B). In the thymus and lymph nodes, approximately 50% of B220+ DCs expressed high CD8α levels, and 50% displayed from low to intermediate levels. Within the spleen, 50% had intermediate to high CD8α levels, whereas the rest were CD8α−. Finally, bone marrow B220+ DCs did not express CD8α. Whether CD8α level expression by B220+ DCs reflects the existence of different B220+ DC subsets has to be defined. Alternatively, CD8α expression by a unique B220+ DC population may be subjected to a differential regulation depending on location. In this sense, high CD8α levels could correlate with their location in relation to T-cell areas. According to this hypothesis, most B220+ DCs from thymus and bone marrow are CD8α+ and CD8α−, respectively. Similarly, CD8α expression by B220− DCs could be subjected to environmental regulation. In this regard, it has been recently reported that splenic CD8α+ DCs originate from the CD8α− subset by a maturation process involving CD8α, DEC-205, and CD24 up-regulation.24 Regulation of CD8α expression by B220+ DCs and B220− DCs is under investigation in our laboratory.
CpG-induced maturation and T-cell stimulatory potential of B220+ DCs
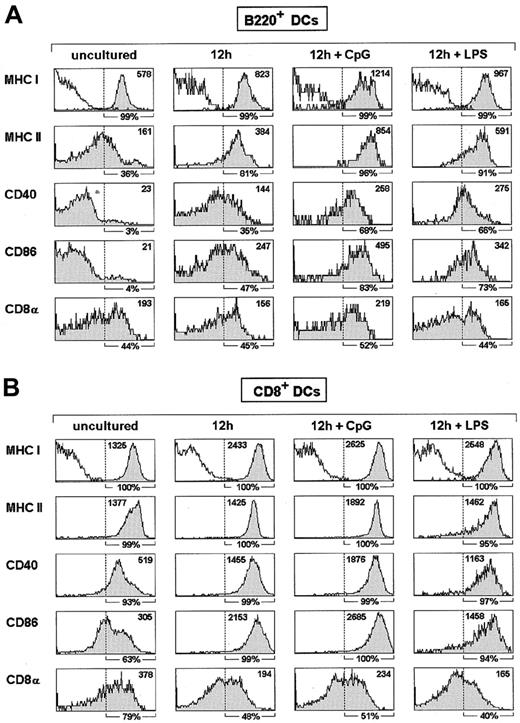
The low or null MHCII, CD40, and CD86 levels expressed by B220+ DCs most likely revealed an immature DC-like state; therefore, it could be hypothesized that up-regulation of these molecules by B220+ DCs could occur on culture in the presence of compounds known to induce a mature DC state. To test this hypothesis MACS-purified thymic B220+ DCs were treated with CpG or bacterial LPS, each of which efficiently promotes DC maturation.25 As illustrated in Figure3A, 12-hour culture of B220+DCs in the presence of CpG determined a strong MHCII up-regulation and the expression of high levels of CD40 and CD86. Comparison of the effect of CpG on thymic B220+ DCs and thymic B220− DCs that express CD8α (CD8+ DCs), revealed that this DC maturation inducer had a comparable effect on the CD8+ DC subset (Figure 3B). CD8α expression by B220+ DCs was not significantly influenced by CpG treatment, but a slight CD8α down-regulation was noticed for CD8+ DCs. A similar effect was produced by LPS, though under the experimental conditions considered in our assay, CpG promoted a stronger CD86 up-regulation by B220+ DCs than LPS (Figure 3A).
Up-regulation of MHC and costimulatory molecules by B220+ DCs induced by CpG and LPS.
Histograms show the expression of the indicated markers by MACS-sorted thymic B220+ DCs (A) or CD8+ DCs (B) before culture and after 12-hour culture in control medium or in the presence of CpG or LPS. Percentage of cells with fluorescence intensity over the dashed vertical lines, corresponding to the upper limit of background staining shown in MHC class I (MHCI) histograms (white profiles), is indicated below each histogram. Mean fluorescence intensity is indicated in the upper right corner of each histogram. Data are representative of 3 experiments with similar results.
Up-regulation of MHC and costimulatory molecules by B220+ DCs induced by CpG and LPS.
Histograms show the expression of the indicated markers by MACS-sorted thymic B220+ DCs (A) or CD8+ DCs (B) before culture and after 12-hour culture in control medium or in the presence of CpG or LPS. Percentage of cells with fluorescence intensity over the dashed vertical lines, corresponding to the upper limit of background staining shown in MHC class I (MHCI) histograms (white profiles), is indicated below each histogram. Mean fluorescence intensity is indicated in the upper right corner of each histogram. Data are representative of 3 experiments with similar results.
When tested in an MLR assay for their ability to stimulate T-cell proliferation, thymic B220+ DCs displayed a low T-cell stimulatory capacity when compared with thymic CD8+ DCs (Figure 4A). This could be related to their low MHCII and costimulatory molecule expression levels, and it could be hypothesized that up-regulation of these molecules on B220+ DCs could endow them with a higher ability to induce T-cell activation and proliferation. According to this view, when thymic B220+ DCs preincubated with CpG were tested in MLR, they displayed a T-cell stimulatory capacity comparable to that of control CD8+ DCs, indicating that their low stimulatory potential in control conditions could be attributed, according to our hypothesis, to a low MHC and costimulatory molecule expression. As expected, CpG-treated CD8+ DCs induced stronger T-cell stimulation than their control counterparts. Therefore, B220+ DCs could be driven to acquire a strong APC potential on maturation. More interestingly, analysis of MHCII, CD40, and CD86 expression by B220+ DCs cultured for 12 hours in the absence of CpG revealed that a remarkable up-regulation of these molecules occurred (Figure 3A), though it was lower than after CpG or LPS treatment. This result revealed that the low T-cell stimulation potential displayed by control, non–CpG-treated B220+ DCs most likely reflected the stimulatory capacity of cultured B220+ DCs acquired as the consequence of the up-regulation of MHCII and costimulation molecules during the first hours of the 4-day MLR assay. Hence, these data suggest that, in fact, under physiological conditions, B220+ DCs have a null or an extremely reduced T-cell stimulatory capacity. In relation to their APC ability, no IL-12 was detected after 12-hour culture of thymic B220+ DCs in control medium, but large amounts of this cytokine were produced after CpG stimulation (Figure 4B). Interestingly, production of IL-12 after CpG stimulation was 2- to 3-fold higher for thymic B220+ DCs than for CD8+ DCs. On the other hand, low but significant amounts of IL-10 were produced by CpG-treated B220+ DCs, though the production level was lower than that detected for peritoneal macrophages used as positive controls for IL-10 secretion (Figure 4C).
Modulation of B220+ DC function by microbial stimulation.
(A) T-cell stimulation capacity of control or CpG-treated MACS-sorted thymic B220+ DCs or CD8+ DCs in a 4 day-MLR assay. T-cell proliferation was determined by [3H] thymidine uptake (error bars represent the SD for triplicate cultures). Data are representative of 3 experiments with similar results. (B) IL-12 production by FACS-sorted thymic B220+ DCs, thymic CD8+ DCs, and peritoneal macrophages (MΦ) after culture in control medium or in the presence of CpG, determined by ELISA (mean ± SD of 3 independent experiments). (C) IL-10 production by FACS-sorted thymic B220+ DCs, thymic CD8+ DCs, and peritoneal macrophages (MΦ) after culture in control medium or in the presence of CpG, determined by ELISA (mean ± SD of 3 independent experiments).
Modulation of B220+ DC function by microbial stimulation.
(A) T-cell stimulation capacity of control or CpG-treated MACS-sorted thymic B220+ DCs or CD8+ DCs in a 4 day-MLR assay. T-cell proliferation was determined by [3H] thymidine uptake (error bars represent the SD for triplicate cultures). Data are representative of 3 experiments with similar results. (B) IL-12 production by FACS-sorted thymic B220+ DCs, thymic CD8+ DCs, and peritoneal macrophages (MΦ) after culture in control medium or in the presence of CpG, determined by ELISA (mean ± SD of 3 independent experiments). (C) IL-10 production by FACS-sorted thymic B220+ DCs, thymic CD8+ DCs, and peritoneal macrophages (MΦ) after culture in control medium or in the presence of CpG, determined by ELISA (mean ± SD of 3 independent experiments).
Expression of CCR5, CCR6, and CCR7 by B220+DCs
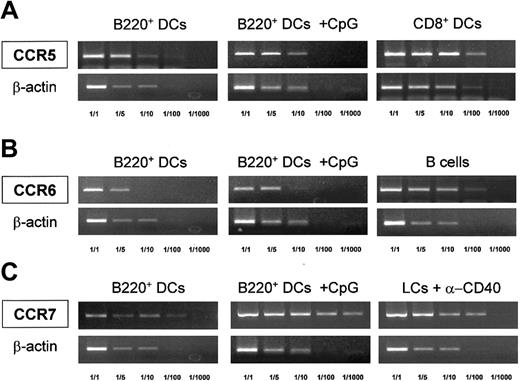
With regard to DC-related chemokine receptors, the expression of CCR5, CCR6, and CCR7 by control or CpG-treated thymic B220+DCs was analyzed by RT-PCR using specific primers. Thymic CD8+ DCs, B cells, and anti–CD40-matured Langerhans cells were used as positive controls for the expression of CCR5,26 CCR6,27 and CCR7,28respectively. Thymic B220+ DCs expressed CCR5 at levels comparable to those of CD8+ DCs, but they expressed significantly lower levels of CCR6 than B cells and lower levels of CCR7-specific mRNA than mature Langerhans cells (Figure5). Interestingly, CpG treatment did not significantly influence CCR5 or CCR6 expression, but it induced the up-regulation of CCR7, reaching expression levels similar to those detected in Langerhans cells stimulated with anti-CD40.
Chemokine receptor expression by B220+ DCs.
Semiquantitative RT-PCR analysis of CCR5, CCR6, and CCR7 mRNA expression in control or CpG-treated FACS-sorted thymic B220+ DCs. Thymic CD8+ DCs, splenic B cells, and anti–CD40-matured epidermal Langerhans (LCs + α-CD40) cells were used as positive controls for the expression of CCR5, CCR6, and CCR7, respectively. RT-PCR was performed on serial dilutions of cDNA from each cell population. β-Actin mRNA levels are shown to control for the relative expression of each cytokine receptor mRNA in the different populations considered.
Chemokine receptor expression by B220+ DCs.
Semiquantitative RT-PCR analysis of CCR5, CCR6, and CCR7 mRNA expression in control or CpG-treated FACS-sorted thymic B220+ DCs. Thymic CD8+ DCs, splenic B cells, and anti–CD40-matured epidermal Langerhans (LCs + α-CD40) cells were used as positive controls for the expression of CCR5, CCR6, and CCR7, respectively. RT-PCR was performed on serial dilutions of cDNA from each cell population. β-Actin mRNA levels are shown to control for the relative expression of each cytokine receptor mRNA in the different populations considered.
In conclusion, with CpG treatment, B220+ DCs can be driven to acquire the capacity to induce T-cell proliferation, produce IL-12, and up-regulate CCR7, endowing them with the potential to migrate to T-cell areas of peripheral lymphoid organs.28
Production of IFN-α by B220+ DCs
Interestingly, B220+ DCs shared with human plasmacytoid cells certain ultrastructural features, especially the development of the protein synthesis machinery (Golgi apparatus and rough endoplasmic reticulum) and, more important, the potential to become competent APCs on microbial stimulation.29 Because an essential role of plasmacytoid cells relies on their capacity to secrete large amounts of type 1 IFN on viral stimulation,30 31 providing a highly efficient antiviral defense mechanism, we tested thymic B220+ DCs for their ability to secrete IFN-α. Although CpG induced the secretion of IFN-α by B220+ DCs, viral stimulation induced a much stronger production of this cytokine, and consequently the production of IFN-α by B220+ DCs after incubation with Sendai virus was 6-fold higher than after CpG stimulation (Figure6).
IFN-α production by B220+ DCs.
FACS-sorted B220+ DCs cultured in control medium, or in the presence of CpG or Sendai virus, were tested for the presence of IFN-α using a mouse of IFN-α ELISA (mean ± SD of 3 independent experiments).
IFN-α production by B220+ DCs.
FACS-sorted B220+ DCs cultured in control medium, or in the presence of CpG or Sendai virus, were tested for the presence of IFN-α using a mouse of IFN-α ELISA (mean ± SD of 3 independent experiments).
T-cell tolerogenic potential of B220+ DCs: induction of Treg cell differentiation
Considered globally, the data presented above dealing with the function of B220+ DCs indicate that this subset of DCs could be induced to play an important defense role against microbial pathogens by fulfilling antigen-presenting and cytokine secretion functions. However, in steady state—when not subjected to bacterial or viral stimulation—B220+ DCs appear to be inefficient APCs because of their low MHC and costimulatory molecule expression levels. This fact, together with their capacity to produce IL-10, prompted us to investigate whether B220+ DCs could be endowed with a tolerogenic potential by inducing T-cell unresponsiveness and the differentiation of Treg cells. The experimental system used for this purpose is described in “Materials and methods” and in Figure 7. In brief, OVA-TCR TCs were first cultured with thymic B220+ DCs or B220−DCs in the presence of OVA. After this first phase, OVA-TCR TCs, precultured with B220+ DCs (OVA-TCs[+B220+DCs]) or B220− DCs (OVA-TCs[+DCs]), were cultured with IL-2 in the absence of OVA and APCs. Importantly, less than 5% of B220+ DCs or B220− DCs survived after the first phase, and they were not detectable after the second culture phase (data not shown). Following this second phase, OVA-TCs[+B220+ DCs] were tested for the capacity of IL-2 to revert their nonresponsive state and for their Treg cell potential. As shown in Figure 7A, B220+ DCs displayed a reduced capacity to induce the proliferation of OVA-TCR TCs in the presence of OVA, according to our previous data from MLR assays (Figure4A). As expected, no cell proliferation was detected in the absence of OVA. When OVA-TCs[+DCs] were restimulated after the second phase with BALB/c splenocytes plus OVA, a strong cell proliferation was induced, and this response was further increased by exogenous IL-2 (Figure 7B). In contrast, no proliferation of OVA-TCs[+B220+ DCs] occurred after restimulation with BALB/c splenocytes plus OVA, and this effect could not be reverted in the presence of IL-2. This result suggests that the nonresponsiveness induced by B220+ DCs on OVA-TCR TCs (ie, the nonresponsiveness of OVA-TCs[+B220+DCs]) did not correspond to an anergic state because the reversal of anergic T cells can be achieved by exogenous IL-2.32 Based on this result, we then tested whether OVA-TCs[+B220+DCs] were endowed with a Treg cell potential by analyzing their capacity to inhibit the proliferation of OVA-TCR TCs cultured with syngeneic splenocytes plus OVA but not previously stimulated (Figure 7C). Interestingly, OVA-TCs[+B220+ DCs] strongly reduced the proliferation of OVA-TCR TCs when compared with that obtained in control conditions (ie, OVA-TCR TCs + splenocytes + OVA). In contrast, when OVA-TCs[+DCs] were used in this assay, thymidine incorporation was almost 2-fold higher than in control conditions as a result of the sum of the proliferation of OVA-TCs[+DCs] and OVA-TCR TCs not previously stimulated.
T-cell tolerogenic potential of B220 DCs.
(A) OVA-TCR TC proliferation after 72-hour culture with either thymic B220+ DCs or B220− DCs (DCs) and OVA. (B) Anergy reversal assay. Proliferation of OVA-TCR TCs precultured for 72 hours with B220+ DCs (OVA-TCs[+B220+ DCs]) or B220− DCs (OVA-TCs[+DCs]) after 48-hour culture with syngeneic splenocytes and OVA in the presence of IL-2. (C) Treg cell assay. Proliferation of OVA-TCR TCs after 36-hour culture with syngeneic splenocytes and OVA in the presence of (OVA-TCs[+B220+ DCs]) or (OVA-TCs[+DCs]). See “Materials and methods” for a detailed explanation of this experimental system. Cell proliferation was assessed by [3H] thymidine (1 μCi/well) uptake in a 16-hour pulse (error bars represent the SD for triplicate cultures). Data are representative of 2 experiments with similar results.
T-cell tolerogenic potential of B220 DCs.
(A) OVA-TCR TC proliferation after 72-hour culture with either thymic B220+ DCs or B220− DCs (DCs) and OVA. (B) Anergy reversal assay. Proliferation of OVA-TCR TCs precultured for 72 hours with B220+ DCs (OVA-TCs[+B220+ DCs]) or B220− DCs (OVA-TCs[+DCs]) after 48-hour culture with syngeneic splenocytes and OVA in the presence of IL-2. (C) Treg cell assay. Proliferation of OVA-TCR TCs after 36-hour culture with syngeneic splenocytes and OVA in the presence of (OVA-TCs[+B220+ DCs]) or (OVA-TCs[+DCs]). See “Materials and methods” for a detailed explanation of this experimental system. Cell proliferation was assessed by [3H] thymidine (1 μCi/well) uptake in a 16-hour pulse (error bars represent the SD for triplicate cultures). Data are representative of 2 experiments with similar results.
To exclude that the inhibition of OVA-TCR TC proliferation induced by OVA-TCs[+B220+ DCs] was only the consequence of a dilution effect resulting from the presence of OVA–nonresponding T cells (in this case OVA-TCs[+B220+ DCs]) among OVA-TCR TCs and splenocytes, an additional control condition was considered. For this purpose, OVA-TCR TCs were cultured with splenocytes plus OVA in the presence of genuine OVA–nonresponding T cells—that is, T cells isolated from BALB/c lymph nodes—in the same proportions as in the experiment described above involving OVA-TCs[+B220+ DCs]. As shown in Figure 7C, compared with the original control conditions, no reduction in cell proliferation was observed. In conclusion, our data suggest that B220+ DCs induced a nonanergic state of T-cell nonresponsiveness involving the differentiation of Treg cells capable of suppressing antigen-specific T-cell proliferation and, therefore, that B220+ DCs could represent a physiological subset of tolerogenic DCs.
Discussion
B220+ DCs, described in this report, constitute a new subset of mouse DCs that, when in steady state, display characteristics of immaturelike DCs, including very low levels of MHC expression and costimulatory molecules and a markedly reduced T-cell stimulation potential compared with conventional murine DCs not expressing B220. As demonstrated by recent data from our laboratory, B220+ DCs could be generated along with CD8α+ and CD8α− DCs from a common dendritic cell precursor population,33 indicating that both B220− and B220+ DCs have a common origin. Interestingly, Lu et al11 have recently reported that DCs obtained in vitro from murine liver lineage–negative cells, in the presence of IL-3 and CD40L, expressed B220 and had a tolerogenic potential because they induced prolonged survival of cardiac allografts on transfer. The ultrastructural features of B220+ DCs resemble those of human plasmacytoid cells in that each cell type has a well-developed protein synthesis machinery, including numerous rough endoplasmic reticulum cisternae.29 Interestingly, on viral stimulation, B220+ DCs produce IFN-α at levels comparable to those of stimulated peritoneal macrophages, which is the hallmark of plasmacytoid cells.30,31 Therefore, B220+ DCs could represent a murine equivalent of human plasmacytoid cells, as proposed in recent reports dealing with the characterization of murine plasmacytoidlike cells in mice.34-36 Moreover, as described for plasmacytoid cells,29 B220+ DCs can be driven to become immunocompetent DCs on maturation. Alternatively, IFN-α production could be related to the proposed role of B220+ DCs in the induction of Treg cells because it has been recently shown that IFN-α and IL-10 are both required for the generation of human Tregcells.37
On stimulation with bacterial agents known to promote DC activation and maturation, such as CpG,25 B220+ DCs acquire a high T-cell stimulation potential, paralleled by a strong up-regulation of MHC and costimulatory molecules, IL-12 production, and expression of the chemokine receptor CCR7, involved in DC migration to lymphoid organ T-cell areas.28 Globally, these functional changes in B220+ DCs endow them with a T-cell stimulatory capacity comparable to that of conventional stimulatory B220− DCs, involving the ability to process and present antigens, migrate to T-cell areas, and induce T-cell activation and proliferation.
Interestingly, our data demonstrate that this reduced T-cell stimulatory capacity of B220+ DCs is paralleled by their potential to induce a nonresponsive state in T cells that does not correspond to T-cell anergy because anergic T cells, but not T cells precultured with B220+ DCs, can be rescued in vitro by the addition of exogenous IL-2.32 More important, our data suggest that nonresponsive T cells generated on culture with B220+ DCs function as Treg cells, as demonstrated by their capacity to exert an inhibitory effect on T-cell proliferation (reviewed in Read and Powrie38). Therefore, our data indicate that B220+ DCs could represent a physiological subset of DCs with tolerogenic potential, not previously described, involved in the generation of Treg cells and thus in the control of autoreactive T-cell clones. Consequently, B220+ DCs could be involved in the maintenance of peripheral tolerance by the induction of Treg cells. On the other hand, given that within the thymus B220+ DCs are as numerous as conventional thymic DCs, indicating that they probably fulfill an important role in T-cell development, it can be hypothesized that thymic B220+ DCs could be involved in intrathymic Treg cell differentiation. In this sense, the CD4+ CD25+ murine Treg cell subset has been claimed to develop in the thymus.4 A great deal of controversy exists regarding the origin, heterogeneity, and mechanism of action of Treg cells, partly because different experimental models have been used in their characterization. Additional experiments are in progress in our laboratory to define the molecular mechanisms controlling the induction of intrathymic and peripheral Treg cell differentiation by B220+DCs and the suppressive effects exerted by these Tregcells. Interestingly, as previously discussed, our phenotypic and functional data support the view that under physiological conditions B220+ DCs constitute a subset of DCs in an immature state that could underlie their possible tolerogenic potential. Experiments carried out in humans and mice have demonstrated the capacity of in vitro–generated immature DCs to induce the differentiation of T anergic and Treg cells (reviewed in Jonuleit et al4).
In conclusion, B220+ DCs represent a specialized subset of constitutively immature DCs that could be involved in intrathymic Treg cell development and in the differentiation of peripheral Treg cells and, consequently, in the control of peripheral T-cell tolerance. On the other hand, on encounter with microbial pathogens, B220+ DCs can function as potent APCs and promote the induction of T-cell defense responses.
We thank Dr A. Rolink for the anti-CD40 hybridoma FGK45 and Dr G. Márquez for the CCR6 and CCR7 primers.
Supported by grants from the European Commission (QLRT-1999-00276), the Comunidad de Madrid of Spain (08.1/0076/2000), and the Ministerio de Ciencia y Tecnologı́a of Spain (BOS 2000-0558).
P.M. and G.M.d.H. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Carlos Ardavı́n, Department of Cell Biology, Faculty of Biology, Complutense University, 28040 Madrid, Spain; e-mail: ardavin@bio.ucm.es.

![Fig. 4. Modulation of B220+ DC function by microbial stimulation. / (A) T-cell stimulation capacity of control or CpG-treated MACS-sorted thymic B220+ DCs or CD8+ DCs in a 4 day-MLR assay. T-cell proliferation was determined by [3H] thymidine uptake (error bars represent the SD for triplicate cultures). Data are representative of 3 experiments with similar results. (B) IL-12 production by FACS-sorted thymic B220+ DCs, thymic CD8+ DCs, and peritoneal macrophages (MΦ) after culture in control medium or in the presence of CpG, determined by ELISA (mean ± SD of 3 independent experiments). (C) IL-10 production by FACS-sorted thymic B220+ DCs, thymic CD8+ DCs, and peritoneal macrophages (MΦ) after culture in control medium or in the presence of CpG, determined by ELISA (mean ± SD of 3 independent experiments).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/2/10.1182_blood.v100.2.383/6/m_h81422828004.jpeg?Expires=1769095062&Signature=ulKTupNghVev2PX7bnYmEqf8oZrL8mCfTAPAqlu8Rlw7OHIflwOTQxEZ50QAcePqPb~QSuHocD07ydxgEGOxCQaUx5xwuhNlr9uDzrobiktB0nhKzdxu6j7WkFVAh-Gp8y-iJYwlljJ26yGiuDecNTSPnFu9xYA6DYgjhpCwjVzCefA4Pnkmj0abWMgeRp62NE0e2HBe1KGcjFkMCHMK542Iji-wamXcRu8XjIicf1c-4QD243GPzUjDQYN7YkbTdGC6MhRxGd3NPHV05n8WnR8LleEmUziLwLxGQDpucJAC0uN7vWyOvrPYARNZsIzt6wyaxcwtLPnDDg4dryVQqg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 7. T-cell tolerogenic potential of B220 DCs. / (A) OVA-TCR TC proliferation after 72-hour culture with either thymic B220+ DCs or B220− DCs (DCs) and OVA. (B) Anergy reversal assay. Proliferation of OVA-TCR TCs precultured for 72 hours with B220+ DCs (OVA-TCs[+B220+ DCs]) or B220− DCs (OVA-TCs[+DCs]) after 48-hour culture with syngeneic splenocytes and OVA in the presence of IL-2. (C) Treg cell assay. Proliferation of OVA-TCR TCs after 36-hour culture with syngeneic splenocytes and OVA in the presence of (OVA-TCs[+B220+ DCs]) or (OVA-TCs[+DCs]). See “Materials and methods” for a detailed explanation of this experimental system. Cell proliferation was assessed by [3H] thymidine (1 μCi/well) uptake in a 16-hour pulse (error bars represent the SD for triplicate cultures). Data are representative of 2 experiments with similar results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/2/10.1182_blood.v100.2.383/6/m_h81422828007.jpeg?Expires=1769095062&Signature=SGGOWVJKAmSlzkVa2wLYb9EAYJUgr0xVylGnhTHf1~CHC3a1Cjg94t~34gcYJe6Wcm4rAsMnWjnDDsaEA8UBzWyICsEw8OQnxtNS7krs7zWIpD0goo~hOnRo38ob-dZTN1wIclQSd1g~LUbeF6C6Mq8Jh2qTs5wuGp0evdXurBxkXffvMNI-AMM-br531mgLkF5Nr4gi72t9qW-raH1WViS14Db024UHsAz3LF-hQ9GFCLA6waOx4fkbBJcAvdTPlr4uf4tQpPoQIKUI2V9J3XDj2w6-gfwsIf44FHX914oesWzj-ULm6CYufj-d8HPC6THyFXRofc~~s7UFit4mKw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal