Abstract
We have hypothesized that in aplastic anemia (AA) the presence of antigen-specific T cells is reflected by their contribution to the expansion of a particular variable beta chain (Vβ) subfamily and also by clonal CDR3 skewing. To determine the role of disease-specific “signature” T-cell clones in AA, we studied preferential Vβ usage by flow cytometry and analyzed Vβ-CDR3 regions for the presence of oligoclonality. We first established the contribution of each Vβ family to the total CD4+ and CD8+ lymphocyte pool; in AA and paroxysmal nocturnal hemoglobinuria, a seemingly random overrepresentation of different Vβ families was observed. On average, we found expansion in 3 (of 22 examined) Vβ families per patient. When the contribution of individual Vβ families to the effector pool was examined, more striking Vβ skewing was found. Vβ-CDR3 size distribution was analyzed for the expanded Vβ families in isolated CD4+ and CD8+ populations; underrepresented Vβ families displayed more pronounced CDR3 skewing. Expanded CD4+Vβ subfamilies showed mostly a polyclonal CDR3 size distribution with only 38% of skewing in expanded Vβ families. In contrast, within overrepresented CD8+Vβ types, marked CDR3 skewing (82%) was seen, consistent with nonrandom expansion of specific CD8+ T-cell clones. No preferential expansion of particular Vβ families was observed, in relation to HLA-type. In patients examined after immunosuppressive therapy, an abnormal Vβ-distribution pattern was retained, but the degree of expansion of individual Vβ was lower. As Vβ skewing may correlate with relative Vβ size, oligoclonality in combination with numerical Vβ expansion can be applied to recognition of disease-specific T-cell receptors.
Introduction
Historically, the unexpected improvement of hematopoietic function in some patients with aplastic anemia (AA) failing engraftment after bone marrow (BM) transplantation1led to the systematic application of immunosuppressive therapies, with most patients now showing hematologic recovery without transplantation.2-4 Many laboratory data have been accumulated to support an immunologic mechanism in idiopathic AA.5-15 Recently, molecular techniques have been applied in the study of normal and pathologic immune responses. These methods allow analysis of the T-cell repertoire using polymorphism within the complementarity-determining region 3 (CDR3) of the beta variable (Vβ) chain of the T-cell receptor (TCR; TCR-Vβ).16-18 Such an “immunoscope” approach provides a more direct view of the pattern of the T-cell response and has been used to study autoimmune diseases, including multiple sclerosis,19 rheumatoid arthritis,20,21 systemic lupus erythematosus,22 type 1 diabetes mellitus,23thyroiditis,24 primary biliary cirrhosis,25psoriasis,26 and graft-versus-host disease.27-30 Subsequently, similar studies have been performed in AA.31-33 Although specific Vβ patterns common to all the patients could not be identified because of HLA heterogeneity, oligoclonality in the TCR-Vβ CDR3 size distribution pattern was documented for certain Vβ families in many patients with AA. Abnormal TCR-Vβ repertoires were also found in other related BM failure syndromes including myelodysplastic syndromes34 and paroxysmal nocturnal hemoglobinuria (PNH),35 suggesting a common immune-mediated pathophysiology. Nonrandom skewing in a large proportion of Vβ families CDR3 size spectrum has suggested that different antigens may drive the immune process in individual patients.33 We have reported that it is possible to identify and characterize dominant T-cell clonotypes that likely represent responses to disease-specific antigens.36However, these initial studies also demonstrated that, given the HLA diversity and number of Vβ families potentially involved, a broad scale analysis of multiple clonotypes in many patients is not practicable using cloning approaches and has prompted us to search for other ways of analyzing the complex nature of changes in the T-cell repertoire in AA.
Our strategy was designed for systematic detection of disease-specific “signature” T-cell clones based on several assumptions, each supported by experimental evidence in human and animal models. We hypothesized that limiting the immunoscope approach to abnormally expanded Vβ families could provide a more specific method to identify pathologic clonotypes, eliminating the bias of skewing because of numerical contraction of the T-cell pool. Using this strategy we first identified overexpanded Vβ families by systematic application of flow cytometry (Vβ skewing), established oligoclonality of expanded Vβ families by using molecular techniques, and, subsequently, characterized the specific predominant CDR3 clonotypic (CDR3 skewing) sequences for both CD4+ and CD8+ lymphocyte subsets. Selection of disease-specific clonotypes may be useful in the analysis of antigens driving the immune process in AA and may be applied to classify distinct forms of AA and to monitor the response of individual patients to therapy.
Patients and methods
Patients and control subjects
We studied samples obtained from 23 subjects with AA and 10 patients with PNH or the AA/PNH syndrome. In 12 cases, serial samples were examined before and after immunosuppressive therapy. Initial samples were peripheral blood (PB) obtained at the time of presentation of the disease, before any treatment (except for transfusional support). Informed consent for venipuncture was obtained according to protocols approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute (Bethesda, MD). The diagnosis of AA was by BM biopsy and PB cell counts according to the International Study of Aplastic Anemia and Agranulocytosis37; severity was classified by the criteria of Camitta.38 For the diagnosis of severe AA, in addition to hypocellular BM without evidence of karyotypic abnormalities or morphologic dysplasia, patients had to fulfill 2 of 3 PB criteria: absolute neutrophil count less than 500/μL, absolute reticulocyte count less than 60 000/μL, and platelet count less than 20 000/μL. Clinical characteristics of the patients studied are summarized in Table1. Patients were subgrouped according to the presence of commonly encountered HLA alleles such as HLA-A2 (HLA-A*02) and HLA-DR2 (HLA-DRB1*15).
Clinical features of patients participating in the study
| . | N . | IS . | Outcome . | No. of expanded Vβ families* . | |
|---|---|---|---|---|---|
| CD4+(28dim) . | CD8+(28dim) . | ||||
| AA | |||||
| sAA | 20 | Yes, n = 15 | R, n = 12 | 3.0 (3.6) | 2.1 (2.1) |
| NR, n = 3 | 1.0 (3.6) | 2.0 (2.3) | |||
| No, n = 5 | NA | 2.0 (3.6) | 2.4 (3.0) | ||
| mAA | 3 | No, n = 3 | NA | 2.6 (2.6) | 2.0 (4.0) |
| PNH | |||||
| Hemolytic | 6 | No, n = 6 | NA | 2.6 (3.0) | 2.8 (2.5) |
| AA/PNH | 4 | No, n = 4 | NA | 2.0 (3.2) | 3.0 (2.5) |
| . | N . | IS . | Outcome . | No. of expanded Vβ families* . | |
|---|---|---|---|---|---|
| CD4+(28dim) . | CD8+(28dim) . | ||||
| AA | |||||
| sAA | 20 | Yes, n = 15 | R, n = 12 | 3.0 (3.6) | 2.1 (2.1) |
| NR, n = 3 | 1.0 (3.6) | 2.0 (2.3) | |||
| No, n = 5 | NA | 2.0 (3.6) | 2.4 (3.0) | ||
| mAA | 3 | No, n = 3 | NA | 2.6 (2.6) | 2.0 (4.0) |
| PNH | |||||
| Hemolytic | 6 | No, n = 6 | NA | 2.6 (3.0) | 2.8 (2.5) |
| AA/PNH | 4 | No, n = 4 | NA | 2.0 (3.2) | 3.0 (2.5) |
For schedules and regimens of immunosuppression, please see “Patients and methods.” Vβ, variable β chain; IS, immunosupression; AA, aplastic anemia; sAA, severe aplastic anemia; mAA, mild aplastic anemia; R, responder; NR, nonresponder; NA, not applicable (patients were not treated or information was not available); PNH, paroxysmal nocturnal hemoglobinuria.
Values represent the average number of pathologically expanded Vβ families found when individual patient groups were analyzed. The number of pathologically expanded Vβ families within the effector cell (CD28dim) pool is shown in parentheses. The definition of a pathologic expansion is given in “Patients and methods.”
Immunosuppressive therapy consisted of a combination of antithymocyte globulin, cyclosporine A, and mycophenolate mofetil. All patients received a course of prednisone during and after antithymocyte globulin. Patients were classified as responders when they no longer fulfilled the severity criteria and became transfusion-independent according to our published definition.37
The presence of a PNH clone was determined by flow cytometry. The test was considered positive when more than 1% of glycosyl-phosphatidylinositol-anchored protein–deficient neutrophils in blood were found as defined by negativity for surface staining for CD66b and CD16 in a distinctive population of CD15+ cells. For the purpose of this study, patients with the presence of PNH clone and otherwise fulfilling criteria of AA were classified as having AA/PNH.39 Nine healthy subjects served as normal controls for flow cytometry experiments.
Vβ cytometry (Vβ skewing)
Fresh PB was stained with a mix of 4 monoclonal antibodies (mAbs): energy-coupled dye (ECD)-conjugated CD4, phycoerythrin cytochrome 5 (PECY5)–conjugated CD8, and a phycoerythrin (PE)- (or fluorescein isothiocyanate [FITC] when appropriate) conjugated CD28 (all these from Beckman-Coulter, Fullerton, CA). For the variable region of the TCR-Vβ), a pool of 22 mAbs (either PE- or FITC-conjugated) was used to quantitate the individual contribution of TCR-Vβ subfamilies to the CD4 and CD8 lymphocyte pool (all from Immunotech [Beckman-Coulter] except mAb anti Vβ6.7 [Endogen, Woburn, MA]). Samples stained with appropriate isotypic PE-, FITC-, PECY-, and ECD-conjugated mAbs served as controls to establish the fluorescence limits of background staining. After a 20-minute incubation at room temperature, erythrocytes were lysed; the remaining cells were fixed with a Q-prep apparatus (Beckman-Coulter) and further analyzed by a Coulter XL flow cytometer equipped with an Epics Elite software. A 4-color protocol was used: the lymphocyte gate was set according to their size and forward scatter properties. TCR-Vβ usage was determined within CD4+CD28low, CD8+CD28low, as well as within total CD4+ and CD8+ populations, by using appropriate gates. In addition, FITC-conjugated α/β mAb (Becton Dickinson, Mountain View, CA) was used to determine the contribution of each Vβ subfamily to the total α/β TCR repertoire. Values obtained for individual Vβ families were expressed as a percentage of α/β TCR-expressing CD4+ or CD8+ cells.
CD4+ and CD8+ lymphocyte purification
Mononuclear cells (MNCs) from PB were separated by density gradient centrifugation (Organon, Durham, NC) and washed twice in phosphate buffered saline (Biosource International, Camarillo, CA). CD4+ and CD8+ cells were directly labeled with mAb conjugated with magnetic microbeads and isolated from BM MNCs by positive or negative selection (MACS; Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions.
RNA isolation and complementary DNA synthesis
Total RNA was extracted from 1 to 2 × 106 PB MNCs with TRIzol reagent (GIBCO-BRL, Bethesda, MD). The SuperScript II RT kit (GIBCO-BRL) was used for first-strand complementary DNA (cDNA) synthesis. Briefly, 1 U SuperScript II Rnase H-reverse transcriptase was used in the presence of 1 μg RNA, 0.5 μg/μL oligo(dT)12-18 at 42°C for 50 minutes and in a final volume of 20 μL.
Polymerase chain reaction and CDR3 size distribution analysis (CDR3 skewing)
Details of the CDR3 size distribution assay have been reported, including reaction conditions and primer sequences.15,38Briefly, cDNA was amplified by using the polymerase chain reaction (PCR) with 22 TCR-Vβ family-specific primers and an antisense, TCR constant β common primer.39 Vβ10 and Vβ19 were excluded from study because they are pseudogenes. Two microliters of 10 × buffer (Takara Biomedicals, Shiga, Japan) containing 15 μmol/L MgCl2, 1.7 μL dNTP (2.5 mmol each), 5 μL of 20 μmol/L of each Vβ subfamily sense primer, 1 μL of 20 μmol/L fluorescent constant β primer, 1 μL cDNA, and 0.18 μL of 5 U/μL TaKaRa Ex Taq (Takara Biomedicals, Shiga, Japan) were mixed with a final volume of 4 μL. PCR was performed in a Peltier Thermal Cycler-200 (MJ Research, Waltham, MA) under the following conditions: 15 cycles of initial touch down was done by denaturation at 94°C for 1 minute, followed by annealing of primers at 60°C for 1 minute with −0.5°C gradient reduction of annealing temperature for the subsequent cycles to 53°C and extension at 72°C for 1 minute. Subsequently, 20 additional amplification cycles (denaturation at 94°C for 1 minute, followed by annealing at 53°C for 1 minute, and extension at 72°C for 1 minute) were performed with a final extension of the primers at 72°C for 10 minutes. Subsequently, 1 μL amplification products was mixed with 12.0 μL deionized formamide (Sigma) and 0.5 μL size standard (Genescan-400 ROX, ABI 310; Perkin-Elmer, Shelton, CT), heated at 90°C for 2 minutes, chilled on ice, and applied to an ABI 310 sequencer to analyze CDR3 size distribution. To classify each individual profile as normal or abnormal (skewed), we adopted a set of numerical standards. The fluorescence intensity of each band was depicted as a peak. CDR3 size patterns that failed to exhibit a bell-shaped distribution because of the appearance of prominent peaks, with or without reduced peak number (< 5 peaks), were judged as abnormal. The analysis was performed by 3 different investigators in blinded fashion, and, in a few cases, when inconsistent results were obtained, the decision as to whether the pattern was skewed or abnormal was based on the agreement of 2 of the 3 investigators.
Statistical analysis
Student t test was used to compare the number of overexpressed (> 2 SD of normal control pool) Vβ subfamilies in AA and PNH patients and healthy control subjects; paired t test was used, in healthy subjects, to compare the percentage contributions of each Vβ class in total lymphocytes versus effector subsets. Chi-squared test was used to determine if specific Vβ subfamilies were nonrandomly overrepresented. Chi-square test was also used to compare the frequency of an abnormal CDR3 size profile in overexpressed Vβ subfamilies in CD4+ and CD8+ subsets and within each Vβ subfamily. All statistical analyses were performed by using Statistica 5.0 software (Statsoft, Cary, NC).
Results
Flow cytometric analysis of Vβ usage
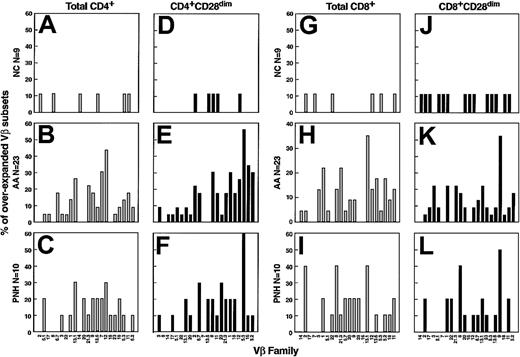
We planned experiments on the premise that either pronounced monoclonal expansion of an individual clone or superantigen stimulation could lead to an increased contribution of a given Vβ family (Vβ skewing) to the whole T-cell repertoire. Therefore, we analyzed preferential usage of 22 different Vβ subfamilies by CD4+and CD8+ cells with the use of flow cytometry. In addition, 9 age-matched healthy individuals were used to establish control values. All 22 Vβ subfamilies were present in PB lymphocytes, each accounting for 0.5% to 9% of total α/β T cells; variability in the percentage representation of each Vβ family among controls was low. To obtain a normal Vβ representation spectrum for CD4+ and CD8+ cells, results from all controls were averaged and ordered according to their contribution to the T-cell repertoire (Figure 1).
Flow cytometric analysis of the TCR-Vβ usage in AA patients.
For controls (n = 9; A,D,G,J), columns and bars represent mean + 2 SD of percentages of lymphocytes belonging to a particular Vβ family as indicated on the x-axis. The values are calculated as percentages of total αβ CD4+ (A,B,C) or CD8+ (G,H,I) lymphocytes. Similarly, contribution of individual Vβ families is represented as percentage of total αβ CD4+CD28dim (D,E,F) and αβ CD8+CD28dim (J,K,L) lymphocytes. For AA (B,E,H,K) and PNH patients (C,F,I,L), 2 representative cases (of a total of 33 studied) are shown.
Flow cytometric analysis of the TCR-Vβ usage in AA patients.
For controls (n = 9; A,D,G,J), columns and bars represent mean + 2 SD of percentages of lymphocytes belonging to a particular Vβ family as indicated on the x-axis. The values are calculated as percentages of total αβ CD4+ (A,B,C) or CD8+ (G,H,I) lymphocytes. Similarly, contribution of individual Vβ families is represented as percentage of total αβ CD4+CD28dim (D,E,F) and αβ CD8+CD28dim (J,K,L) lymphocytes. For AA (B,E,H,K) and PNH patients (C,F,I,L), 2 representative cases (of a total of 33 studied) are shown.
Subsequently, we analyzed 23 AA and 10 PNH patients: for comparison purposes, values more than mean + 2 × SD of controls were considered abnormal. In AA, from a total of 22 Vβ families, on average we found expansion in 3 ± 2 and 2 ± 2 Vβ families in CD4+ and CD8+ subsets, respectively (P = .029 and P = .023). Similar results (3 ± 2 for CD4+ and 3 ± 1 for CD8+lymphocytes; P = .019 and P < .001) were obtained in PNH (Figures 1 and 2). Most of the AA patients had received some transfusions before the time of blood sampling, but 5 were analyzed before any transfusions were given. In addition, there was 1 patient with PNH who did not receive any transfusions. These patients showed Vβ oligoclonality patterns similar to those who received transfusion support, with no differences with regard to the degree or extent of Vβ family expansions (P > .05). No correlation was found between the numbers of expanded Vβ families per patient (degree of Vβ skewing) and clinical parameters such as absolute neutrophil count, and reticulocyte and platelet counts at presentation (data not shown). The frequency of skewing of a particular family was not homogeneous (P < .001, chi-squared test); in AA, some Vβ subfamilies appeared to be more frequently overused (Figure3). However, no consistent overrepresentation of specific Vβ subfamilies was found when patients were grouped by HLA-A or -B (analyzing CD8+ subset,P > .05) and DR or DP phenotype (for CD4+subset, P > .05; chi-squared test).
Nonparametric analysis of the abnormal TCR-Vβ repertoire in AA patients.
Columns represent the mean numbers + SD of overexpressed Vβ-families of a total of 22 screened. Pathologic overrepresentation of a particular Vβ was defined as described in “Patients and methods.” Analysis of total αβ T cells is shown (A,B), whereas effector lymphocytes were analyzed (C,D). By analogy, analysis of αβ CD4+ (A,C) and αβ CD8+ (B,D) cells (in both total and effector [CD28dim] compartment) is depicted.
Nonparametric analysis of the abnormal TCR-Vβ repertoire in AA patients.
Columns represent the mean numbers + SD of overexpressed Vβ-families of a total of 22 screened. Pathologic overrepresentation of a particular Vβ was defined as described in “Patients and methods.” Analysis of total αβ T cells is shown (A,B), whereas effector lymphocytes were analyzed (C,D). By analogy, analysis of αβ CD4+ (A,C) and αβ CD8+ (B,D) cells (in both total and effector [CD28dim] compartment) is depicted.
Frequency of expansion of individual Vβ families in AA.
Results represent a summary of all patients analyzed by flow cytometry. Columns correspond to percentage of cases in which the pathologic expansion was found. Results in total αβ CD4+ (A,B,C,D) and αβ CD8+ (G,H,I) cells are represented. By analogy, results within the effector (CD28dim) pool for CD4+ (D,E,F) and CD8+ (J,K,L) lymphocytes are depicted.
Frequency of expansion of individual Vβ families in AA.
Results represent a summary of all patients analyzed by flow cytometry. Columns correspond to percentage of cases in which the pathologic expansion was found. Results in total αβ CD4+ (A,B,C,D) and αβ CD8+ (G,H,I) cells are represented. By analogy, results within the effector (CD28dim) pool for CD4+ (D,E,F) and CD8+ (J,K,L) lymphocytes are depicted.
Preferential Vβ usage by effector CD4+ and CD8+ T-cell subpopulations
According to our initial hypothesis that effector lymphocyte subsets were more likely to contain specific Vβ families involved in the pathologic process, we had determined the Vβ spectrum of effector T cells. CD28 down-modulation was used as a marker of the effector phenotype (CD4+CD28dim and CD8+CD28dim). In controls, effector cells showed deviations from the usual distribution of total CD4+or CD8+ cells. For example, within CD4+ cells, Vβ1, Vβ2, Vβ5.1, and Vβ16 were underrepresented (P < .05, t test), whereas Vβ14 was expanded. In CD8+ cells, Vβ5.2, Vβ6.7, Vβ9, Vβ13.1, Vβ13.6, and Vβ17 were all underused in the effector pool as compared with total CD8+ T cells. In patients, the average number of overrepresented Vβ subfamilies constituting the effector pool did not differ when compared with total CD4+ and CD8+ lymphocytes (on average, about 3 of 22 Vβ families studied were overrepresented). However, certain Vβ families showed a higher degree of the expansion within effector T cells as compared with the total lymphocyte pool; in some patients, 1 or 2 Vβ individual subfamilies represented more than 50% of the total effector T cells (Figure 1). Again, no consistent expansion of individual Vβ families was found among clinically similar patients, and there was no correlation with HLA class-I and class-II type (Figure 3).
CDR3 size distribution analysis of overrepresented Vβ subfamilies
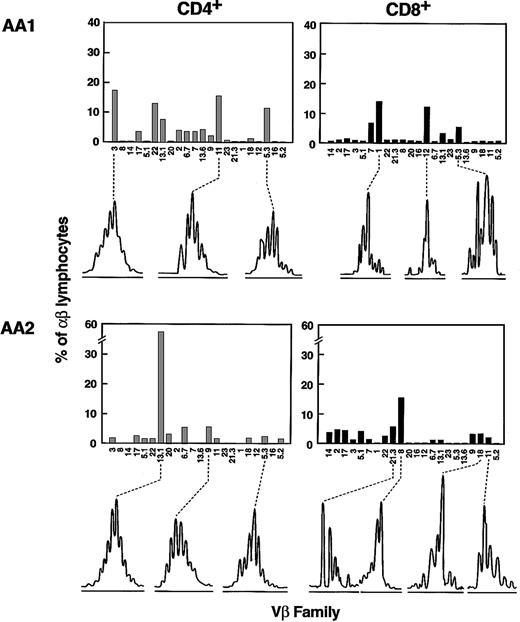
From the flow cytometry data, all Vβ families overexpressed in the CD28dim compartment (effector pool) were selected as likely to represent oligoclonal or monoclonal expansion of disease-specific CD4+ or CD8+ T cells. For these Vβ families, size distribution analysis of their CDR3, amplified by PCR, was performed. For that purpose, isolated CD4+ and CD8+ cell populations were used for the amplification with specific Vβ primer pairs.29Surprisingly, we found that the majority (72%, 35 of a total of 57 expanded Vβ-families [in 20 patients]) of the expanded CD4+ Vβ subfamilies showed a Gaussian-like distribution (Figure4), indicating a normal polyclonal expansion within these families. In contrast, almost all (82%, 38 of 44) Vβ subfamilies overused by CD8+ cells demonstrated a skewed profile, with oligoclonal and often monoclonal patterns (Figure 4). The difference in the degree of Vβ skewing between CD4+- and CD8+-expanded Vβ families was highly significant (P < .001, chi-squared test). In addition, for CD4+ cells, skewing was observed predominantly in Vβ11, Vβ12, Vβ16, and Vβ21. However, these Vβ families are relatively underrepresented within the entire normal T-cell pool. When the specific contribution of each Vβ subset to the patient lymphocyte pool was analyzed, the numbers of Vβ-specific CD4+ cells were significantly lower in families showing abnormal spectratyping as compared with those with polyclonal CDR3 spectrums (18 ± 13 and 46 ± 39 CD4+/L; P = .001, t test). Moreover, subfamilies accounting for less than 30 CD4+ T cells/L blood were more likely to show a skewed CDR3 size distribution pattern (20 of 35 versus 3 of 22; P = .002, chi-squared test).
TCR-Vβ CDR3 size distribution analysis of expanded Vβ families in AA.
Two representative examples (patients AA1 and AA 2) are shown (of 20 performed). Columns represent percentages of lymphocytes belonging to a particular Vβ family as indicated on the x-axis. For overexpanded families, the results of the Vβ CDR3 size distribution analysis are depicted below each panel. For the spectrograms, the x-axis represents size of the PCR amplification product fragments as determined using fluorescent sequencing technique.
TCR-Vβ CDR3 size distribution analysis of expanded Vβ families in AA.
Two representative examples (patients AA1 and AA 2) are shown (of 20 performed). Columns represent percentages of lymphocytes belonging to a particular Vβ family as indicated on the x-axis. For overexpanded families, the results of the Vβ CDR3 size distribution analysis are depicted below each panel. For the spectrograms, the x-axis represents size of the PCR amplification product fragments as determined using fluorescent sequencing technique.
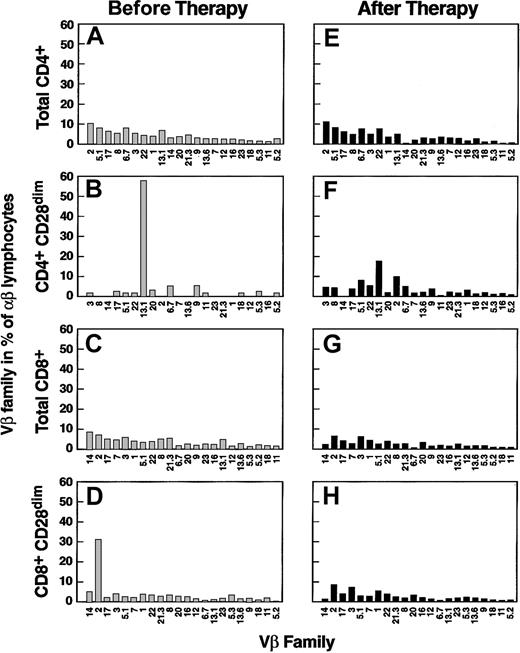
Clinical response and Vβ usage after immunosuppressive treatment
Twelve of the 23 AA patients were analyzed prior to immunosuppression and 3 to 6 months after treatment. Although we expected normalization of the Vβ family and CDR3 skewing patterns seen at initial presentation, no major changes in the number of expanded Vβ classes were found after therapy. However, numerically, overrepresention of the Vβ subfamilies was less pronounced in most of the cases (Figure 5), although their percentages remained more than 2 SD of controls regardless of clinical response (Table 1). When parametric analysis was used to summarize the results obtained in 12 patients studied, some Vβ families showed lower percentages after therapy (t test, P < .05): for total CD4+ lymphocytes, Vβ5.2, Vβ6.7, Vβ11, Vβ14, Vβ18, and Vβ21.3 decreased, whereas for CD8+ T-cells, Vβ5.2, Vβ14, and Vβ18 were lower and Vβ1 was higher. When the effector pool was analyzed, CD4+Vβ9 T cells showed a higher contribution to the total α/β T-cell pool than was observed before therapy, whereas CD4+Vβ11+, CD8+Vβ11+, and CD8+Vβ14+ lymphocytes contracted.
Preferential usage of Vβ families in AA patients prior to and after immunosuppressive therapy.
Two representative cases are shown, one for CD4+lymphocytes (A,B,E,F) and one for CD8+ lymphocytes (C,D,G,H); results in total cells (A,E,C,G) and effector pool (B,D,F,H) are depicted. Vβ usage prior to immunosuppressive treatment (B,F,D,H) is represented, whereas the corresponding results after therapy was administered (E,F,G,H) according to the regimen described in “Patients and methods” are shown.
Preferential usage of Vβ families in AA patients prior to and after immunosuppressive therapy.
Two representative cases are shown, one for CD4+lymphocytes (A,B,E,F) and one for CD8+ lymphocytes (C,D,G,H); results in total cells (A,E,C,G) and effector pool (B,D,F,H) are depicted. Vβ usage prior to immunosuppressive treatment (B,F,D,H) is represented, whereas the corresponding results after therapy was administered (E,F,G,H) according to the regimen described in “Patients and methods” are shown.
Discussion
In AA, the evidence of an autoimmune pathophysiology is mostly indirect, as the offending antigens have not been identified. Demonstration of oligoclonal T-cell responses may serve as a surrogate for the recognition of the antigens that drive the immune process.40 41 Our strategy of characterization of clones responsible for immune-mediated damage to hematopoietic tissue included a combination of 2 different concepts: that immunodominant antigens (determining disease) will produce both qualitative (CDR3-skewing pattern) and quantitative (expansion of Vβ class) changes in each TCR-Vβ family.
Because of the wide spectrum of antigens encountered by the immune system under physiologic conditions, the normal TCR-Vβ repertoire would be expected to be similar among healthy individuals, but it is possible that the diversity of HLA backgrounds could result in a variably distributed Vβ spectrum. We found that the contribution of each specific Vβ class to the total αβ+ lymphocyte pool showed little variability, results comparable to data published by others.42 Therefore, it is likely that the similar utilization of individual Vβ chains among healthy control subjects reflects preferential molecular rearrangements of particular variable segments43 rather than responses to ubiquitous but different antigens.44 45
In healthy donors, BM T cells are likely representative of preferential homing processes rather than peripheral mechanisms of selection and expansion. In previous studies, no differences in Vβ skewing were demonstrated when blood and BM were simultaneously analyzed.35 Utilization of marrow introduces an artifact related to variable PB contamination and low cellularity of marrow specimens. To avoid this problem we based our studies on PB. Although this approach may be less sensitive, it allows for a more systematic and frequent analysis and better correlation with clinical parameters. In addition, Vβ families and clonotypes identified in blood can be selectively analyzed in the marrow.
When Vβ usage was studied in 33 AA and PNH patients, marked differences in the contribution of individual Vβ families to the T-cell pool were measured by flow cytometry; in particular, most of the patients showed overexpansion of specific Vβ families. This effect was unlikely to be due to a superantigen but rather suggestive of an antigen-driven clonal response. The differences from the normal Vβ repertoire were even more pronounced when the effector lymphocyte pool was studied, as effector T-cell subsets are more likely to include the disease-specific, antigen-primed T cells. In our study, overrepresented Vβ families were found in both CD4+ and CD8+ effector lymphocytes and are compatible with specific T-cell responses. However, commonly expanded specific Vβ classes were not found even if patients were grouped according to their HLA typing.
Previous studies included CDR3 analysis performed using total T-cell populations.31-33 However, analysis of Vβ in CD4+ and CD8+ cells is more rational because CDR3 spectra of cytotoxic T lymphocytes and helper T cells would have different HLA restrictions and might overlap when analyzed in combination. We used expansion of specific Vβ families to limit the search for the disease-specific clones distinguished by unique CDR3 nucleotide sequences. Consequently, we have analyzed CDR3 size distribution only within the numerically overrepresented Vβ families. We found that, although the expansion of certain Vβ families was associated with oligoclonality in the CD8+ subset, Vβ expansions in CD4+ cells were more likely to be polyclonal. These results indicate that our method is more useful in recognizing disease-specific cells within the CD8+ subset as cytotoxic T lymphocytes contain distinct T-cell clones. By comparison, CD4+ responses may be more complex and Vβ CDR3 size analysis may not be helpful.
The analysis of clonality in the context of an immune response differs from the definition used in malignant processes. Unlike in T-cell lymphomas, oligoclonal response to an immunodominant antigen does not exclude otherwise polyclonal T-cell populations. Perhaps the most overlap between the malignancy and immuonodominant clonal expansion can be postulated for large granular lymphocytosis (LGL). Consequently, our findings could also reflect detection of subclinical, yet pathophysiologically relevant, LGL-like process. Although spectratyping may suggest oligoclonality as a reflection of immunodominance, this method is likely not sensitive enough to detect all T-cell responses. Similarly, individual clonal expansion may not be extensive enough to skew the quantitative composition of the entire Vβ repertoire. Such a situation may specifically apply to CD4 responses. For example, a quantitatively weak clonal CD4 expansion may serve to amplify the final CD8 effector pathway.
Our previous studies have shown that Vβ CDR3 skewing can also be related to the contraction in the total T-cell compartment and a reflection of clonal sparing.33 Many different factors may contribute to TCR repertoire impairment in AA. First, cytopenia may also involve lymphocytes, with reduction of specific T-cell subsets. Second, the underlying immunologic disorder itself may be responsible for the contracted T-cell repertoire. Third, contraction can result from immunosuppressive therapy33 or after BM transplantation.46 Instead, we observed that within the CD4 T-cell population, the size of a particular Vβ family appears to correlate with the likelihood of skewing.
At least in theory, our findings might be due to transfusions or infections, and it is not possible to rigorously exclude this possibility. However, in our previous studies, we did not observe increased CDR3 skewing in poly-transfused patients, and none of our current patients were clinically infected at the time of blood sampling. Moreover, in this study a few patients were analyzed before any transfusion, and they showed no differences with regard to the degree or extent of Vβ expansion compared with others who received transfusion support. Lack of direct clinical correlations (such as total normalization of Vβ repertoire for patients in remission) may be due to persistence of the antigenic drive or to lack in the selectivity of our current immunosuppressive regimens, allowing T-cell regeneration according to the original Vβ class distribution. Instead, major expansions usually are reduced after antithymocyte globulin therapy, but do not disappear, consistent with the high relapse rate. Eradication of pathologic clones may be difficult. However, because of the short follow-up, we have not yet observed relapses in the group of AA patients who responded to immunosuppression, and comparisons of Vβ spectrum between the time of relapse and initial presentation were not yet available.
Identification of disease-specific clonotypes can be followed by their characterization on the molecular level. Ultimately, our approach will serve the goal of recognition of specific clonotypic sequences by analogy to autoantibody detection. TCR clonotypes should serve as surrogate molecular markers for specific etiologies or pathophysiologic pathways in autoimmune diseases.
Prepublished online as Blood First Edition Paper, [April 30, 2002]; DOI 10.1182/blood-2002-01-0236.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jaroslaw P. Maciejewski, Experimental Hematology and Hematopoiesis Division, Taussig Cancer Center, R40, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: maciejj@cc.ccf.org.


![Fig. 2. Nonparametric analysis of the abnormal TCR-Vβ repertoire in AA patients. / Columns represent the mean numbers + SD of overexpressed Vβ-families of a total of 22 screened. Pathologic overrepresentation of a particular Vβ was defined as described in “Patients and methods.” Analysis of total αβ T cells is shown (A,B), whereas effector lymphocytes were analyzed (C,D). By analogy, analysis of αβ CD4+ (A,C) and αβ CD8+ (B,D) cells (in both total and effector [CD28dim] compartment) is depicted.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/1/10.1182_blood-2002-01-0236/7/m_h81322784002.jpeg?Expires=1767748820&Signature=PQWEN1nDnkGmQnGPLbn8~Etk4n~G132ERBRPLJKgd4Z9MQZT1cGLKnfFArl~Pr6OWgvBHYLrzu7VtoKAgH~x5KftnukxNyMPqobKzhClsbC817-juT6SBuvPV9NW8rf31qxDIKEEESXMDxHWUzaKr0w9Q9fWQKXyL6XYd7y9bbwOWOoVrOoUp1XQl6WWDz40UbH5o4F6Tg30592hLBUhkaPCzRIFZIaB1mOuUi2Q1lQIa4fnKyau7M1MgYtlHl-1SmkIs7XamxUcTSyHOy7VD5BI-udfRgkA8CoqG3eVjd8kgygwNT2K03GgOmU3Ca-gjB0cFl2ttjXZ-rLk~WdHFg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal