To determine the clinical significance of BCL-2 expression in Hodgkin-Reed-Sternberg (HRS) cells of classical Hodgkin disease (cHD), we correlated its expression with presenting clinical and laboratory features and failure-free survival (FFS). Eligible patients were untreated and negative for HIV-1; they had biopsy-proven cHD. BCL-2 expression was determined immunohistochemically in available pretreatment tissue biopsy specimens without knowledge of clinical outcome. Tumors were considered positive if any HRS cells expressed BCL-2. We identified 707 patients with cHD, whose median age was 30 years; 54% were men. HRS cells expressed BCL-2 in 359 (65%) of 551 nodular sclerosis, 67 (47%) of 143 mixed cellularity, and all 5 lymphocyte depletion. For all patients, the 5-year FFS was 74% versus 84% for tumors with versus without BCL-2 expression (P = .0016, by log-rank test). For the 412 patients treated with adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) or equivalent regimens, the 5-year FFS for tumors with versus without BCL-2 expression was 74% versus 88% (P = .001, by log-rank test); for the 233 patients with Ann Arbor stage I or II, FFS was 84% versus 92% (P = .04, by log-rank test); and for the 179 patients with Ann Arbor stage III or IV, FFS was 62% versus 81% (P = .006, by log-rank test). Multivariate analysis confirmed that BCL-2 expression is independently associated with inferior FFS along with age 45 or older, Ann Arbor stage IV, low serum albumin and high serum lactate dehydrogenase levels. We conclude that BCL-2 is frequently expressed by HRS cells in cHD and is associated with inferior FFS in patients treated with ABVD or equivalent regimens.
Introduction
Even though Hodgkin disease (HD) is curable, approximately 30% of all patients relapse and eventually die of progressive disease or complications of therapy.1 Several clinical and laboratory features have been used to predict failure-free survival (FFS) and overall survival (OS) to adjust therapy according to the predicted risk of relapse. These features include age, sex, bulky peripheral or mediastinal disease, Ann Arbor stage IV, bone marrow or inguinal lymph node involvement, anemia, leukocytosis, lymphocytopenia, low serum levels of albumin, high serum levels of lactate dehydrogenase (LDH) or β2-microglobulin,2-5 serum interleukin 10, and soluble CD30.6-10 However, most models, including the International Prognostic Score (IPS),5 fail to identify a sizable fraction of patients whose chance of cure is less than 50%. Therefore, we decided to determine whether features directly related to the biology of HD can predict clinical outcome. These features may also provide a rational basis for investigational therapy.
Immunologic and molecular studies have shown that in classical HD (cHD) most Hodgkin and Reed-Sternberg (HRS) cells are derived from germinal center B cells with rearranged immunoglobulin genes but without cell surface expression of B-cell receptor.11,12This has been attributed by some investigators to crippling mutations11,13 and by others to transcriptional defects of the rearranged immunoglobulin genes in HRS cells.14 15Either mechanism would preclude the expression of functional B-cell receptor on the surface of HRS cells, which would be expected to induce apoptosis in normal germinal center cells. However, HRS cells escape apoptotic death despite no functional B-cell receptor, and the underlying salvage mechanisms for their escape are not yet known.
BCL2, first identified by its involvement in the t(14;18)(q32;q21) characteristic of follicular lymphomas,16 is a major negative regulator of apoptosis. The t(14;18) results in overexpression of BCL-2 protein and represents the first example of oncogenesis mediated by decreased cell death.17,18 The mechanism of the antiapoptotic function of BCL-2 is only partially understood, involving decreased mitochondrial release of cytochrome c, which is, in turn, required for procaspase-9 activation and initiation of the apoptotic cascade.19
Previous studies have shown that HRS cells express BCL-2 mRNA and protein20-22 even though they lack t(14;18)(q32;q31).23-25 The expression of BCL-2 by HRS cells may prevent apoptosis caused by the absence of functional B-cell receptor and, thus, explain tumorigenesis. In addition, BCL-2 expression may explain resistance to treatment-induced apoptosis of HRS cells and eventual clinical treatment failure. However, the clinical significance of BCL-2 expression in uniformly treated patients with cHD remains undefined.
Therefore, we decided to determine BCL-2 expression in HRS cells of previously untreated patients with cHD and correlate its expression with presenting clinical and laboratory features and clinical outcome. We used FFS as the primary end point, censoring all events other than tumor progression. However, we also examined OS. To minimize the effect of regimens with different efficacy, we also analyzed FFS and OS of patients treated with adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) or equivalent regimens. Our findings demonstrate that BCL-2 expression in HRS cells of cHD is an adverse biologic prognostic factor for FFS and OS and suggest that modulation of BCL-2 function may have future therapeutic implications.
Patients and methods
Patients
Patients were eligible if they were previously untreated and presented with cHD between 1984 and 2001 to one of the following institutions: University of Texas M. D. Anderson Cancer Center, United States; Istituto Nazionale Tumori, Milan and University of Verona, Italy; and the National and Kapodistrian University of Athens, Greece. This retrospective study did not require approval by the Institutional Review Boards of the participating institutions at the time the project was initiated. It was required that pathologic diagnosis be established on the basis of tissue biopsy, that tumor tissue be available for immunohistochemical determination of BCL-2 expression, and that the histologic diagnosis be confirmed by review of available slides according to criteria outlined in the World Health Organization (WHO) classification.26 In all cases, the neoplastic cells were positive for CD30 or CD15 or both. Patients with antibodies to HIV-1 by standard enzyme-linked immunoassays were excluded from analysis.
Staging
All patients had physical examination, chest radiograph, bone marrow biopsy, and computed tomography of chest, abdomen, and pelvis. When clinically indicated, lymphangiogram, gallium scan, and computed tomography of the head and neck were also obtained according to the individual practices of the participating institutions. The ratio of mediastinal mass to thoracic diameter was measured at the T4-5 interspace as previously described,2 and was considered high if 0.45 or above. The Ann Arbor stage27 and treatment according to either standard or investigational protocols were determined by the attending physician at each institution. Serum LDH levels were considered high if they exceeded the upper normal limit at each institution. Serum albumin levels were defined as low if less than 3.5 g/dL, and anemia was defined as hemoglobin less than 12 g/dL for women and less than 14 g/dL for men.2 The serum β2-microglobulin level was measured by radioimmunoassay (Pharmacia Diagnostics, Uppsala, Sweden) and was considered high if the level exceeded the upper normal limit at each institution. We also correlated FFS with these laboratory values using the cutoffs defined by Hasenclever and Diehl.5
Therapy
Treatment was either standard or according to investigational protocols that were active during the time the patient was diagnosed at the participating institutions. Informed consent (signed or oral) was obtained before all procedures and before the administration of all investigational therapy according to local practice guidelines.
Regimens included nitrogen mustard, vincristine, procarbazine, and prednisone (MOPP)28 in 61 patients; doxorubicin or epirubicin, bleomycin, vinblastine, and dacarbazine (ABVD or EBVD)29,30 in 224; MOPP alternating with ABVD1,31 in 101; cyclophosphamide, vinblastine, prednisone, procarbazine (CVPP) alternating with doxorubicin, bleomycin, dacarbazine, prednisone, and carmustine (ABDIC)32 in 69; vinblastine, etoposide, prednisone, epirubicin, and bleomycin (VEBEP)33 in 18; and mitoxantrone, vincristine, vinblastine, and prednisone (NOVP) followed by variable radiotherapy ports32 in 157 patients. Radiotherapy was administered after completion of chemotherapy in 464 patients with various ports and dosages according to local standard or investigational protocols. Fifty-nine highly selected patients with perceived favorable prognosis were treated only with curative radiotherapy according to the policies of each center. For the purposes of this analysis ABVD, EBVD, CVPP/ABDIC, and MOPP/ABVD were considered equivalent regimens.1,34 35 Kaplan-Meier analysis also revealed that patients treated with VEBEP had identical FFS with those treated with ABVD. This was confirmed by multivariate analysis adjusting for other prognostic features, in which VEBEP was not statistically different from ABVD (data not shown). Therefore, for the purposes of the present analysis, VEBEP was also considered equivalent to ABVD.
Complete remission (CR) was defined as absence of disease for at least 1 month as determined by physical examination and appropriate laboratory and imaging studies. Partial remission (PR) was defined as more than 50% reduction of tumor load, estimated as the sum of the products of 2 perpendicular diameters of all measurable lesions. Progressive disease (PD) was defined as more than 25% reduction of tumor load or development of disease in a previously uninvolved site. Primary treatment failure was defined as failure to achieve CR or PR during initial therapy. Relapse was defined as progression occurring at least 1 month after CR or PR.
Immunohistochemistry
Method.
The immunohistochemical method used to detect BCL-2 expression in HRS cells has been described previously.36 Tissue sections were deparaffinized in xylene and rehydrated in a graded series of ethanols. Endogenous peroxidase was blocked by 3% hydrogen peroxide in 140 mM NaCl, 2 mM KCl, 12 mM NaPO4, 1.7 mM KH2PO4, pH 7.4 (phosphate-buffered saline [PBS]) for 10 minutes at room temperature. Heat-induced epitope retrieval and a monoclonal antibody specific for BCL-2 (clone 124, Dako, Carpinteria, CA) in a dilution of 1:50 were used.36Each reaction set included external controls that were a BCL-2+ follicular lymphoma, and a cell block of K562 cells, which do not express BCL-2. In addition, coexisting reactive lymphocytes served as internal positive and negative controls in each slide.
Evaluation.
Evaluation of all slides was performed by 2 hematopathologists (G.Z.R. and L.J.M.) without knowledge of the clinical outcome. All slides were reviewed at the time of immunohistochemical analysis for confirmation of the diagnosis of cHD according to criteria outlined by the WHO classification.26 Slides were considered evaluable for BCL-2 if all concurrent internal and external immunohistochemistry controls stained appropriately. Any cytoplasmic BCL-2 staining of HRS cells was considered positive, based on the assumption that even few cells expressing BCL-2 might be resistant to therapy-induced apoptosis and thus cause clinical relapse. At least 100 HRS cells in representative fields were counted in each tumor to determine the percentage of HRS cells expressing BCL-2. Staining intensity was considered as absent, when no HRS cells expressed BCL-2; weak, when HRS cells stained for BCL-2 less intensely than the small reactive lymphocytes; and strong, when HRS cells stained for BCL-2 with equal or greater intensity than small reactive lymphocytes.
Statistical analysis
Failure-free survival (FFS) was measured from the beginning of treatment to primary treatment failure, relapse, or last follow-up. Patients who died during treatment without evidence of PD or after the end of therapy without prior evidence of relapse were censored at the time of these events. This censoring was also used by Hasenclever and Diehl, in their development of the IPS.5 Its purpose is to eliminate the confounding effect of deaths from neutropenia, second malignancies, cardiac or pulmonary complications of treatment, and other causes that do not provide insight in the biology of the disease. OS was measured from the beginning of treatment to the time of last follow-up or death from any cause. Actuarial survival was measured by the method of Kaplan and Meier.37 The statistical significance of differences in FFS between groups of patients was estimated by the log-rank test.38 The statistical independence between prognostic variables was evaluated by multivariate analysis using the Cox proportional hazards model.39 The comparisons between BCL-2 expression and presenting clinical and laboratory features were based on χ2 and Fisher exact tests, as appropriate. The nonparametric Mann-Whitney test was used to evaluate the correlation between patient age and BCL-2 expression. All statistical calculations were performed using StatView (Abacus Concepts, Berkeley, CA).
Results
Study group
We identified 1695 untreated patients with cHD in the participating institutions between 1984 and 2001. Archival pretreatment biopsy material was available for 707 patients who constitute the study group with known BCL-2 expression status. Most presenting clinical and laboratory features of patients with known versus unknown BCL-2 expression are similar (Table 1). However, patients with known BCL-2 status were more likely to have higher serum β2-microglobulin levels than those without available tissue, but the difference was marginally significant (P = .04, by Fisher exact test; Table 1). Therefore, we consider the analyzed group to be representative of the whole patient population. The histologic types of cHD in this study group were nodular sclerosis in 551, mixed cellularity in 143, lymphocyte depletion in 5 patients, and cHD not further classified in 8 patients.
Presenting clinical and laboratory characteristics of patients with cHD
| . | BCL-2 expression . | P* . | |||
|---|---|---|---|---|---|
| Known . | Unknown . | ||||
| No. . | % . | No. . | % . | ||
| All patients | 707 | 100 | 988 | 100 | |
| Age, y | |||||
| Median | 31 | NA | 30 | NA | .8† |
| Range | 4-88 | NA | 5-82 | NA | |
| Male sex | 383 | 54 | 540 | 55 | .8 |
| B symptoms | 258/707 | 36 | 362/985 | 37 | .9 |
| Histology | |||||
| Nodular sclerosis | 551 | 78 | 680 | 69 | |
| Mixed cellularity | 143 | 20 | 239 | 24 | |
| Lymphocyte depletion | 5 | 1 | 15 | 2 | |
| Lymphocyte-rich cHD | 0 | 0 | 3 | 0.3 | |
| cHD not further classified | 8 | 1 | 51 | 5 | |
| Ann Arbor stage | .6‡ | ||||
| I | 94 | 13 | 156 | 16 | |
| II | 358 | 51 | 482 | 49 | |
| III | 142 | 20 | 196 | 20 | |
| IV | 113 | 16 | 154 | 16 | |
| High serum LDH level | 213/627 | 34 | 204/650 | 31 | .3 |
| High serum β2-microglobulin level | 161/434 | 37 | 148/483 | 31 | .04 |
| Bone marrow involvement | 55/707 | 8 | 55/983 | 6 | .09 |
| Inguinal or iliac node involvement | 131/705 | 19 | 165/966 | 17 | .4 |
| Mediastinal mass ratio at least 0.45 | 77/502 | 15 | 70/434 | 16 | .8 |
| Low serum albumin level | 96/648 | 15 | 141/782 | 18 | .1 |
| Anemia | 313/702 | 45 | 469/968 | 48 | .1 |
| Leukocytosis | 67/702 | 10 | 116/963 | 12 | .1 |
| Lymphocytopenia | 181/637 | 28 | 216/863 | 25 | .2 |
| . | BCL-2 expression . | P* . | |||
|---|---|---|---|---|---|
| Known . | Unknown . | ||||
| No. . | % . | No. . | % . | ||
| All patients | 707 | 100 | 988 | 100 | |
| Age, y | |||||
| Median | 31 | NA | 30 | NA | .8† |
| Range | 4-88 | NA | 5-82 | NA | |
| Male sex | 383 | 54 | 540 | 55 | .8 |
| B symptoms | 258/707 | 36 | 362/985 | 37 | .9 |
| Histology | |||||
| Nodular sclerosis | 551 | 78 | 680 | 69 | |
| Mixed cellularity | 143 | 20 | 239 | 24 | |
| Lymphocyte depletion | 5 | 1 | 15 | 2 | |
| Lymphocyte-rich cHD | 0 | 0 | 3 | 0.3 | |
| cHD not further classified | 8 | 1 | 51 | 5 | |
| Ann Arbor stage | .6‡ | ||||
| I | 94 | 13 | 156 | 16 | |
| II | 358 | 51 | 482 | 49 | |
| III | 142 | 20 | 196 | 20 | |
| IV | 113 | 16 | 154 | 16 | |
| High serum LDH level | 213/627 | 34 | 204/650 | 31 | .3 |
| High serum β2-microglobulin level | 161/434 | 37 | 148/483 | 31 | .04 |
| Bone marrow involvement | 55/707 | 8 | 55/983 | 6 | .09 |
| Inguinal or iliac node involvement | 131/705 | 19 | 165/966 | 17 | .4 |
| Mediastinal mass ratio at least 0.45 | 77/502 | 15 | 70/434 | 16 | .8 |
| Low serum albumin level | 96/648 | 15 | 141/782 | 18 | .1 |
| Anemia | 313/702 | 45 | 469/968 | 48 | .1 |
| Leukocytosis | 67/702 | 10 | 116/963 | 12 | .1 |
| Lymphocytopenia | 181/637 | 28 | 216/863 | 25 | .2 |
NA indicates not applicable.
Fisher exact test was used for all comparisons except for mean age and Ann Arbor stage.
Mean age was evaluated by Mann-Whiney test.
Ann Arbor stage was evaluated by χ2 test.
BCL-2 expression
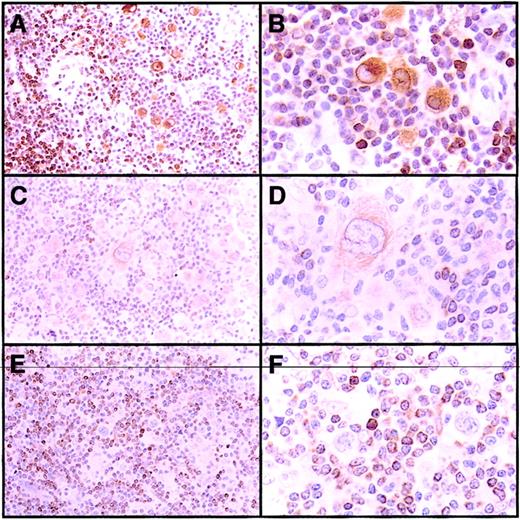
BCL-2 was detected in HRS cells of 433 (61%) of 707 cHD tumors (Table 2), where it was present in the cytoplasm of HRS cells with variable staining intensity. Reactive small lymphocytes, present in variable numbers in all tumors, were strongly positive for BCL-2 and served as internal positive controls (Figure 1).
BCL-2 expression in HRS cells according to the histologic type
| Histologic type . | No. . | BCL-2+tumors . | Intensity of BCL-2 expression . | ||
|---|---|---|---|---|---|
| No. . | % . | Weak, % . | Strong, % . | ||
| All tumors | 707 | 433 | 61 | 36 | 25 |
| Nodular sclerosis | 551 | 359 | 65* | 38 | 27† |
| Mixed cellularity | 143 | 67 | 47* | 31 | 16† |
| Lymphocyte depletion | 5 | 5 | 100 | 40 | 60 |
| Unclassified cHD | 8 | 1 | 13 | 13 | 0 |
| Histologic type . | No. . | BCL-2+tumors . | Intensity of BCL-2 expression . | ||
|---|---|---|---|---|---|
| No. . | % . | Weak, % . | Strong, % . | ||
| All tumors | 707 | 433 | 61 | 36 | 25 |
| Nodular sclerosis | 551 | 359 | 65* | 38 | 27† |
| Mixed cellularity | 143 | 67 | 47* | 31 | 16† |
| Lymphocyte depletion | 5 | 5 | 100 | 40 | 60 |
| Unclassified cHD | 8 | 1 | 13 | 13 | 0 |
P < .0001 by Fisher exact test (nodular sclerosis versus mixed cellularity).
P = .0006 by χ2 test (strong versus weak versus negative).
BCL-2 expression in HRS cells of cHD.
(A-B) Nodular sclerosis with strong expression of BCL-2 in HRS cells. (C-D) Mixed cellularity with weak BCL-2 expression in HRS cells. (E-F) Nodular sclerosis with BCL-2− HRS cells. The small reactive lymphocytes stained positively for BCL-2 and served as internal positive controls in all panels. Original magnifications A, C, E, × 100; B, D, F, × 400.
BCL-2 expression in HRS cells of cHD.
(A-B) Nodular sclerosis with strong expression of BCL-2 in HRS cells. (C-D) Mixed cellularity with weak BCL-2 expression in HRS cells. (E-F) Nodular sclerosis with BCL-2− HRS cells. The small reactive lymphocytes stained positively for BCL-2 and served as internal positive controls in all panels. Original magnifications A, C, E, × 100; B, D, F, × 400.
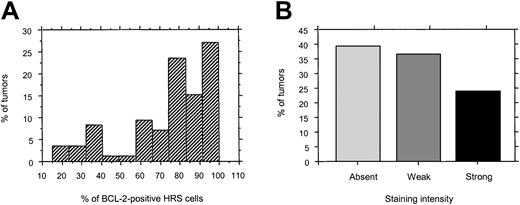
The mean percentage of BCL-2+ HRS cells (mean ± SD) was 74.3% ± 17% and ranged from 15% to 100% (median, 80%). In most positive tumors, BCL-2 was detected in the majority of HRS cells (Figure 2A). However, the intensity of BCL-2 staining in HRS cells was more variable among tumors, and expression was weak in 36% and strong in 25% (Table 2 and Figure 2B). Strong intensity of BCL-2 staining was associated with expression in a high fraction of HRS cells (P < .0001 by Mann-Whitney test).
Histograms of frequency and intensity of BCL-2 expression in HRS cells.
(A) Frequency of HRS cells expressing BCL-2 in positive tumors. (B) Staining intensity of BCL-2 in HRS cells.
Histograms of frequency and intensity of BCL-2 expression in HRS cells.
(A) Frequency of HRS cells expressing BCL-2 in positive tumors. (B) Staining intensity of BCL-2 in HRS cells.
The frequency of BCL-2 staining intensity in histologic types of HD is shown in Table 2. BCL-2 expression was more frequent (65% versus 47%;P < .0001 by Fisher exact test) and stronger in nodular sclerosis (27% versus 16%; P = .0006 by χ2test) than in mixed cellularity. BCL-2 was more frequently detected in HRS cells of younger patients, women, and patients with stage II disease or B symptoms (Table 3). The frequency of BCL-2 expression was also determined for each parameter of the IPS. As shown in Table 4, the expression of BCL-2 in HRS cells is essentially constant for all IPSs.
Correlation of BCL-2 expression in HRS cells with clinical and laboratory findings
| Parameter . | All patients . | BCL-2+ . | P3-150 . | |
|---|---|---|---|---|
| No. . | % . | |||
| Age, y | ||||
| 45 or older | 143 | 72 | 50 | .003 |
| Younger than 45 | 564 | 323 | 64 | |
| Sex | ||||
| Male | 383 | 217 | 57 | .005 |
| Female | 324 | 217 | 67 | |
| Stage | ||||
| I | 94 | 50 | 53 | .008 |
| II | 358 | 238 | 66 | |
| III | 142 | 74 | 52 | |
| IV | 113 | 72 | 64 | |
| B symptoms | ||||
| Present | 258 | 173 | 68 | .02 |
| Absent | 449 | 261 | 58 | |
| Serum albumin level | ||||
| Low3-151 | 96 | 64 | 67 | .3 |
| Normal | 552 | 336 | 61 | |
| Anemia | ||||
| Present | 313 | 198 | 63 | .4 |
| Absent | 389 | 232 | 60 | |
| Leukocytosis3-152 | ||||
| Present | 67 | 48 | 72 | .09 |
| Absent | 635 | 383 | 60 | |
| Lymphocytopenia3-152 | ||||
| Present | 63 | 40 | 63 | .7 |
| Absent | 574 | 347 | 60 | |
| Serum β2-microglobulin level | ||||
| High3-151 | 161 | 92 | 57 | .3 |
| Normal | 273 | 172 | 63 | |
| Serum LDH level | ||||
| High3-151 | 213 | 139 | 65 | .17 |
| Normal | 414 | 246 | 59 | |
| Bone marrow involvement | ||||
| Present | 55 | 29 | 53 | .2 |
| Absent | 652 | 405 | 62 | |
| Mediastinal mass ratio | ||||
| At least 0.45 | 77 | 52 | 68 | .2 |
| Lower than 0.45 | 425 | 254 | 60 | |
| Inguinal or iliac nodes | ||||
| Involved | 131 | 79 | 60 | .8 |
| Uninvolved | 574 | 354 | 62 | |
| Parameter . | All patients . | BCL-2+ . | P3-150 . | |
|---|---|---|---|---|
| No. . | % . | |||
| Age, y | ||||
| 45 or older | 143 | 72 | 50 | .003 |
| Younger than 45 | 564 | 323 | 64 | |
| Sex | ||||
| Male | 383 | 217 | 57 | .005 |
| Female | 324 | 217 | 67 | |
| Stage | ||||
| I | 94 | 50 | 53 | .008 |
| II | 358 | 238 | 66 | |
| III | 142 | 74 | 52 | |
| IV | 113 | 72 | 64 | |
| B symptoms | ||||
| Present | 258 | 173 | 68 | .02 |
| Absent | 449 | 261 | 58 | |
| Serum albumin level | ||||
| Low3-151 | 96 | 64 | 67 | .3 |
| Normal | 552 | 336 | 61 | |
| Anemia | ||||
| Present | 313 | 198 | 63 | .4 |
| Absent | 389 | 232 | 60 | |
| Leukocytosis3-152 | ||||
| Present | 67 | 48 | 72 | .09 |
| Absent | 635 | 383 | 60 | |
| Lymphocytopenia3-152 | ||||
| Present | 63 | 40 | 63 | .7 |
| Absent | 574 | 347 | 60 | |
| Serum β2-microglobulin level | ||||
| High3-151 | 161 | 92 | 57 | .3 |
| Normal | 273 | 172 | 63 | |
| Serum LDH level | ||||
| High3-151 | 213 | 139 | 65 | .17 |
| Normal | 414 | 246 | 59 | |
| Bone marrow involvement | ||||
| Present | 55 | 29 | 53 | .2 |
| Absent | 652 | 405 | 62 | |
| Mediastinal mass ratio | ||||
| At least 0.45 | 77 | 52 | 68 | .2 |
| Lower than 0.45 | 425 | 254 | 60 | |
| Inguinal or iliac nodes | ||||
| Involved | 131 | 79 | 60 | .8 |
| Uninvolved | 574 | 354 | 62 | |
All P values were calculated with the Fisher exact test, except for the P between BCL-2 expression and stage I versus II versus III versus IV, which was calculated with the χ2 test.
As defined in “Patients and methods.”
As defined by in IPS.5
Correlation of BCL-2 expression with the IPS
| IPS . | All patients . | Positive for BCL-2 . | 95% CI . | |
|---|---|---|---|---|
| No. . | % . | |||
| 0 | 95 | 61 | 64 | 54-74 |
| 1 | 231 | 137 | 59 | 53-65 |
| 2 | 120 | 76 | 63 | 55-70 |
| 3 | 97 | 56 | 58 | 47-68 |
| 4 | 35 | 22 | 63 | 45-79 |
| 5 or greater | 16 | 10 | 63 | 35-86 |
| IPS . | All patients . | Positive for BCL-2 . | 95% CI . | |
|---|---|---|---|---|
| No. . | % . | |||
| 0 | 95 | 61 | 64 | 54-74 |
| 1 | 231 | 137 | 59 | 53-65 |
| 2 | 120 | 76 | 63 | 55-70 |
| 3 | 97 | 56 | 58 | 47-68 |
| 4 | 35 | 22 | 63 | 45-79 |
| 5 or greater | 16 | 10 | 63 | 35-86 |
CI indicates confidence interval.
Survival analysis
In the entire study group of 707 patients, the 5-year FFS was 74% for tumors with BCL-2–positive HRS cells compared with 84% for tumors with HRS cells negative for BCL-2 (P = .0016 by log-rank test). Treatment was with ABVD or equivalent regimens in 412 patients and various other approaches for the remaining 295 patients. Therefore, a more detailed analysis for FFS and OS was performed using the 412 patients treated with ABVD or equivalent regimens, to eliminate the confounding effects of treatments of variable efficacy.
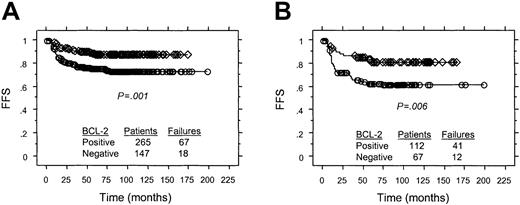
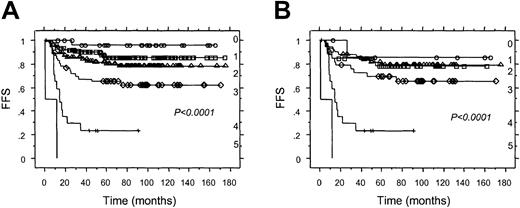
After a median follow-up of 83 months for survivors, 85 (21%) of the 412 patients treated with ABVD or equivalent regimens had refractory disease or relapsed after an initial CR or PR. The 5-year FFS for patients with cHD with BCL-2+ versus BCL-2−HRS cells was 74% versus 88% (P = .001 by log-rank test; Figure 3A). When the analysis was restricted to the 233 patients with stage I or II disease, the 5-year FFS for patients with cHD with BCL-2+ versus BCL-2− HRS cells was 84% versus 92% (P = .04 by log-rank test). Similarly, for the 179 patients with stage III or IV disease, the 5-year FFS for patients with tumors with BCL-2+ versus BCL-2− HRS cells was 62% versus 81% (P = .006 by log-rank test; Figure3B).
Associations between FFS and BCL-2 expression in HRS cells for patients treated with ABVD or equivalent regimens.
(A) All patients. (B) patients with Ann Arbor stage III or IV. ○ indicates BCL-2+ cHD; ⋄, BCL-2−cHD.
Associations between FFS and BCL-2 expression in HRS cells for patients treated with ABVD or equivalent regimens.
(A) All patients. (B) patients with Ann Arbor stage III or IV. ○ indicates BCL-2+ cHD; ⋄, BCL-2−cHD.
At 5 years, OS for patients with cHD with BCL-2+ versus BCL-2− HRS cells was 87% versus 96% (P = .005 by log-rank test); for those with stage I or II, OS was 94% versus 99% (P = .05 by log-rank test); and for those with stage III or IV the corresponding OS was 79% versus 94% (P = .008 by log-rank test).
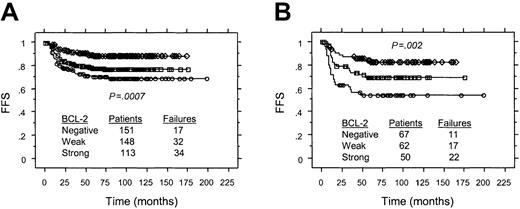
The intensity of BCL-2 staining in HRS cells also correlated with FFS in the 412 patients treated with ABVD or equivalent regimens. The 5-year FFS for patients with cHD in which HRS cells exhibited strong versus weak versus undetectable BCL-2 expression was 70%, 78%, and 89%, respectively (P < .0007 by log-rank test; Figure4A). For those patients with stage III or IV disease, the corresponding FFS was 54%, 69%, 82%, respectively (P = .002 by log-rank test; Figure 4B). Similarly, OS at 5 years for this group of patients in which HRS cells expressed BCL-2 strongly versus weakly, or were negative was 83%, 92%, and 96%, respectively (P = .001 by log-rank test).
Associations between FFS and staining intensity of BCL-2 expression in HRS cells for patients of all stages treated with ABVD or equivalent regimens.
(A) All patients. (B) patients with Ann Arbor stage III or IV. ○ indicates strong BCL-2 expression; ■, weak BCL-2 expression; ⋄, no BCL-2 expression.
Associations between FFS and staining intensity of BCL-2 expression in HRS cells for patients of all stages treated with ABVD or equivalent regimens.
(A) All patients. (B) patients with Ann Arbor stage III or IV. ○ indicates strong BCL-2 expression; ■, weak BCL-2 expression; ⋄, no BCL-2 expression.
Univariate and multivariate analysis
Univariate statistical analysis for FFS confirmed the significance of most established clinical and laboratory prognostic factors (Table5). Serum LDH levels, serum β2-microglobulin levels, anemia, and serum albumin levels were analyzed using the appropriate lower or higher limits as cutoffs. Albumin levels with a cutoff of 4.0 g/dL (which is higher than lower normal level) and hemoglobin levels at the cutoff of 10.5 g/dL (which is lower than lower normal limit) as used by the IPS5 were also prognostic in our patient population (data not shown).
Univariate statistical analysis of prognostic factors for patients with cHD treated with ABVD or equivalent regimens
| Factor . | No. of patients . | % 5-y FFS . | P5-150 . |
|---|---|---|---|
| Age, y | 997 | .005 | |
| 45 or older | 73 | ||
| Younger than 45 | 82 | ||
| Sex | 997 | .5 | |
| Male | 80 | ||
| Female | 81 | ||
| Stage | 997 | <.0001 | |
| I-III | 85 | ||
| IV | 63 | ||
| B symptoms | 997 | <.0001 | |
| Present | 73 | ||
| Absent | 86 | ||
| Serum albumin level | 821 | <.0001 | |
| Low5-151 | 67 | ||
| Normal | 83 | ||
| Anemia | 983 | <.0001 | |
| Present | 76 | ||
| Absent | 86 | ||
| Leukocytosis5-152 | 980 | .0006 | |
| Present | 70 | ||
| Absent | 82 | ||
| Lymphocytopenia5-152 | 840 | .02 | |
| Present | 71 | ||
| Absent | 80 | ||
| Serum β2-microglobulin level | 486 | .0008 | |
| High5-151 | 73 | ||
| Normal | 85 | ||
| Serum LDH level | 731 | .004 | |
| High5-151 | 76 | ||
| Normal | 84 | ||
| Bone marrow involvement | 996 | <.0001 | |
| Present | 62 | ||
| Absent | 82 | ||
| Mediastinal mass ratio | 436 | .9 | |
| At least 0.45 | 80 | ||
| Lower than 0.45 | 80 | ||
| Inguinal or iliac nodes | 987 | <.0001 | |
| Involved | 70 | ||
| Uninvolved | 84 | ||
| BCL-2 | 412 | .001 | |
| Positive | 74 | ||
| Negative | 88 |
| Factor . | No. of patients . | % 5-y FFS . | P5-150 . |
|---|---|---|---|
| Age, y | 997 | .005 | |
| 45 or older | 73 | ||
| Younger than 45 | 82 | ||
| Sex | 997 | .5 | |
| Male | 80 | ||
| Female | 81 | ||
| Stage | 997 | <.0001 | |
| I-III | 85 | ||
| IV | 63 | ||
| B symptoms | 997 | <.0001 | |
| Present | 73 | ||
| Absent | 86 | ||
| Serum albumin level | 821 | <.0001 | |
| Low5-151 | 67 | ||
| Normal | 83 | ||
| Anemia | 983 | <.0001 | |
| Present | 76 | ||
| Absent | 86 | ||
| Leukocytosis5-152 | 980 | .0006 | |
| Present | 70 | ||
| Absent | 82 | ||
| Lymphocytopenia5-152 | 840 | .02 | |
| Present | 71 | ||
| Absent | 80 | ||
| Serum β2-microglobulin level | 486 | .0008 | |
| High5-151 | 73 | ||
| Normal | 85 | ||
| Serum LDH level | 731 | .004 | |
| High5-151 | 76 | ||
| Normal | 84 | ||
| Bone marrow involvement | 996 | <.0001 | |
| Present | 62 | ||
| Absent | 82 | ||
| Mediastinal mass ratio | 436 | .9 | |
| At least 0.45 | 80 | ||
| Lower than 0.45 | 80 | ||
| Inguinal or iliac nodes | 987 | <.0001 | |
| Involved | 70 | ||
| Uninvolved | 84 | ||
| BCL-2 | 412 | .001 | |
| Positive | 74 | ||
| Negative | 88 |
Multivariate analysis using the Cox proportional hazard model revealed that BCL-2 expression in HRS cells was an independent prognostic factor. The other factors that entered the final model, along with BCL-2 expression, were age 45 years or older, Ann Arbor stage IV, low serum albumin level, and high serum LDH level (Table6 and Figure5). Identical results were obtained using stepwise forward and backward variable selection with the Cox proportional hazards model (data not shown). The variables and their cutoffs as used by Hasenclever and Diehl in the IPS5 were also tried in the multivariate analysis. Once BCL-2 was entered in the model, it was not possible to enter hemoglobin levels (at the cutoff of 10.5 g/dL), albumin levels (at the cutoff of 4.0 g/dL), leukocytosis, lymphocytopenia, and sex (data not shown). The combination of BCL-2 expression with these 4 adverse clinical features identified 68 (22%) of 314 patients of all stages who have 3 or more adverse factors and 31 of them (10%) who have a 5-year FFS lower than 60% (Figure5A). If the analysis is restricted to patients with stage III and IV disease, the combination of all 5 adverse prognostic factors revealed that 54 of 151 patients (36% of the population) had 3 or more adverse prognostic factors and 27 of them (18%) had 5-year FFS lower than 60% (Figure 5B). The model developed for FFS also identified groups of patients with statistically different OS (P < .0001 by log-rank test).
Multivariate analysis of prognostic factors for FFS in 314 patients with cHD treated with ABVD or equivalent regimens who had complete data
| Prognostic factor . | Relative risk (RR) of failure . | ||
|---|---|---|---|
| RR . | 95% CI . | P6-150 . | |
| BCL-2+ | 3.0 | 1.6-5.7 | .0005 |
| Age 45 y or older | 2.1 | 1.2-3.8 | .01 |
| Ann Arbor stage IV | 2.2 | 1.3-3.5 | .003 |
| Low serum albumin6-151 | 2.8 | 1.7-4.6 | <.0001 |
| High serum LDH6-151 | 1.9 | 1.1-3.0 | .01 |
| Prognostic factor . | Relative risk (RR) of failure . | ||
|---|---|---|---|
| RR . | 95% CI . | P6-150 . | |
| BCL-2+ | 3.0 | 1.6-5.7 | .0005 |
| Age 45 y or older | 2.1 | 1.2-3.8 | .01 |
| Ann Arbor stage IV | 2.2 | 1.3-3.5 | .003 |
| Low serum albumin6-151 | 2.8 | 1.7-4.6 | <.0001 |
| High serum LDH6-151 | 1.9 | 1.1-3.0 | .01 |
Cox proportional hazards model.
As defined in “Patients and methods.”
Association between number of adverse features and FFS in patients treated with ABVD or equivalent regimens.
(A) FFS, all Ann Arbor stages. (B) FFS, Ann Arbor stage III or IV. ○ indicates no adverse features; □, 1 adverse feature; ▵, 2 adverse features; ⋄, 3 adverse features; +, 4 adverse features; and no symbol, 5 adverse features.
Association between number of adverse features and FFS in patients treated with ABVD or equivalent regimens.
(A) FFS, all Ann Arbor stages. (B) FFS, Ann Arbor stage III or IV. ○ indicates no adverse features; □, 1 adverse feature; ▵, 2 adverse features; ⋄, 3 adverse features; +, 4 adverse features; and no symbol, 5 adverse features.
Discussion
We report that BCL-2 is expressed by HRS cells in 61% of all patients with cHD and its expression is significantly more frequent in nodular sclerosis than mixed cellularity histology. The expression of BCL-2 in HRS cells is associated with inferior FFS and OS in patients treated with ABVD or equivalent regimens. Similarly, the intensity of BCL-2 expression is associated with statistically significant different FFS and OS.
This conclusion is based on analysis of 707 patients derived from an international database that included 1695 previously untreated patients with biopsy-proven cHD. This study group was selected on the basis of the availability of archival pretreatment tissue for determination of BCL-2 expression. Statistical analysis showed that the presenting clinical and laboratory features of patients with known versus unknown BCL-2 expression were very similar. Therefore, we considered the group with known BCL-2 expression by HRS cells to be representative of the entire patient population.
For the purpose of statistical analysis, we considered tumors with any HRS cells that express BCL-2 to be positive. This choice was based on the biologic assumption that even a few BCL-2+ HRS cells might survive chemotherapy-induced apoptosis and cause a relapse. Thus, we avoided choosing any arbitrary percentage of positive HRS cells as a cutoff to define BCL-2 expression. This choice is further supported by the distribution of BCL-2+ HRS cells among tumors as shown in Figure 2B. Using these criteria, BCL-2 was expressed more frequently and more intensely in HRS cells of patients with nodular sclerosis than mixed cellularity histology. Expression of BCL-2 was also associated with age younger than 45 years, female sex, and stage II disease. These associations are expected because nodular sclerosis HD is more frequent in patients younger than 45 years, with female sex and stage II disease in our patient population (data not shown). Perhaps these associations can account for the similar FFS between nodular sclerosis and mixed cellularity histology, despite the more frequent BCL-2 expression in the former.
For FFS, we considered as events only failure to control tumor, as was done in the generation of the IPS.5 However, for OS any deaths were considered as events, even after highly variable salvage therapy. BCL-2 expression by HRS cells of cHD was associated with significantly lower FFS and OS in all patients and in those treated with ABVD or equivalent regimens. This association was also significant for patients with either early or advanced Ann Arbor stages, and cannot be explained by an association of BCL-2 expression with high IPS as shown in Table 4. Because of the small patient numbers and highly variable radiotherapy ports and treatment cycles, it was not possible to meaningfully analyze patients treated only with radiotherapy, NOVP and radiotherapy, or a variable number of MOPP cycles with or without radiotherapy.
For patients treated with ABVD or equivalent regimens, multivariate analysis demonstrated that BCL-2 expression by HRS cells in cHD was independently associated with inferior FFS (Table 6 and Figure 5). However, other factors contributed significantly to outcome. The combination of BCL-2 expression in HRS cells with age older than 45 years, stage IV, low serum albumin, and high serum LDH identifies 18% of patients with stage III or IV disease with 5-year FFS lower than 60%.
Smolewski et al40 analyzed BCL-2 expression in HRS cells of 194 patients with HD, including patients with lymphocyte-predominant histology. Using a 10% cutoff for positivity, BCL-2 was expressed in 47% of tumors.40 Although the authors did not report FFS, their analysis for all patients who received variable initial and variable salvage therapy demonstrated that BCL-2 expression was associated with statistically inferior OS.40 In another study, using a cutoff of 25% for positivity, BCL-2 was more frequently expressed in HRS cells of those patients with nodular sclerosis HD who did not achieve CR.41
Single-cell polymerase chain reaction and immunohistochemical studies have shown that HRS cells express BCL-2 mRNA and protein without carrying the t(14;18).23-25 Although previous studies demonstrated the rare presence of t(14;18) or BCL2gene rearrangements in HD,42-45 more refined analysis has revealed that these rearrangements are not present in HRS cells but in bystander small reactive B lymphocytes. The molecular basis underlying BCL-2 expression in HRS cells is not yet clarified, cannot be established by this study, and merits further investigation.
BCL-2 expression has been detected frequently in aggressive non-Hodgkin lymphomas, occurs independently of the t(14;18), and is similarly associated with unfavorable prognosis.46-49Therefore, it appears that expression of BCL-2 is associated with inferior clinical outcome in patients with either cHD or non-Hodgkin lymphoma.
We conclude that BCL-2 is expressed in HRS cells in 61% of patients with cHD. In positive tumors, the majority of HRS cells express BCL-2 but the intensity of expression is variable. BCL-2 expression is an independent adverse prognostic factor that may contribute to the failure of primary therapy and also the inability to save these patients after relapse. Other prognostic factors, in addition to BCL-2 expression in HRS cells, also contribute to the inferior clinical outcome. Furthermore, our results suggest that modulation of BCL-2 function50-52 may have a future role in the treatment of cHD.
Supported in part by Cancer Center support grant CA-16672 to the University of Texas M. D. Anderson Cancer Center. G.Z.R. is a recipient of an Alexander S. Onassis Foundation scholarship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andreas H. Sarris, c/o George Z. Rassidakis, University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Box 72, Houston, TX 77030; e-mail:gzrassidakis@mail.mdanderson.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal