We have characterized 2 distinct mechanisms through which infectious agents may promote platelet adhesion and thrombus formation in flowing blood, thus contributing to the progression of disease. In one case, the process initiates when the integrin αIIbβ3 mediates platelet arrest onto immobilized bacterial constituents that have bound plasma fibrinogen. If blood contains antibodies against the bacteria, immunoglobulin (Ig) G may cluster on the same surface and activate adherent platelets through the FcγRIIA receptor, leading to thrombus growth. As an alternative, bacteria that cannot bind fibrinogen may attach to substrates, such as immobilized plasma proteins or components of the extracellular matrix, which also support platelet adhesion. As a result of this colocalization, IgG bound to bacteria can activate neighboring platelets and induce thrombus growth regardless of their ability to initiate platelet-surface contact. Our results demonstrate that intrinsic constituents of infectious agents and host proteins play distinct but complementary roles in recruiting platelets into thrombi, possibly contributing to complications of acute and chronic infections.
Introduction
Platelet adhesion and aggregation at sites of tissue trauma involve interactions of membrane receptors with constituents of extracellular matrices, such as collagen, and circulating macromolecules, such as von Willebrand factor (VWF) and fibrinogen,1 which contribute to arrest bleeding during hemostasis. Bacteria, too, can induce platelet aggregation2-4 or uncontrolled clotting with disseminated intravascular coagulation (DIC),5,6 which may become disease mechanisms when the causative agent intermittently invades the bloodstream. For example, coagulation and hemostasis are activated in some localized infections, such as necrotizing fasciitis, resulting in extensive thrombosis of arterioles and veins in and around lesions.7,8 Moreover, experimental and clinical observations have demonstrated that platelets play a key pathogenetic role when certain microorganisms establish infection in the bloodstream, as in the case of bacterial endocarditis.4,9In particular, platelet aggregates may allow bacteria to settle and remain at the site of infection withstanding the shear forces of flowing arterial blood. In experimental models of this disease, early vegetations grow by accretion of layers of fibrin and platelets with bacterial colonies sandwiched between them.10 Similar mechanisms may facilitate the establishment of bacteria on artificial devices, such as arterial grafts.11 The number of strains that can settle in the arterial circulation is limited, but an array of different species can cause septic venous thrombosis,12 13a condition in which platelet activation may be involved.
In these studies, we have used 2 invasive species, Streptococcus pyogenes (also designated group A streptococcus) andStaphylococcus aureus, as models to examine the mechanisms involved in bacteria-induced thrombus formation under conditions mimicking the macromolecular, cellular, and hemodynamic complexity of blood circulating in different vessels. Only bacteria capable of binding a platelet-reactive factor from blood, such as fibrinogen, could initiate adhesion on a surface not intrinsically conducive to platelet deposition. This step, however, was not required on substrates that could directly support platelet adhesion, such as immobilized fibrinogen, fibronectin, or subendothelial matrix. In either case, specific antibodies bound to surface-immobilized bacterial antigens were necessary to induce platelet aggregation into thrombi through an interaction with the FcγRIIA IgG receptor. Thus, the interplay of bacterial and host proteins elicits platelet responses with a potential pathogenetic role during infections.
Materials and methods
Blood and blood cells
Blood from healthy donors contained the thrombin inhibitord-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone dihydrochloride (PPACK; 80 μM) as anticoagulant. Institutional review boards from the University of Lund and the Scripps Research Institute approved these studies, and all volunteer donors gave their informed consent according to the Declaration of Helsinki. Blood cells were washed free of plasma constituents by centrifugation and resuspension in Tyrode-HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer containing 1.0 mM Ca++ and 0.5 mM Mg++, as described previously.14
Bacterial strains and culture conditions
AP1, M3, and M5 Manfredo are S pyogenes strains expressing the M1, M3, and M5 protein, respectively. ΔM1, ΔM3, and ΔM5 strains carrying inactivated emm genes have been described previously,15-17 as have the clumping factor–expressing strain, S aureus Newman, and its clumping factor–negative isogenic derivative, ΔClfA.18 Bacteria were grown in Todd-Hewitt (TH) broth and 5% CO2 at 37°C for 16 hours. ΔM5 transformed with the shuttle plasmid pLZ12(spec), or derivatives thereof, were grown in a medium supplemented with spectinomycin at 100 mg/L. Bacteria were harvested by centrifugation, washed twice in 0.15 M NaCl, 0.03 M sodium phosphate, pH 7.4 (phosphate-buffered saline [PBS]), and finally resuspended in PBS. Transformed Escherichia coli DH5α was grown in Luria-Bertani (LB) broth supplemented with spectinomycin (20 mg/L), or ampicillin (100 mg/L), depending on the plasmid used.
Proteins
To generate the M5ΔB-encoding construct, the parts of theemm5 gene located 5′ and 3′ of the B-repeats were amplified by polymerase chain reaction (PCR) and then cloned into the shuttle vector pLZ12(spec). The resulting construct,emm5ΔB, was used to express rM5ΔB in E coliand in ΔM5 streptococci.19 To generate the chimeric proteins M4/M1 and M4/M5, the regions encoding the fibrinogen-binding B-repeats in emm1 and emm5 were amplified by PCR and cloned into emm4 in the shuttle plasmid pJRS264, a derivative of pLZ12(spec).19 Cloning of M1 in the E coli expression vector pHD389, of M5 in pLZ12(spec), and of the fibrinogen-binding region of clumping factor (rClfA) in pQE30 have been described previously.17,20,21 Recombinant M1, M5, and M5ΔB were purified on agarose coupled with human serum albumin from lysates of overnight cultures of E coli transformed with the corresponding genes. Human serum albumin was coupled to agarose columns activated with N-hydroxysuccinimide ester (Amersham Pharmacia Biotech, Uppsala, Sweden). Proteins bound to the affinity columns were eluted using 0.1 M glycine, pH 2.0, and dialyzed against PBS. The synthetic oligopeptide N23 (AVTRGTINDPQRAKEALDKYELE) corresponds to the unique N-terminal end of the M5 protein and was synthesized using fluorenyl-methoxycarbonyl (F-moc) chemistry. The peptide was purified by high-performance liquid chromatography (HPLC) and characterized by mass spectrometry. rClfA expressed in pQE30 contains an N-terminal extension of 6 His residues. The fusion protein was purified using chelating Ni-Sepharose Fast Flow (Amersham Pharmacia Biotech). Bound proteins were eluted with imidazole (200 mM) and dialyzed against PBS. Fibrinogen was purified from blood collected in acid-citrate-dextrose anticoagulant containing 0.1 M (final concentration) ε-aminocaproic acid, using the glycine precipitation method22 as previously reported.23High-molecular-weight contaminants were removed by gel permeation chromatography through a Sepharose CL-4B column. Human VWF was purified and characterized using methods previously described in detail.24 Absence of fibronectin and fibrinogen contamination was verified by immunoblotting with monospecific antibodies. Human plasma fibronectin was generously provided by Dr Mark Ginsberg (Scripps Research Institute). Bovine fibrillar type I collagen was purchased from Sigma (St Louis, MO).
Antibodies
The monoclonal antibodies IV.3 (American Type Culture Collection no. HB-217, Manassas, VA), BIIG2 and P5D2 (both obtained from the Developmental Studies Hybridoma Bank at the University of Iowa, Iowa City, IA), LJ-CP823 and LJ-Ib125were purified on protein A agarose. IgG and M5-specific antibodies from human plasma were purified on protein A agarose and agarose coupled with rM5, respectively. Anti-M antibody titers in plasma against 3 different recombinant M proteins (M1, M5, and M53) were determined by enzyme-linked immunosorbent assay (ELISA) using microtiter plates coated with 2.5 μg purified protein. After adding blocking buffer (PBS containing 1% bovine serum albumin [BSA]) overnight, plasma diluted in the same buffer was added to the wells. Following incubation for 1 hour at room temperature and subsequent washing, horseradish peroxidase–conjugated goat antihuman immunoglobulin (Zymed Laboratories, San Francisco, CA), diluted 1:2000, was added. After incubation at room temperature for 2 hours, the wells were washed repeatedly with blocking buffer. Finally 200 μL of a developing buffer containing 1 M citrate, pH 4.0, 0.3% H2O2 and 1/10 ABTS (2,2-azino-di (3-ethylbenzthiazoline) sulfonic acid; Zymed Laboratories) was added. The optical density was measured at 405 nm and plotted versus the dilution. The presence of M5-specific antibodies in plasma was determined by ELISA, using the peptide N23 (see above) as antigen.
Preparation of glass coverslips
Bacterial proteins were used at a concentration of 100 μg/mL in PBS, and 300 μL of the solution was applied on a glass coverslip (24 × 50 mm, Corning, Vineland, NJ) as previously described.26 Bovine fibrillar type I collagen was used at 200 μg/mL. Human serum albumin, fibrinogen, and fibronectin were used at 50 μg/mL. Bacteria, 300 μL of 108 cfu/mL in PBS, were added either directly to the coverslip or after coating the glass surface with various human plasma proteins or extracellular matrix (ECM). Bacteria were allowed to attach for 30 minutes at room temperature, and after thorough rinses the coverslip was placed in the flow chamber. For coating with ECM, pooled human umbilical vein endothelial cells (Clonetics, San Diego, CA) were cultured as previously described.1 After reaching confluence in T-75 tissue culture flasks, cells were plated and cultured on sterile glass coverslips. Confluent monolayers were washed repeatedly with PBS and detached by incubation in 20 mM NH4OH and 0.5% Triton X-100 in PBS for 3 minutes at 37°. The matrix remaining attached to the glass slides was washed with PBS. The matrix-coated glass slides were assembled as the base of a parallel plate perfusion chamber and used immediately in blood flow studies.
Epifluorescence videomicroscopy
Platelet interaction with immobilized proteins, ECM, or bacteria under various flow conditions was studied using a modification of a parallel flow chamber as previously described.1,26,27 A syringe pump (Harvard Bioscience, South Natick, MA) was used to aspirate blood through the flow chamber. Platelets were labeled in whole blood by direct incubation with the fluorescent dye mepacrine (quinacrine dihydrochloride, 10 μM). The flow chamber, mounted on an epifluorescence microscope (Axiovert 135 M inverted microscope; Carl Zeiss, Thornwood, NY), allowed direct visualization in real time of platelet adhesion and thrombus formation, which were recorded on videotape. The total volume occupied by thrombi in a given area was measured while blood was flowing from a series of confocal sections at 1.0-μm intervals in the z axis (LSM confocal microscope; Carl Zeiss), and calculated as described previously1 14using Metamorph (Universal Imaging Corporation, Downingtown, PA) for image analysis.
Results
S aureus and S pyogenes cause platelet thrombus formation under flow conditions
The ability of bacteria to induce aggregation of flowing platelets was analyzed using 2 different invasive species: S aureus, which causes endovascular infections, and S pyogenes, which can cause derangement of hemostasis following transient invasion of the bloodstream. The bacteria were allowed to adhere to a glass slide and then exposed to human blood perfused at wall shear rates as high as 2000 s−1 to mimic the hemodynamic conditions of the arterial circulation. Both strains were able to induce platelet thrombus formation (Figure 1).
Induction of platelet thrombi by S aureusand S pyogenes.
(A) Human blood (donor 1) containing PPACK and mepacrine was perfused at 37°C over glass coverslips coated with the S aureusstrain Newman or the S pyogenes strain M5 Manfredo (coating suspension: 108 bacteria/mL). The 2 images, obtained by epifluorescence videomicroscopy, show platelets and platelet thrombi on an area of 45 000 μm2 after 8 minutes of perfusion at 400 s−1. (B) The corresponding surface coverage was measured after 7.5 to 8.5 minutes of perfusion at the indicated wall shear rates. The data are representative of 4 experiments with blood from different donors.
Induction of platelet thrombi by S aureusand S pyogenes.
(A) Human blood (donor 1) containing PPACK and mepacrine was perfused at 37°C over glass coverslips coated with the S aureusstrain Newman or the S pyogenes strain M5 Manfredo (coating suspension: 108 bacteria/mL). The 2 images, obtained by epifluorescence videomicroscopy, show platelets and platelet thrombi on an area of 45 000 μm2 after 8 minutes of perfusion at 400 s−1. (B) The corresponding surface coverage was measured after 7.5 to 8.5 minutes of perfusion at the indicated wall shear rates. The data are representative of 4 experiments with blood from different donors.
Fibrinogen binds to streptococcal M proteins and supports platelet adhesion and thrombus formation
Initial studies on bacteria-induced platelet aggregation were focused on S pyogenes, which expresses on its surface a class of type-specific virulence factors designated M proteins.28 Most M proteins bind fibrinogen, and all group A streptococcal strains express at least one fibrinogen-binding M protein (Figure 2A-B). After immobilization onto a glass surface, recombinant M1 and M5 proteins, which bind fibrinogen, supported platelet adhesion when exposed to whole blood perfused at a wall shear rate between 600 and 1500 s−1 (Figure 2C-D). Platelet aggregates began to form in 3 to 4 minutes and became progressively larger as perfusion continued. After 7 to 9 minutes, the area covered by platelets was similar on surfaces coated with either rM1 or rM5. In contrast, there was no platelet interaction with rM5ΔB, which lacks the fibrinogen-binding B repeats of the wild-type protein (Figure 2).19
Immobilized streptococcal M proteins support platelet adhesion and thrombus formation under flow.
(A) Schematic representation of 3 streptococcal proteins. The variant M5ΔB lacks the fibrinogen-binding B repeats. (B) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis of the recombinant proteins used to coat glass coverslips at a concentration of 100 μg/mL, visualized with Coomassie blue (stain) and radiolabeled fibrinogen binding (blot). (C) Single platelets and thrombi on an area of 45 000 μm2 after blood perfusion (donor 2) over the 3 different M proteins for 8 minutes at 1500 s−1. (D) Measurement of surface coverage by single platelets and thrombi after 8 minutes of perfusion at the indicated wall shear rates. The results shown are the average of 3 (rM1) or 2 (rM5 and rM5ΔB) experiments with blood from different donors; bars show the range of values observed.
Immobilized streptococcal M proteins support platelet adhesion and thrombus formation under flow.
(A) Schematic representation of 3 streptococcal proteins. The variant M5ΔB lacks the fibrinogen-binding B repeats. (B) Sodium dodecyl sulfate–polyacrylamide gel electrophoresis of the recombinant proteins used to coat glass coverslips at a concentration of 100 μg/mL, visualized with Coomassie blue (stain) and radiolabeled fibrinogen binding (blot). (C) Single platelets and thrombi on an area of 45 000 μm2 after blood perfusion (donor 2) over the 3 different M proteins for 8 minutes at 1500 s−1. (D) Measurement of surface coverage by single platelets and thrombi after 8 minutes of perfusion at the indicated wall shear rates. The results shown are the average of 3 (rM1) or 2 (rM5 and rM5ΔB) experiments with blood from different donors; bars show the range of values observed.
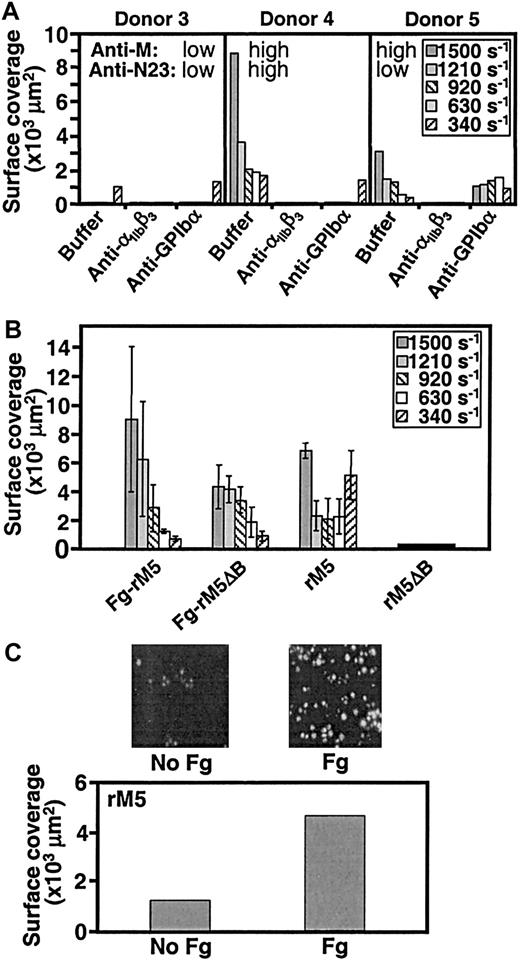
Analysis of blood from different individuals revealed a considerable variability in the extent of platelet adhesion to and thrombus formation on immobilized rM1 and rM5 (Figure3A). In some cases there was no response at the higher shear rates tested, but some degree of platelet deposition always occurred at the lower shear rates. Table1A presents a summary of the results obtained in different donors. Platelet adhesion to and aggregation on rM5 were completely abolished by the monoclonal antibody LJ-CP8, which blocks ligand binding to the platelet integrin αIIbβ3, regardless of the shear rate tested. Because the latter receptor interacts with fibrinogen but not M proteins, this result indicates that fibrinogen adsorbed from blood is primarily responsible for initiating platelet deposition onto M proteins. In contrast, the monoclonal antibody LJ-Ib1, which inhibits glycoprotein (GP) Ibα binding to VWF, had a more selective effect. In individuals who showed platelet deposition on rM5 only at low shear rate, the antibody caused no inhibition (donor 1, Figure 3A). On the other hand, the anti–GP Ibα antibody variably reduced surface coverage when platelet deposition occurred at higher shear rates, but was always without effect at the lower shear rates in the same individuals (compare donors 4 and 5, Figure 3A). Streptococcal M proteins are not known to bind VWF, thus GP Ibα is not expected to support platelet adhesion to rM5. After surface contact is established, however, plasma-derived or α granule–released VWF binds to the adherent platelets and is a required substrate for the recruitment of circulating platelets into developing thrombi under conditions of rapid blood flow.1 14 Thus, even in the absence of an effect on initial platelet-surface interactions, by inhibiting VWF-mediated platelet-platelet cohesion at the higher shear rates the anti–GP Ibα antibody may interfere with the firm attachment and growth of thrombi on immobilized streptococcal M proteins, in effect reducing surface coverage.
Role of αIIbβ3, GP Ibα, and fibrinogen in M protein–induced platelet thrombus formation.
(A) Blood (donors 3-5) was mixed for 15 minutes at room temperature (22-25°C) with buffer, or 50 μg/mL of the anti-αIIbβ3 antibody LJ-CP8, or 100 μg/mL of the anti–GP Ibα antibody LJ-Ib1, and then perfused at 37°C over streptococcal M5 protein (coating solution: 100 μg/mL). The surface coverage in an area of 45 000 μm2 was measured at the indicated shear rates after 8 minutes of perfusion. Antibody titers against rM5 (anti-M5) and an M5-specific peptide sequence (anti-N23) were determined by ELISA. (B) Blood was perfused over coverslips coated first with fibrinogen (200 μg/mL) and then rM5 or rM5ΔB (100 μg/mL), or coated directly with the bacterial proteins without fibrinogen. The results represent the mean values of 2 experiments with blood from different donors (6 and 7); bars show the range of values observed. (C) Blood cells from donor 1 were washed free of plasma proteins and then resuspended with Tyrode-HEPES buffer alone or supplemented with 2 mg/mL fibrinogen before perfusion at a shear rate of 340 s−1 over glass coverslips coated with rM5. The images show the extent of surface coverage after 8 minutes of perfusion. These results are representative of those observed in 3 separate experiments.
Role of αIIbβ3, GP Ibα, and fibrinogen in M protein–induced platelet thrombus formation.
(A) Blood (donors 3-5) was mixed for 15 minutes at room temperature (22-25°C) with buffer, or 50 μg/mL of the anti-αIIbβ3 antibody LJ-CP8, or 100 μg/mL of the anti–GP Ibα antibody LJ-Ib1, and then perfused at 37°C over streptococcal M5 protein (coating solution: 100 μg/mL). The surface coverage in an area of 45 000 μm2 was measured at the indicated shear rates after 8 minutes of perfusion. Antibody titers against rM5 (anti-M5) and an M5-specific peptide sequence (anti-N23) were determined by ELISA. (B) Blood was perfused over coverslips coated first with fibrinogen (200 μg/mL) and then rM5 or rM5ΔB (100 μg/mL), or coated directly with the bacterial proteins without fibrinogen. The results represent the mean values of 2 experiments with blood from different donors (6 and 7); bars show the range of values observed. (C) Blood cells from donor 1 were washed free of plasma proteins and then resuspended with Tyrode-HEPES buffer alone or supplemented with 2 mg/mL fibrinogen before perfusion at a shear rate of 340 s−1 over glass coverslips coated with rM5. The images show the extent of surface coverage after 8 minutes of perfusion. These results are representative of those observed in 3 separate experiments.
Anti-M5 titer and platelet adhesion to rM5 in different donors
| Donor . | Surface coverage (× 103 μm2) on immobilized rM5 . | Anti-M5 titer . | Figure . | |
|---|---|---|---|---|
| 1500s − 1 . | 340s − 1 . | |||
| 1 | 10 | 6.1 | 0.9 | 1; 4A,C; 5; 6B-D |
| 2 | 1.7 | ND | 0.2 | 2, 6E |
| 3 | 0 | 1 | <0.1 | 3A |
| 4 | 8.8 | ND | 1 | 3A |
| 5 | 3.0 | ND | 0.8 | 3A |
| 6 | 6.2 | ND | 0.8 | 3B |
| 7 | 7.2 | 3.3 | 0.8 | 3B |
| 8 | ND | 0.6 | 0.2 | 4B |
| Donor . | Surface coverage (× 103 μm2) on immobilized rM5 . | Anti-M5 titer . | Figure . | |
|---|---|---|---|---|
| 1500s − 1 . | 340s − 1 . | |||
| 1 | 10 | 6.1 | 0.9 | 1; 4A,C; 5; 6B-D |
| 2 | 1.7 | ND | 0.2 | 2, 6E |
| 3 | 0 | 1 | <0.1 | 3A |
| 4 | 8.8 | ND | 1 | 3A |
| 5 | 3.0 | ND | 0.8 | 3A |
| 6 | 6.2 | ND | 0.8 | 3B |
| 7 | 7.2 | 3.3 | 0.8 | 3B |
| 8 | ND | 0.6 | 0.2 | 4B |
ND indicates not determined.
Antibodies bound to streptococcal proteins contribute to thrombus formation by interacting with the IgG Fc receptor independently of fibrinogen binding
As shown above (Figure 2), immobilized rM5ΔB by itself failed to support platelet adhesion, indicating that streptococcal M proteins depend on bound fibrinogen to initiate platelet tethering when they are the only substrate presented to flowing blood. In contrast, rM5ΔB elicited thrombus formation when immobilized on a surface with fibrinogen (Figure 3B), although the latter by itself supports adhesion of single platelets without inducing aggregation.26Furthermore, the removal of plasma proteins from a blood cell suspension reduced the interaction of platelets with immobilized rM5, and the addition of soluble fibrinogen enhanced adhesion but was not sufficient to restore thrombus formation (Figure 3C). Together, these findings suggest that the role of M proteins in eliciting platelet aggregation cannot be explained solely by their ability to bind fibrinogen. Given the variable response observed in different individuals, and considering that FcγRIIA (the predominant Fc receptor on platelets29,30) has been implicated in the induction of aggregation by anti–platelet IgG,31 we hypothesized that antibacterial antibodies were involved in the activation of platelets exposed to immobilized M proteins. Indeed, the size of platelet thrombi formed on immobilized rM5 was directly related to the anti-M5 antibody titer in the perfused blood (Figure 3A). Of note, these antibodies were directed against conserved epitopes of M proteins and, except in one donor (donor 4; Figure 3A), failed to react against a specific sequence of M5 (anti-N23; Figure 3A).
To confirm the role of anti-M IgG and FcγRIIA in the thrombogenic response induced by streptococcal M proteins, blood from a highly reactive donor was incubated with the monoclonal antibody IV.3 to inhibit IgG binding to FcγRIIA. The antibody completely blocked rM5-induced thrombus formation (Figure4A), and the effect was specific because there was no influence on the response induced by fibrillar collagen type I (data not shown). Furthermore, when blood from an individual with a low anti-M5 titer was supplemented with M protein–specific IgG, but not IgG depleted of anti-M protein antibodies, platelet adhesion and aggregation induced by perfusion on immobilized rM5 were enhanced (Figure 4B). Finally, washed blood cells suspended in plasma depleted of anti-M5 IgG exhibited markedly reduced platelet reactivity on perfusion on immobilized rM5, but subsequent addition of anti-M5 antibodies restored platelet response almost to the levels seen with control plasma (Figure 4C).
Role of specific antibodies in M protein–induced platelet aggregation.
Surface coverage by platelets and platelet thrombi was measured in an area of 45 000 μm2 after blood perfusion at 340 s−1 over glass coverslips coated with 100 μg/mL rM5. (A) Blood from the highly reactive donor 1 was perfused after mixing with buffer (control) or the anti- FcγRIIA antibody IV.3 (20 μg/mL). (B) Blood from the poorly reactive donor 8 was perfused after mixing with buffer (control), or with polyclonal anti-M5 IgG purified from donor 1, or with the polyclonal IgG from which the anti-M protein antibodies had been removed by immunoadsorbtion. (C) Plasma-free blood cells from donor 1 were reconstituted with homologous plasma, or plasma depleted of antistreptococcal antibodies plus buffer, or depleted plasma plus either polyclonal anti-M5 IgG or immunoadsorbed IgG lacking anti-M5 antibodies, prepared as described in the legend to panel B. Where bars are displayed, the results represent the average and range of values observed in different fields of view during the same experiment.
Role of specific antibodies in M protein–induced platelet aggregation.
Surface coverage by platelets and platelet thrombi was measured in an area of 45 000 μm2 after blood perfusion at 340 s−1 over glass coverslips coated with 100 μg/mL rM5. (A) Blood from the highly reactive donor 1 was perfused after mixing with buffer (control) or the anti- FcγRIIA antibody IV.3 (20 μg/mL). (B) Blood from the poorly reactive donor 8 was perfused after mixing with buffer (control), or with polyclonal anti-M5 IgG purified from donor 1, or with the polyclonal IgG from which the anti-M protein antibodies had been removed by immunoadsorbtion. (C) Plasma-free blood cells from donor 1 were reconstituted with homologous plasma, or plasma depleted of antistreptococcal antibodies plus buffer, or depleted plasma plus either polyclonal anti-M5 IgG or immunoadsorbed IgG lacking anti-M5 antibodies, prepared as described in the legend to panel B. Where bars are displayed, the results represent the average and range of values observed in different fields of view during the same experiment.
Whole streptococci induce platelet thrombus formation with the same mechanism as isolated M proteins
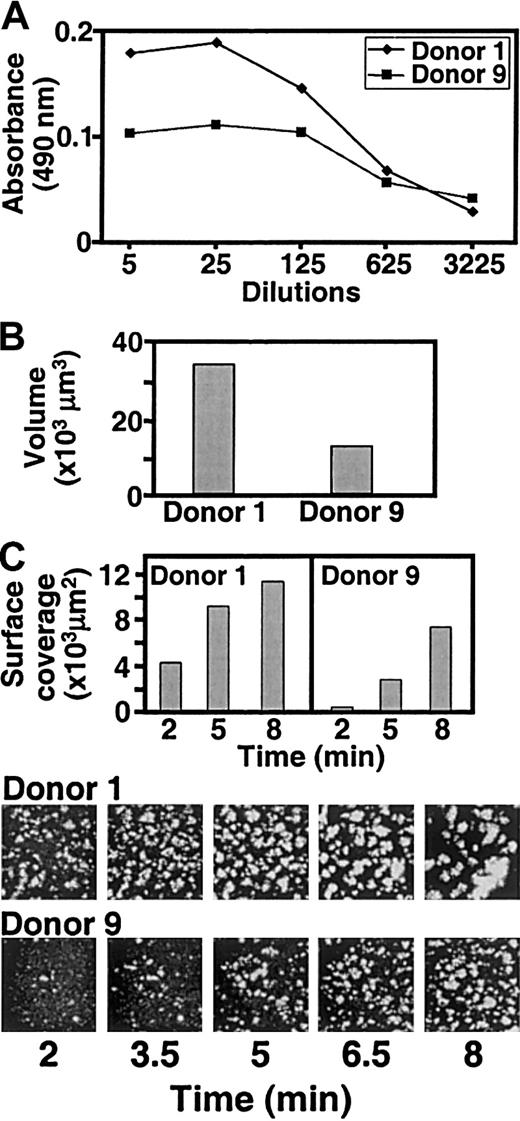
The results obtained with recombinant proteins were confirmed by experiments with S pyogenes, immobilized on glass slides coated with human serum albumin. M5 streptococci induced thrombus formation with all blood samples tested. When the anti-M5 antibody titer was low (Figure 5A), both total surface coverage by platelets (Figure 5B) and thrombus volume (Figure5C) were less than in samples with high antibody titer. Platelet adhesion and aggregation occurred on or in the immediate vicinity of streptococci, both at the highest (1600 s−1) and lowest (140 s−1) shear rates tested (data not shown). Thrombi formed faster on M5 streptococci (2-3 minutes) than on rM5 protein (6-8 minutes).
Individual variation in platelet thrombus formation induced by S pyogenes.
(A) Anti-M5 antibody titer in 2 individuals with a high (donor 1) or low (donor 9) platelet reactivity toward immobilized M5 protein. (B) M5 streptococci (coating suspension: 108 bacteria/mL) were immobilized on glass coverslips coated with human serum albumin (100 μg/mL) and then exposed to blood from the 2 donors with different anti-M5 antibody titer perfused at the wall shear rate of 340 s−1. The total volume of platelet thrombi on an area of 45 000 μm2 was measured after 9 minutes. (C) Surface coverage by platelets and platelet thrombi measured at the indicated time points using the same conditions as described in the legend to panel B. Images of the surface at different time points are also shown.
Individual variation in platelet thrombus formation induced by S pyogenes.
(A) Anti-M5 antibody titer in 2 individuals with a high (donor 1) or low (donor 9) platelet reactivity toward immobilized M5 protein. (B) M5 streptococci (coating suspension: 108 bacteria/mL) were immobilized on glass coverslips coated with human serum albumin (100 μg/mL) and then exposed to blood from the 2 donors with different anti-M5 antibody titer perfused at the wall shear rate of 340 s−1. The total volume of platelet thrombi on an area of 45 000 μm2 was measured after 9 minutes. (C) Surface coverage by platelets and platelet thrombi measured at the indicated time points using the same conditions as described in the legend to panel B. Images of the surface at different time points are also shown.
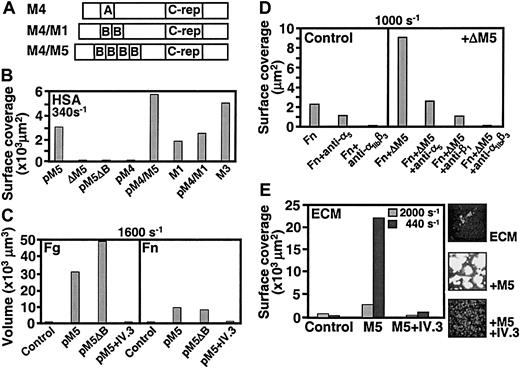
Similar to M5, streptococci expressing the fibrinogen-binding M1 and M3 proteins supported thrombus formation, as did strains expressing chimerical proteins in which the fibrinogen-binding B repeats from M1 or M5 had been grafted into the M4 protein that cannot bind fibrinogen (Figure 6A-B). In contrast, strains expressing M4, M5ΔB, or lacking expression of M proteins, such as the ΔM5 strain, failed to support platelet deposition when immobilized on a surface with albumin (Figure 6B). To study the requirements for bacteria-induced thrombus formation on a substrate capable of supporting platelet adhesion, streptococci were immobilized on slides precoated with fibrinogen. As previously observed,26 a single layer of platelets attached to fibrinogen but without development of thrombi. However, as seen with purified M proteins, streptococci expressing M5ΔB as well as M5 were able to induce thrombus formation, which was blocked by addition to the blood of the antibody IV.3 (Figure 6C). These findings support the notion that, even when whole bacteria are coimmobilized with fibrinogen, activation is solely dependent on ligation of the platelet FcγRIIA. Many bacteria, including streptococci and staphylococci, express surface structures that bind to fibronectin, a plasma and matrix protein that, by itself, has limited capacity to support adhesion of unstimulated platelets in flowing blood (Figure 6C). Nevertheless, when coimmobilized with fibronectin, group A streptococci expressing M5 or M5ΔB induced thrombus formation albeit less efficiently than when coimmobilized with fibrinogen. In either case, blocking FcγRIIA on platelets prevented thrombus formation (Figure 6C). Function-blocking monoclonal antibodies against the α5 or β1 subunits of the major fibronectin-binding integrin on platelets also inhibited thrombus formation induced by the M protein–negative strain ΔM5 immobilized on fibronectin, indicating that the latter is responsible for the initial recruitment of platelets to the bacterial environment. The inhibitory effect of the anti-αIIbβ3antibody (LJ-CP8), on the other hand, can be explained by a dual action on both adhesion to fibronectin32 and aggregation. To mimic the substrate exposed at sites of tissue injury, we also used extracellular matrix derived from human umbilical vein endothelial cells as the surface onto which bacteria were presented to flowing blood. Under these conditions, the matrix of unstimulated cells by itself supported platelet adhesion but minimal aggregation (Figure 6E). After M5 streptococci were allowed to adhere, however, platelet thrombus formation was greatly enhanced, and blocking FcγRIIA inhibited this effect (Figure 6E).
Thrombus formation induced by S pyogenesimmobilized on different substrates.
(A) Schematic representation of the chimerical proteins used to complement ΔM5 streptococci. The IgA-binding region (indicated by “A”) of the M4 protein was replaced by the 2 B-repeats (indicated by “B”) from the M1 protein or the 4 B-repeats from the M5 protein. (Panel B) Different streptococcal strains (108 bacteria/mL) were immobilized on glass coverslips coated with human serum albumin (HSA) at 100 μg/mL. Strains pM5 and pM5ΔB are ΔM5 streptococci complemented with plasmids encoding M5 and M5ΔB, respectively; strains pM4, pM4/M5, and pM4/M1 are complemented, respectively, with plasmids encoding M4 or the chimerical proteins shown in panel A; M1 and M3 are wild-type strains expressing the corresponding M proteins. Blood was perfused at 340 s−1, and surface coverage by platelets and platelet thrombi was measured after 8 minutes on an area of 45 000 μm2. (C) Strains M5 or M5ΔB, or no bacteria in the control, were immobilized on coverslips coated with fibrinogen (Fg; 200 μg/mL) or fibronectin (Fn; 100 μg/mL). Blood from the same donor as in panel B was perfused with or without addition of the anti-FcγRIIA antibody IV.3 (20 μg/mL, incubated for 15 minutes). The total volume occupied by platelet thrombi on an area of 45 000 μm2 was measured after 10 minutes of perfusion at 1600 s−1. (D) Strain ΔΜ5, or no bacteria in the controls, was immobilized on coverslips coated with fibronectin (Fn; 100 μg/mL). Blood from the same donor as in panel B was perfused with or without addition of antibodies (100 μg/mL each) against α5 (BIIG2), β1 (P5D2), or αIIbβ3 (LJ-CP8). Surface coverage by platelets and thrombi was measured after 8 minutes of perfusion at 1000 s−1. The data are representative of 3 separate experiments. (E) Strain M5, or no bacteria in the control, was immobilized on extracellular matrix (ECM) deposited by endothelial cells. Other conditions are the same as those as described in the legend to panel C, with the exception that blood was perfused at the 2 indicated wall shear rates. Surface coverage was measured as described in the legend to panel B. Representative images of the surface are also shown.
Thrombus formation induced by S pyogenesimmobilized on different substrates.
(A) Schematic representation of the chimerical proteins used to complement ΔM5 streptococci. The IgA-binding region (indicated by “A”) of the M4 protein was replaced by the 2 B-repeats (indicated by “B”) from the M1 protein or the 4 B-repeats from the M5 protein. (Panel B) Different streptococcal strains (108 bacteria/mL) were immobilized on glass coverslips coated with human serum albumin (HSA) at 100 μg/mL. Strains pM5 and pM5ΔB are ΔM5 streptococci complemented with plasmids encoding M5 and M5ΔB, respectively; strains pM4, pM4/M5, and pM4/M1 are complemented, respectively, with plasmids encoding M4 or the chimerical proteins shown in panel A; M1 and M3 are wild-type strains expressing the corresponding M proteins. Blood was perfused at 340 s−1, and surface coverage by platelets and platelet thrombi was measured after 8 minutes on an area of 45 000 μm2. (C) Strains M5 or M5ΔB, or no bacteria in the control, were immobilized on coverslips coated with fibrinogen (Fg; 200 μg/mL) or fibronectin (Fn; 100 μg/mL). Blood from the same donor as in panel B was perfused with or without addition of the anti-FcγRIIA antibody IV.3 (20 μg/mL, incubated for 15 minutes). The total volume occupied by platelet thrombi on an area of 45 000 μm2 was measured after 10 minutes of perfusion at 1600 s−1. (D) Strain ΔΜ5, or no bacteria in the controls, was immobilized on coverslips coated with fibronectin (Fn; 100 μg/mL). Blood from the same donor as in panel B was perfused with or without addition of antibodies (100 μg/mL each) against α5 (BIIG2), β1 (P5D2), or αIIbβ3 (LJ-CP8). Surface coverage by platelets and thrombi was measured after 8 minutes of perfusion at 1000 s−1. The data are representative of 3 separate experiments. (E) Strain M5, or no bacteria in the control, was immobilized on extracellular matrix (ECM) deposited by endothelial cells. Other conditions are the same as those as described in the legend to panel C, with the exception that blood was perfused at the 2 indicated wall shear rates. Surface coverage was measured as described in the legend to panel B. Representative images of the surface are also shown.
A mechanism for bacterial induction of platelet thrombus formation shared by streptococci and staphylococci
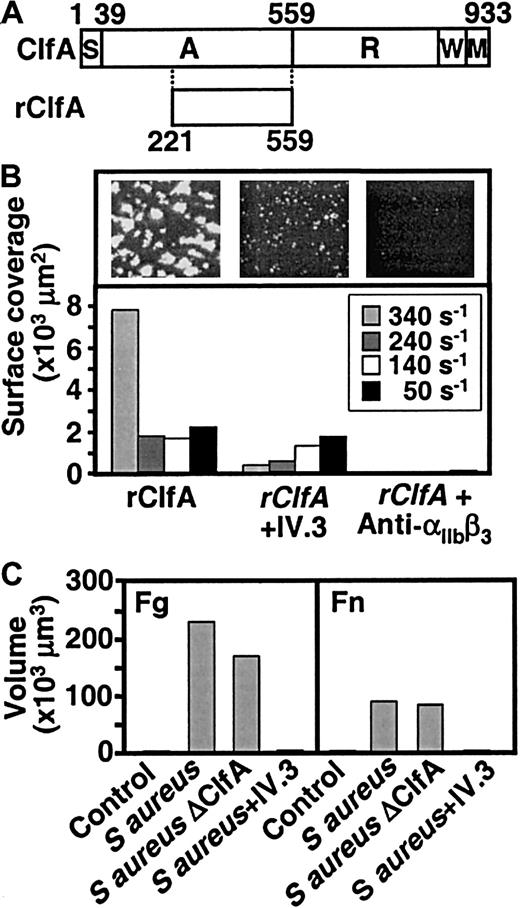
Experiments were carried out to evaluate whether thrombus formation on S aureus is induced by mechanisms similar to those that determine a platelet response to streptococci. S aureus produces several molecules capable of interacting with fibrinogen. The best known is clumping factor, a surface-associated protein that interacts with the same regions in fibrinogen that bind to αIIbβ3.18,26,33 Exposure to flowing blood of an immobilized recombinant 35-kDa fragment of clumping factor containing the fibrinogen-binding region (Figure7A)20 resulted in the formation of thrombi, which after 6 to 8 minutes were comparable in size to those induced by rM5 (Figure 7B). As with the latter, the extent of aggregation was donor dependent (data not shown), and both the anti-αIIbβ3 antibody, LJ-CP8, and the anti-FcγRIIA antibody, IV.3, inhibited the response (Figure 7B). We then tested a wild-type S aureus strain (Newman) or its isogenic derivative, ΔClfA, which contains an inactivated clumping factor gene.18 Before blood perfusion, the strains were immobilized on slides coated with either fibrinogen or fibronectin that, as noted above, by themselves support single-platelet adhesion but no significant thrombus formation in flowing blood. Both the wild-type S aureus strain and the ΔClfA strain induced platelet adhesion and aggregation (Figure 7C). Incubation of blood with the antibody IV.3 before perfusion markedly reduced aggregation induced by the wild-type S aureus strain (Figure 7C). Together, these data suggest that platelet aggregation by S aureus andS pyogenes involve similar mechanisms, including the bridging of platelets to bacteria through adsorbed fibrinogen if the substrate cannot tether platelets directly, and activation through the IgG-FcγRIIA interaction.
Platelet thrombus formation induced by S aureus and its fibrinogen-binding clumping factor.
(A) Schematic representation of clumping factor A (ClfA) and of the recombinant fragment containing the fibrinogen-binding region (rClfA) used in these experiments. (B) Blood, without or with addition of the anti-FcγRIIA antibody IV.3 (20 μg/mL) or the anti-αIIbβ3 antibody LJ-CP8 (50 μg/mL), as indicated (incubation: 15 minutes), was perfused over coverslips coated with a solution of rClfA (100 μg/mL). Surface coverage by platelets and platelet thrombi on an area of 45 000 μm2was measured after 8 minutes of perfusion at the indicated wall shear rates. Single frame images show the surface after 8 minutes of perfusion at 340 s−1. (C) Coverslips were first coated with fibrinogen (200 μg/mL) or fibronectin (100 μg/mL), followed by wild-type or ΔClfA strains of S aureus(108 bacteria/mL), or no bacteria in the control. Blood, treated as described in the legend to panel B, was perfused at 1500 s−1 for 10 minutes before measuring the volume of platelet thrombi on an area of 45 000 μm2.
Platelet thrombus formation induced by S aureus and its fibrinogen-binding clumping factor.
(A) Schematic representation of clumping factor A (ClfA) and of the recombinant fragment containing the fibrinogen-binding region (rClfA) used in these experiments. (B) Blood, without or with addition of the anti-FcγRIIA antibody IV.3 (20 μg/mL) or the anti-αIIbβ3 antibody LJ-CP8 (50 μg/mL), as indicated (incubation: 15 minutes), was perfused over coverslips coated with a solution of rClfA (100 μg/mL). Surface coverage by platelets and platelet thrombi on an area of 45 000 μm2was measured after 8 minutes of perfusion at the indicated wall shear rates. Single frame images show the surface after 8 minutes of perfusion at 340 s−1. (C) Coverslips were first coated with fibrinogen (200 μg/mL) or fibronectin (100 μg/mL), followed by wild-type or ΔClfA strains of S aureus(108 bacteria/mL), or no bacteria in the control. Blood, treated as described in the legend to panel B, was perfused at 1500 s−1 for 10 minutes before measuring the volume of platelet thrombi on an area of 45 000 μm2.
Discussion
Our studies define the sequential steps of an immune system–mediated mechanism of platelet thrombus formation that may be relevant to understand the pathogenic properties of microbes that invade the bloodstream transiently, such as S pyogenes, or persist in specific locations within the vascular system, such asS aureus. In the first step of this process, initial platelet arrest at the site of bacterial presentation can be mediated directly by surface-immobilized microorganisms that bind plasma fibrinogen, to which platelets adhere through the integrin αIIbβ3.26 This property, however, may not be necessary when invading bacteria are presented to flowing blood bound to components of an altered vascular wall or wounded tissue that can directly initiate platelet tethering. In such a situation, platelets and infectious agents become colocalized on a surface even without interacting directly with one another. Regardless of how the initial arrest takes place, adherent platelets are activated through an interaction of their IgG receptor, FcγRIIA, with specific antibodies bound to the microorganisms,34 thus initiating the recruitment of additional platelets into a developing thrombus. This latter stage of the process involves the same pathways of platelet aggregation that have been identified previously for physiologic substrates of hemostasis, because platelet-platelet cohesion in bacteria-induced thrombi requires the distinct adhesive properties of fibrinogen, VWF, and their respective membrane receptors depending on flow conditions.1,14 The present findings, therefore, clarify that fibrinogen binding to bacteria is not required for the induction of platelet aggregation, an issue that is still being debated,35 but may have a role in initiating adhesion depending on the nature of the substrate involved.
The concepts discussed here must be viewed in relation to established mechanisms that explain the potential pathogenetic role played by hemostatic alterations during invasive bacterial infections. Complications such as DIC are attributed to highly toxic species of lipopolysaccharide (endotoxin), a cell wall constituent that is released by gram-negative pathogenic bacteria such as E coliand Neisseria meningitidis.36 The role of endotoxin in DIC caused by gram-negative organisms can be mimicked in experimental animal models by injection of small quantities of the purified bacterial product.37 The effects of endotoxin on the host organism are multiple, and some are mediated through activation of hematopoietic cells or cells in the vessel lining. A key element in these processes is tissue factor released from monocytes and endothelial cells in response to endotoxin.38 It is also apparent that platelets can play an important role in endotoxin-induced DIC, not only as a catalytic surface for the generation of procoagulant species such as thrombin, but also as enhancers of tissue factor release and presentation by monocytes.39,40 Gram-positive bacteria such as S pyogenes and S aureus do not express endotoxin, but can still give rise to DIC.41Although these organisms produce substances that may mimic the effects of endotoxin, our findings demonstrate an alternative mechanism through which bacteria may interfere with the normal processes of hemostasis. The pathogenetic relevance of platelet activation by antibacterial antibodies remains to be verified in an appropriate animal model. It should be noted that S pyogenes, the focus of this study, is a strict human pathogen and the symptoms produced in experimental models may not be relevant for the human forms of disease. In fact, lethality is usually the only parameter measured following injection of very large inocula (108-1010bacteria).15,42 At the molecular level, the inability ofS pyogenes to cause infections in species other than human parallels the failure to interact with specific ligands of nonhuman origin. Studies in nonhuman primates, limited by their complexity, have been performed with S aureus41 and group A streptococci43 and, along with the results obtained in dogs exposed to S aureus,44 have typically demonstrated a complex pathology but quite clearly distinct from a typical endotoxin-induced DIC. Our present findings, therefore, provide background information that underlines the need for future experiments addressed at establishing the pathogenic role of direct platelet interactions with bacteria in vivo.
As shown here, circulating antibodies and the IgG receptor, FcγRIIA, may be the key determinants of a host response that leads to platelet aggregation at a site of bacterial invasion, even when exposed to rapidly flowing blood. The possible pathogenic role of an IgG-platelet Fc receptor pathway has been highlighted by studies with transgenic mice expressing human FcγRIIA. In these animals, administration of platelet-activating antibodies caused thrombosis accompanied by platelet consumption, a response that was absent in the control mice lacking the receptor.45 Moreover, under certain conditions, the mice expressing FcγRIIA developed shock, supporting the proposition that Fc receptor–induced platelet aggregation may contribute to the severe manifestations of bacterial invasion of the bloodstream, such as DIC and septic shock. It is important to note that these reactions required antibodies against specific platelet “activating” epitopes, in agreement with the notion that most immune-mediated platelet responses, such as autoimmune thrombocytopenic purpura and heparin-induced thrombocytopenia, involve antibodies against native or modified platelet antigens.46 47 The situation is different in the mechanism described here, because there is no evidence that the antibacterial antibodies responsible for platelet activation bind to any other surface protein except the FcγRIIA, an interaction that does not involve the specific antigen-combining site. In this regard, our results prove that the 2 stages of platelet thrombus formation on bacteria, initial adhesion and subsequent activation, are distinct, and antibodies have no necessary role in primary platelet recognition and tethering. In fact, bacteria and bacterial proteins recognized by specific IgG can activate platelets immobilized on an appropriate substrate, but cannot by themselves initiate adhesion unless they also bind a platelet-reacting protein. This observation is compatible with the concept that no primary recognition of platelet surface antigens by selected IgG is required for thrombus formation induced by infectious agents.
The ability of platelets to become activated by invading pathogens targeted by the immune system may be a relevant host defense mechanism, because platelets release antimicrobial proteins.48,49 It is now apparent, however, that this homeostatic finality may turn into a pathogenic process because any invasive microorganism that becomes immobilized, transiently or permanently, onto a platelet-reactive surface exposed to blood and is recognized by specific IgG may induce or amplify thrombus formation. Even more important, we show here that the presence of intact organisms is not necessary to induce platelet thrombi, because isolated microbial proteins against which the host has mounted an immune response are sufficient to support thrombus formation. In fact, a single antigen without direct platelet-binding capacity, here exemplified by rM5ΔB, is sufficient to trigger thrombus formation provided it is presented on the proper substrate. Hence, one can now envision a mechanism through which microbes or their remnants contribute to platelet activation with a relevance that can vary in time in different individuals depending, among other factors, on specific antibody titers that may be influenced by the recurrence of infections. As an example, these concepts add a new dimension to our understanding of the involvement that bacteria may have in the onset or progression of cardiovascular disease, including acute thrombotic events. Attention in this regard has been addressed to the isolation from atherosclerotic plaques of certain microorganisms responsible for periodontitis,50 in view of the fact that individuals with the latter oral condition have been found to be at increased risk for myocardial infarction.51 Other species implicated in the pathogenesis of cardiovascular disease and stroke areHelicobacter pylori52 and Streptococcus sanguis,53 the latter also known as the leading cause of endocarditis affecting previously damaged heart valves. In this regard, epidemiologic and clinical studies have focused on isolation of the different microorganisms and demonstration of their ability to interact with platelets and induce aggregation. Our findings indicate that the immune response to specific bacterial antigens may be an equally important parameter to evaluate in the effort to understand the pathogenetic link between infection and thrombosis.
We thank Dr Tim Foster, Trinity College, Dublin, Ireland, for supplying the bacterial strains Newman, Newman ΔclfA, and the plasmid encoding the fibrinogen-binding region of clumping factor; Dr Cameron Ashbaugh, The Channing Institute, Harvard University, Cambridge, Massachusetts, for the M3-deficient streptococcal strain; Dr Fanny Almus-Jacobs for the preparation of extracellular matrix; and Judith Dent for technical assistance.
Prepublished online as Blood First Edition Paper, August 1, 2002; DOI 10.1182/blood-2002-01-0069.
Supported by grants HL-31950, HL-42846, and HL-48728 from the National Institutes of Health; RR0833 to the General Clinical Research Institute; and by the Stein Endowment Fund (Z.M.R.); the Swedish Medical Research Council (grant no. 9926); the 80 years' Trust of King Gustaf V; the Swedish Society for Medical Research; and the Swedish Heart and Lung Association (U.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Zaverio M. Ruggeri, Roon Research Center for Arteriosclerosis and Thrombosis, Division of Experimental Hemostasis and Thrombosis, Departments of Molecular and Experimental Medicine and of Vascular Biology, MEM 175, Scripps Research Institute, 10550 N Torrey Pines Rd, La Jolla, CA 92037; e-mail: ruggeri@scripps.edu; or Ulf Sjöbring, MIG, Institute of Laboratory Medicine, BMC B14, Tornav. 10, S-221 84 Lund, Sweden; e-mail:ulf.sjobring@mig.lu.se.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal