Insulinlike growth factor 1 (IGF-1) has growth-promoting effects on myeloma cells in vitro as well as in vivo. In this study, we measured the concentration of IGF-1 and its major binding protein, IGF- binding protein 3 (IGFBP-3), in serum from 127 patients with multiple myeloma. Serum had been drawn at the time of diagnosis, before treatment with high-dose melphalan. IGFBP-3 in myeloma patients (1.6 ± 0.73 μg/mL; mean ± SD) was significantly decreased compared to healthy age- and sex-matched controls (2.2 ± 0.42 μg/mL). However, IGFBP-3 had no prognostic value in this study. The mean IGF-1 level did not differ between myeloma patients (17.8 ± 7.7 nM) and controls (17.3 ± 5.6 nM). Nevertheless, IGF-1 was a strong indicator of prognosis. After 80 months of follow-up, myeloma patients with low levels (< 13 nM) of serum IGF-1 had not reached median survival. In the patient group with IGF-1 levels above 13 nM, median survival was 62 months (P = .006). These findings support the hypothesis of a role for IGF-1 in myeloma disease progression.
Introduction
Multiple myeloma (MM) is a B-cell malignancy that is characterized by accumulation of clonal plasma cells in the bone marrow. The disease is associated with production of monoclonal immunoglobulins and painful bone destruction. In recent years, the role of cytokines in the development of MM has been the focus of many studies. Although interleukin 6 (IL-6) is the best documented growth factor in this malignancy, it is now clear that several cytokines induce MM cell proliferation and possibly promote disease development.
Insulinlike growth factor (IGF) is mainly produced by hepatocytes in response to growth hormone stimulation but is also produced locally in many other tissues (eg, by chondroblasts, fibroblasts, and osteoclasts).1 IGF-1 can be detected in high (nanomolar) concentrations in serum, where 90% is bound to IGF-binding protein 3 (IGFBP-3). This protein inhibits IGF-1 by rendering it inaccessible to the receptor.1 Substantial evidence suggests a role for IGF-1 in the growth and survival of MM cells. In vitro,IGF-1 induces proliferation of several MM cell lines.2-4 IGF-1 can also enhance the growth-promoting effect of IL-6 on MM cells.2,3,5 MM cell lines express the IGF-1 receptor, and stimulation of the receptor activates a distinct signal transduction cascade. This results in proliferation of MM cells as well as protection against apoptosis.6 A mouse model of myeloma indicates that IGF-1 in the bone marrow can act as a chemoattractant for myeloma cells in vivo.7 This suggests that IGF-1 may contribute to the recruitment and homing of myeloma cells to the bone marrow compartment. Moreover, in mice with severe combined immunodeficiency inoculated with MM cells, administration of IGF-1 increases the growth rate of the tumor cells.6
The probable functional interaction between IGF-1 and MM cells made us examine the levels of IGF-1 and IGFBP-3 in serum of patients with MM and to compare them to the concentration in healthy controls. We further wished to relate serum levels of IGF-1 and IGFBP-3 to other disease variables at the time of diagnosis and to disease outcome. In a few patients, serum samples drawn at intervals during the course of the disease were analyzed retrospectively. We here demonstrate that in a large, well-defined population of patients with MM the levels of IGFBP-3 in serum are decreased. We further show that patients with low IGF-1 levels have a favorable disease prognosis.
Patients, materials, and methods
Treatment protocol
The Nordic Myeloma Study Group (NMSG) 5/94 protocol is described in detail elsewhere.8 Briefly, induction therapy with 3 courses of vincristine/vinblastine and dexamethasone was followed by peripheral stem cell harvest. After high-dose therapy with a single dose of intravenous melphalan, stem cells were reinfused. Interferon-α2b was given as maintenance therapy.
Patients
Fourteen centers in Denmark, Norway, and Sweden participated in the study. In the regions served by the centers all newly diagnosed, symptomatic myeloma patients younger than 60 years of age were registered during the inclusion period; 348 patients were registered. Of these, 274 patients were entered in the specified intensive-therapy protocol in the period from March 1994 until June 1997. Of the 274 patients entered, 214 followed the intended high-dose protocol with autologous stem cell support. The diagnostic and eligibility criteria, reasons for nonentry, and results are described elsewhere.8 Serum samples from 127 of the 274 entered patients were available, and these constitute the study population. Of these 127 patients, 94 followed the intended high-dose protocol with stem cell support.
The median age of the patients (82 men, 45 women) in the study population was 52 years (range, 28-59 years). The distribution of myeloma characteristics with respect to monoclonal component (M-protein) type was IgG in 66%, IgA in 18%, IgD in 1.6%, and light chains only in 14%. According to the staging of Durie and Salmon,34 1.5% of the patients were in stage I, 29.5% in stage II, and 69% in stage III disease. The median survival was 68 months. None of these characteristics differed significantly from the original population of 274 patients.
Registered parameters at diagnosis were age, sex, Durie-Salmon stage, World Health Organization (WHO) performance status, grade of bone morbidity (3 severity levels as judged by x-ray analyses), percentage of plasma cells in the bone marrow, immunoglobulin class, urine immunoglobulin per 24 hours and serum M-protein concentration, blood hemoglobin, serum albumin, serum calcium, serum creatinine, serum lactate dehydrogenase, serum β2-microglobulin, C-reactive protein in serum, number of leukocytes and thrombocytes, and hepatocyte growth factor (HGF).
Four additional patients from whom serum had been drawn at regular intervals (> 1 sample/10 months) were included for a detailed analysis of change in IGF-1 and IGFBP-3 over time. Serum M-protein concentration and treatment periods were registered from their medical records. These patients received treatment according the NMSG 5/94 protocol and samples from them were obtained from the Section of Hematology, University Hospital of Trondheim.
Control samples were obtained from 42 healthy age- and sex-matched individuals.
IGF-1 and IGFBP-3 measurements
Serum IGFBP-3 was measured by enzyme-linked immunosorbent assay (ELISA). An antibody pair (R & D Systems, Minneapolis, MN) was used according to the manufacturer's instructions, with the modification that horseradish peroxidase (HRP)–conjugated streptavidin from Diaclone (Besançon, France) was used to increase sensitivity. The standard curve was linear between 2 and 40 ng/mL, and samples were diluted to concentrations within this range. All samples were run in duplicate. Human IgG does not interfere with the assay. The intra-assay and interassay variation coefficients were 3.3% and 5.5%, respectively. Total serum IGF-1 was measured by IGF-1 immunoradiometric assay (IRMA; Nichols Institute Diagnostics, San Juan Capistrano, CA) at the Department of Clinical Chemistry at the University Hospital of Trondheim, Norway. The detection limit was 7 nM IGF-1. IGFBP-3 does not interfere with analysis. Intra-assay and interassay variation coefficients were 4% and 11.6%, respectively.
Statistical analyses
All statistical analyses were done with the SPSSX/PC computer program (SPSS, Chicago, IL). Results were considered statistically significant when P ≤ .05. Skewed variables were transformed by the natural logarithm before making analyses requiring normal distribution. Comparisons between groups were performed with the Student t test or one-way ANOVA analyses. Correlation between 2 parameters was estimated by the Spearman rank correlation analysis. For investigation of linear correlations multiple regression analysis was performed. Survival was modeled with the Cox regression analysis, variables were entered by forward selection where entry required a maximum P of .05. The method of Kaplan and Meier was used to compute the survival curves and to estimate the median survival. To compare the survival curves (test for significance) the log-rank test was used.
Results
Serum analyses
Serum IGF-1 and IGFBP-3 levels in patients at the time of diagnosis and in controls are shown in Figure1. The IGF-1 concentrations in myeloma and control sera were 17.8 ± 7.7 nM (mean ± SD) and 17.3 ± 5.6 nM, respectively (Figure 1A). This difference was not significant. The mean IGFBP-3 concentration was 1.6 ± 0.73 μg/mL in myeloma patients and 2.2 ± 0.42 μg/mL in control sera (Figure1B). This difference was highly significant (P < .001). Serum levels in 41% of the patients were lower than mean −2 SD in the control population (< 1.4 μg/mL), and could therefore be considered abnormal by conventional criteria.
Serum IGF-1 and IGFBP-3 levels in patients with MM and healthy controls.
(A) Serum IGF-1 levels in 127 patients with MM (17.8 ± 7.7 nM) and 42 healthy controls (17.3 ± 5.6 nM). This difference was not significant. Horizontal lines indicate mean levels. (B) Serum IGFBP-3 levels in 127 patients with multiple myeloma (1.6 ± 0.73 μg/mL) and 42 healthy controls (2.2 ± 0.42 μmL). This difference was highly significant (P < .001). Horizontal lines indicate mean levels.
Serum IGF-1 and IGFBP-3 levels in patients with MM and healthy controls.
(A) Serum IGF-1 levels in 127 patients with MM (17.8 ± 7.7 nM) and 42 healthy controls (17.3 ± 5.6 nM). This difference was not significant. Horizontal lines indicate mean levels. (B) Serum IGFBP-3 levels in 127 patients with multiple myeloma (1.6 ± 0.73 μg/mL) and 42 healthy controls (2.2 ± 0.42 μmL). This difference was highly significant (P < .001). Horizontal lines indicate mean levels.
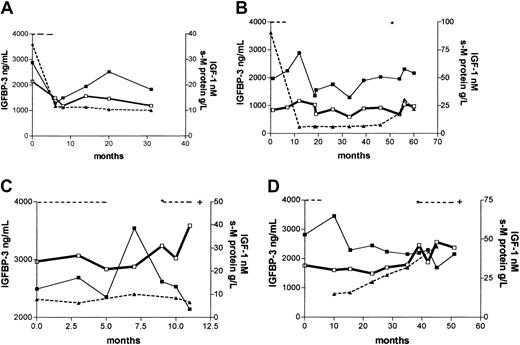
In 4 patients, consecutive serum samples from the time of diagnosis until point of death or last follow-up were analyzed. Patient no. 1 (Figure 2A) was followed for 2.5 years and had normal IGFBP-3 (2.9 μg/mL) and IGF-1 (21.4 nM) levels at diagnosis. The patient responded to treatment with a fall in serum M-protein levels of more than 50%, and a concomitant reduction in IGF-1 levels of 30% was observed. IGFBP-3 levels temporarily decreased (1.3 μg/mL), rising to levels within “normal range” 4 months after treatment. Levels of serum M-protein and IGF-1 remained stable throughout the follow-up period.
Serial measurements of IGF-1 and IGFBP-3 from diagnosis to death or last follow-up in 4 MM patients.
Serum M-protein levels were derived from medical records. For details, see “Results.” Dotted line indicates treatment periods; asterisk, initiation of new treatment; IGFBP-3, ▪; s-M protein, ▴; IGF-1, ■; and +, death.
Serial measurements of IGF-1 and IGFBP-3 from diagnosis to death or last follow-up in 4 MM patients.
Serum M-protein levels were derived from medical records. For details, see “Results.” Dotted line indicates treatment periods; asterisk, initiation of new treatment; IGFBP-3, ▪; s-M protein, ▴; IGF-1, ■; and +, death.
Patient no. 2 (Figure 2B) had normal IGFBP-3 (2 μg/mL) and IGF-1 (21 nM) at the time of diagnosis. He responded to treatment with a fall in serum M-protein level of 90%. The serum M-protein levels remained stable for 4 years after treatment, but then increased (relapse). IGFBP-3 and IGF-1 levels varied within normal range during the follow-up period of 5 years.
Patient no. 3 (Figure 2C) had normal IGFBP-3 (2.5 μg/mL) and IGF-1 (24.1 nM) levels at the time of diagnosis. The serum M-protein levels were stable until death 11 months after diagnosis. The patient responded to treatment as judged by clinical observations, but within 2 months after termination of treatment new treatment (chemotherapy) was initiated due to relapse. IGFBP-3 levels increased in the responsive period (3.5 μg/mL), and thereafter tended to decline. IGF-1 levels increased the last month before death.
Patient no. 4 (Figure 2D) had a normal IGFBP-3 level (2.8 μg/mL) at diagnosis. IGF-1 concentration was 33 nM, which is above “normal range.” The patient did not respond to treatment, and both serum M-protein levels and IGF-1 levels increased until death 4 years after diagnosis. IGFBP-3 levels declined, but remained within “normal range” (above 1.4 μg/mL) during the whole period.
Correlation with other parameters
We wished to correlate IGF-1 and IGFBP-3 at diagnosis to other registered parameters (see “Patients, materials, and methods”). IGF-1 correlated significantly only to IGFBP-3 (r = 0.39,P < .001). IGF-1 was likewise correlated to IGFBP-3 in the healthy population (r = 0.45, P = .004).
IGFBP-3 correlated significantly to IGF-1 (r = 0.39,P < .001), the concentration of M-component in serum (r = −0.23, P = .008), serum lactate dehydrogenase (r = 0.20, P = .03), concentration of light chain in urine (r = 0.2, P = .03), and serum albumin (r = 0.18,P = .05). IGFBP-3 was also related to categorical variables. IGFBP-3 levels were higher in myeloma patients with “light chains only” (n = 18, mean 2.3 ± 0.8 μg/mL) than in patients with a serum M-component (n = 110, mean 1.6 ± 0.7 μg/mL;P = .003). Also, IGFBP-3 was slightly higher in female patients (mean 1.9 ± 0.8 μ/mL) than in male patients (1.5 ± 0.7 μg/mL, P = .03). This phenomenon was not seen in the control group (data not shown). No significant relations were found to the other categorical variables, including type of light chain in urine, disease stage, WHO status, or number of radiographic skeletal lesions.
By forward selection of significantly related variables, a multivariate linear regression analysis yielded IGF-1 and presence or absence of serum M-component as the best predictors of IGFBP-3 (adjusted r2 = 0.19).
Overall survival
IGFBP-3 did not predict mortality in a univariate Cox regression analysis. Patients with low levels of IGFBP-3 had a survival similar to those with normal IGFBP-3 levels (data not shown).
When IGF-1 values for all 127 included patients were entered in a univariate Cox regression analysis, IGF-1 was a significant predictor of overall survival (P = .004). Of the 127 patients, 94 received the intended high-dose protocol with stem cell support, and IGF-1 was likewise a predictor of mortality in this group (P = .02). Therefore, IGF-1 was entered as a continuous variable in a multivariate Cox regression analysis together with the other factors that held significant (P < .05) prognostic information: natural logarithm (Ln) of β2-microglobulin (P < .001), hemoglobin (P = .001), and albumin (P = .03). Data from 108 patients were available for this analysis. Table 1 demonstrates the result of the multivariate Cox regression. Only 3 factors retained prognostic significance: Ln (β2-microglobulin;P = .01), hemoglobin (P = 0.01), and IGF-1 (P = .02). The χ2 of the model was 25.
Variables with independent prognostic importance for survival according to a multivariate Cox regression analysis
| Variable . | β coefficient . | SE . | P . |
|---|---|---|---|
| Ln (β2-microglobulin) | 0.53 | 0.21 | .01 |
| Hemoglobin | −0.02 | 0.008 | .01 |
| IGF-1 | 0.04 | 0.02 | .02 |
| Variable . | β coefficient . | SE . | P . |
|---|---|---|---|
| Ln (β2-microglobulin) | 0.53 | 0.21 | .01 |
| Hemoglobin | −0.02 | 0.008 | .01 |
| IGF-1 | 0.04 | 0.02 | .02 |
IGF-1 was further evaluated as a dichotomous variable with respect to survival. The best separation of curves was seen with the cutoff point at the 25th percentile of IGF-1 levels. As shown in Figure3A, there was a highly significant survival difference between patients with “low” IGF-1 (< 13 nM) compared to the remaining patients (IGF-1 ≥ 13 nM). Median survival was not reached in the patient group with low IGF-1, compared to a median survival of 62 months in the remaining patients (P = .006). This difference was also evident in the 94 patients treated with the intended protocol (median survival not reached compared to 70 months; P = .03; Figure 3B). Follow-up periods did not differ between the groups.
Kaplan-Meier survival plots for patients with MM.
The curves are separated by IGF-1 levels: “low” IGF-1 (25th percentile, < 13 nM) compared to remaining patients (IGF-1 ≥ 13 nM). Follow-up periods did not differ between the groups. (A) The 127 patients entered in the study. Median survival was not reached in the patient group with low IGF-1 (n = 32), compared to a median survival of 62 months in the remaining 95 patients (P = .006). (B) This difference was also evident in the 94 patients treated with the intended protocol. Median survival was not reached in the patient group with low IGF-1 (n = 27) compared to a median survival of 70 months in the remaining 67 patients; P = .03).
Kaplan-Meier survival plots for patients with MM.
The curves are separated by IGF-1 levels: “low” IGF-1 (25th percentile, < 13 nM) compared to remaining patients (IGF-1 ≥ 13 nM). Follow-up periods did not differ between the groups. (A) The 127 patients entered in the study. Median survival was not reached in the patient group with low IGF-1 (n = 32), compared to a median survival of 62 months in the remaining 95 patients (P = .006). (B) This difference was also evident in the 94 patients treated with the intended protocol. Median survival was not reached in the patient group with low IGF-1 (n = 27) compared to a median survival of 70 months in the remaining 67 patients; P = .03).
Discussion
IGF-1 is an important growth and antiapoptotic factor for the cancer cells in several malignancies.9-14 In colorectal adenomas and breast cancer IGF-1 in serum has prognostic value.15 16 Here we demonstrate that IGF-1 is a prognostic factor in MM.
We found that healthy individuals and myeloma patients at diagnosis have similar amounts of IGF-1 in the circulation. Nevertheless, MM patients with low serum IGF-1 levels have a favorable prognosis. This finding is in keeping with the observed biologic effects of IGF-1, which includes promotion of myeloma cell growth and survival. It is possible that in patients with low levels of IGF-1, paracrine growth stimulation of myeloma cells is minimal due to reduced availability of IGF-1 in the bone marrow microenvironment. In this way reactivation of the malignant clone after completion of chemotherapy could possibly be delayed.
Serum levels of IGF-1 are increased in patients with breast cancer compared to healthy individuals16,17 although this is not the case in all studies.18 Also, in patients with high-risk colorectal adenomas, serum IGF-1 levels seem to be elevated compared to patients with normal colonoscopy.15 In this study IGF-1 levels hold prognostic value even though the serum IGF-1 concentrations in the group of patients with MM did not differ from those of the healthy population. This is in contrast to other prognostic markers in myeloma, for example, IL-6,19 serum β2-microglobulin,20syndecan-1,21 and HGF,22 where serum levels are elevated in the patient group. Possibly patients with an innate high IGF-1 production have a microenvironment favorable for MM cell growth. Several population/epidemiologic studies have suggested that high premorbid serum levels of IGF-1 may predispose to some types of cancer, for example, breast cancer in premenopausal women,23 colon cancer,24 and prostate cancer.25 Whether this is also true in MM remains to be elucidated.
We correlated IGF-1 to a number of disease parameters registered at diagnosis, including the concentration of serum M-component, β2-microglobulin in serum, and the percentage of plasma cells in the bone marrow. The fact that IGF-1 did not correlate to markers of tumor burden or clinical status may suggest that levels of this cytokine do not reflect the intrinsic malignancy of the myeloma clone. Indeed, IGF-1 is not known to be produced by myeloma cells, and it is likely that the IGF-1 to which myeloma cells respond is derived from stromal cells and osteoblastic cells in the bone marrow microenvironment. In this study IGF-1 correlated significantly to its binding protein IGFBP-3, both in patients and controls, even though IGFBP-3 is reduced in the patient group.
The bioavailability of IGF-1 is in part regulated by binding proteins. Of these IGFBP-3 is the most abundant, binding 90% of IGF-1 in the circulation. IGF-1 action is inhibited when it is bound to IGFBP-3. Enhanced proteolysis of IGFBP-3 has been observed in patients with breast cancer,26 colon cancer,27 and prostate cancer.28 The resulting fragments have a reduced binding affinity for IGF-1, as seen when IGFBP-3 is cleaved by, for example, plasmin.29 The expression of proteases cleaving IGFBP-3 by MM cells could provide an explanation for the reduced levels of IGFBP-3 in patients with MM found in this study. Urokinase-type plasminogen activator (uPA) is a serine protease capable of converting plasminogen to its active form plasmin, which then cleaves IGFBP-3. Myeloma cells express both uPA and its receptor uPAR.30 Another protease associated with MM31 and known to digest IGFBP-3 is ADAM 12 (adisintegrin andmetalloprotease domain 12).32 33 One could speculate that reduced levels of IGFBP-3 increase the amount of biologically active IGF-1 and in this way contribute to increased cancer cell growth. However, the biologic relevance of the reduced levels of IGFBP-3 is uncertain, because IGFBP-3 had no prognostic value in this study.
IGF-1 and IGFBP-3 in serial samples from a small number of myeloma patients were also studied. We wished to examine if there was a trend toward increasing IGF-1 levels and decreasing IGFBP-3 levels in periods of active disease. However, as seen in Figure 2, no clear picture emerged from these analyses. As for IGF-1 this may not be surprising because IGF-1 is probably not produced by the myeloma cells and not related to tumor burden. However, a well-defined, prospective approach in the examination of serial patient samples is needed to reach a conclusion on this matter.
This study highlights the importance of IGF-1 and its binding protein in the setting of MM. The in vivo and in vitro biologic effects of IGF-1 in MM seem to translate into a favorable prognosis for patients with low IGF-1 levels in serum.
We are grateful to Anne Hole at the Department of Clinical Chemistry at the University Hospital of Trondheim, Norway for IGF-1 laboratory analyses.
Other members of the Nordic Myeloma Study Group responsible for trial no. 5/94, include the following individuals. Sweden: Martin Hjorth, Lidköping; Ingemar Turesson, Malmö; Jan Westin, Lund; Kristina Carlson, Uppsala; Margaretha Carlsson, Lindköping; Eva Löfvenberg, Umeå; Stig Rödjer, Göteborg.
Norway: Lorentz Brinch, Oslo; Inger Marie S. Dahl, Tromsø; Jon Lamvik, Trondheim; Ingerid Nesthus, Bergen.
Denmark: Johan Lanng Nielsen, Århus; Peter Gimsing, København; Erik Hippe, Herlev; Hans Johnsen, Herlev.
Prepublished online as Blood First Edition Paper, July 18, 2002; DOI 10.1182/blood-2002-05-1406.
A complete membership list of the Nordic Myeloma Study Group appears in the “.”
Supported by grants from the Norwegian Cancer Society (T.S., C.S., M.B., A.S.); the Norwegian Research Council (grant 139615/300); the Cancer Fund, St Olav's Hospital, Trondheim; the Swedish Cancer Society (C.S.); and the Wallenberg Foundation (C.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Therese Standal, Norwegian University of Science and Technology, Institute of Cancer Research and Molecular Biology, Olav Kyrresgt 3, N-7489 Trondheim, Norway; e-mail:therese.standal@medisin.ntnu.no.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal