Abstract
Chronic lymphocytic leukemia (CLL) cells are ineffective antigen-presenting cells (APCs) although CD40-activated CLL cells can stimulate proliferation of autologous and allogeneic T cells. We examined the antigen-presenting capacity of CD40-activated CLL cells as well as dendritic cells pulsed with apoptotic bodies of CLL cells to generate autologous and allogeneic immune responses against CLL cells. Both APC types were capable of generating T-cell lines that proliferate specifically in response to unstimulated CLL cells. Whereas cytotoxic responses against stimulated and unstimulated CLL cells could be repeatedly generated by allogeneic healthy donors, autologous cytotoxic immune responses against CD40-activated and native CLL cells were rarely detected. However, T cells isolated from patients with CLL could recognize and lyse allogeneic stimulated and unstimulated CLL cells, demonstrating that cytotoxic T cells from these tumor-bearing patients are functionally intact.
Introduction
Chronic lymphocytic leukemia (CLL) is an attractive candidate to examine immunotherapeutic approaches because it is a slow-growing tumor, allowing time for the generation of an immune response against the tumor cells. CLL cells express major histocompatibility complex (MHC) class I and class II molecules and also express a specific tumor antigen, the idiotype, which has been shown to be a target of T- and B-cell–mediated immune responses.1-5 Following allogeneic stem cell transplantation, CLL cells appear to be good targets of a graft-versus-leukemia effect.6 However, a number of immune dysregulations occur in CLL with patients often exhibiting severe hypogammaglobulinemia, impaired immunoglobulin class switching, and diminished germinal center formation. This results in an increased susceptibility to bacterial infections that contributes greatly to morbidity and mortality in CLL.7-9
It is not clear what role T cells play in the observed B-cell defects and immunosuppression in CLL. A number of T-cell defects have been well characterized including inversion of the CD4/CD8 ratio,10enhanced susceptibility of CD4 cells to FAS (CD95) ligand–induced apoptosis,11 and expression of an oligoclonal T-cell receptor (TCR) Vβ gene pattern.12-14 In addition, there are increased absolute numbers of phenotypically activated CD4+ and CD8+ T cells,15alteration of production of cytokines including interleukin 4 (IL-4) and interferon γ (IFN-γ), and reduced expression of CD40L, ζ-chain of the TCR, and CD28.16,17 These defects likely explain the reduced mitogenic and allogeneic T-cell responses that have been observed in this disease.18,19 More recently it has been reported that CD30 is up-regulated on T cells of patients with CLL by an IL-4 and OX40L-dependent mechanism and that these T cells might be involved in both defective IgG class switching and accumulation of leukemic B cells.20 Finally, therapy for CLL, especially the use of purine analogues, is associated with decreased lymphocyte count and function, resulting often in further immunosuppression.
Despite these T-cell alterations, we have previously demonstrated that autologous cytotoxic T-cell lines can be generated against immunoglobulin-derived peptides when these immunogenic peptides are presented by antigen-presenting cells (APCs) and that once established these T-cell lines are capable of killing CLL cells.21,22These data demonstrate that because cytotoxic T cells can be expanded and are capable of recognizing and killing autologous tumor cells, they must be present within the T-cell repertoire. Although CLL cells can be a target of a T-cell–mediated cytotoxic response once these cytotoxic T-cell lines have been established ex vivo, CLL cells are ineffective stimulator cells in allogeneic as well as autologous mixed lymphocyte cultures and it has been extremely difficult to initiate autologous T-cell responses against unstimulated CLL cells.23 The low immunogenicity of the CLL cells is due at least in part to their low levels of expression of costimulatory and adhesion molecules required for the initiation of an effective immune response. CD40 has been shown to be an effective tool to up-regulate expression of these molecules on both normal and malignant B cells including CLL cells.24-26 Moreover, CD40-activated normal and malignant B cells induce T-cell responses27-29 and transfection of CLL cells with replication-defective adenovirus encoding murine CD154 induced immune responses against unmodified leukemia cells.30 31
Although CD40-activated normal and lymphoma B cells are effective APCs, CD40-activated CLL cells might still have defects in antigen presentation and induction of antitumor responses, because the CLL cells themselves might be involved in dysregulation of autologous T cells. Dendritic cells (DCs) have been shown to be effective in inducing antitumor responses in many cancers including B-cell lymphoma.2,4,32 33 However, the role of DCs to initiate T-cell immune responses has not previously been investigated in patients with CLL. In the present report we used both CD40-activated CLL cells as well as DCs pulsed with apoptotic bodies of CLL cells as APCs to test their capacity to induce proliferative and cytotoxic autologous and allogeneic T-cell responses against CLL cells. Both APC types induced specific proliferating T lymphocytes in response to unstimulated CLL cells that could be partially blocked by antibodies against MHC class I and class II molecules suggesting that CLL-associated antigens are presented by both pathways. Cytotoxicity and specific proliferation could be induced against unstimulated allogeneic CLL cells. However, autologous cytotoxic T-cell responses could rarely be generated against naturally presented peptides. In keeping with the notion that there are T-cell alterations in the autologous tumor-bearing host, prior to stimulation we observed enhanced expression of the chemokine receptors CXCR4 and reduced expression of CCR2 on CD4+ cells of patients with CLL compared to healthy donors. However, T cells isolated from patients with CLL are capable of mounting cytotoxic immune responses against allogeneic CLL cells. Therefore, although a number of defects have been demonstrated in the T cells of CLL patients, including defective granzyme-perforin pathway and reduced cell numbers, CD8+cytotoxic T cells from CLL appear to be functionally capable of killing CLL cells. Taken together these data are consistent with the notion that to induce effective autologous immune responses against CLL cells, it will likely be necessary to enhance antigen presentation, perhaps to overcome tolerance mechanisms on both the antigen-presenting and effector pathways.
Patients, materials, and methods
Patients and donors
Peripheral blood samples were obtained from 12 patients with B-cell CLL (B-CLL) and 6 healthy donors by phlebotomy or leukopheresis. All specimens were obtained after informed consent was obtained and following approval by the Dana-Farber Cancer Institute institutional review board. Patient data are shown in Table1.
Patient data
| . | Sex . | Age . | Stage (Binet) . | Peripheral white blood cell count (per μL) . |
|---|---|---|---|---|
| CLL1 | F | 59 | C | 158 |
| CLL3 | M | 70 | C | 30 |
| CLL4 | M | 52 | A | 37 |
| CLL6 | M | 45 | A | 10 |
| CLL8 | M | 48 | A | 24 |
| CLL9 | M | 61 | A | 45 |
| CLL11 | M | 49 | A | 29 |
| CLL12 | F | 56 | A | 10 |
| CLL13 | F | 71 | A | 11 |
| CLL14 | F | 50 | A | 17 |
| CLL16 | M | 48 | C | 147 |
| CLL17 | M | 41 | C | 17 |
| CLL21 | M | 49 | A | 16 |
| . | Sex . | Age . | Stage (Binet) . | Peripheral white blood cell count (per μL) . |
|---|---|---|---|---|
| CLL1 | F | 59 | C | 158 |
| CLL3 | M | 70 | C | 30 |
| CLL4 | M | 52 | A | 37 |
| CLL6 | M | 45 | A | 10 |
| CLL8 | M | 48 | A | 24 |
| CLL9 | M | 61 | A | 45 |
| CLL11 | M | 49 | A | 29 |
| CLL12 | F | 56 | A | 10 |
| CLL13 | F | 71 | A | 11 |
| CLL14 | F | 50 | A | 17 |
| CLL16 | M | 48 | C | 147 |
| CLL17 | M | 41 | C | 17 |
| CLL21 | M | 49 | A | 16 |
Patient data including sex, age, clinical stage, and peripheral white blood cell count are shown. All patients are untreated with exception of patient CLL3 and CLL17. Patient CLL3 was treated with fludarabine for 5 months and patient CLL17 for 3 months prior to cell harvest. These 2 patients were excluded for autologous T-cell function assays (specific proliferation and cytotoxicity) after stimulation.
Cell preparation and isolation of different cell types
Mononuclear cells from peripheral blood samples were obtained by density gradient centrifugation on Ficoll/Hypaque (Pharmacia, Peapack, NJ). T cells and B cells were separated by negative depletion using several separation steps, including E-rosetting with sheep erythrocytes (Biowhittacker, Walkersville, MD)34 and magnetic bead (Polysciences, Warrington, PA) depletion using monoclonal antibodies (mAbs) against cell line-specific cell surface markers.35Generation of DCs was performed using immunomagnetic bead depletion of CD19+ cells followed by a 1-hour adherence step. Culture plates were then rinsed to deplete nonadherent cells and adherent cells were cultured as described elsewhere.36
Cell culture and cytokines
Cells were cultured at 37°C in a 5% CO2humidified atmosphere. To generate DCs, adherent cells were cultured in Iscoves modified Dulbecco medium (IMDM; Life Technologies, Rockville, MD) supplemented with 10% human serum, 50 μg/mL human transferrin (Boehringer Mannheim, Indianapolis, IN), 5 μg/mL human insulin (Sigma Chemical, St Louis, MO), 15 μg/mL gentamicin (Life Technologies), 2.4 mg/mL HEPES (Mediatech-Cellgro, Herndon, VA), and 2.9 mg/mLl-glutamine (Mediatech-Cellgro), and addition of 10 ng/mL recombinant human IL-4 (rhIL-4; R & D Systems, Minneapolis, MN) and 50 ng/mL recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF; Immunex, Seattle, WA) for 5 days. Cells were then loaded with apoptotic bodies of CLL cells in a DC—apoptotic cell ratio of 1:4. Apoptotic bodies were generated by irradiation of freshly thawed CLL cells with 50 Gy. Apoptotic cell death was assayed by using annexin V–fluorescein isothiocyanate (FITC) and propidium iodide staining, according to the manufacturer's instructions. For maturation of DCs, 1 μg/mL soluble CD40L (Immunex) was added 24 hours before stimulation of T cells. Prior to T-cell stimulation residual apoptotic bodies were depleted by rinsing the cell culture plates. DCs were then harvested using a cell scraper, irradiated with 32 Gy, and used for T-cell activation in a ratio of 1:5 to 1:10.
Murine NIH3T3 fibroblasts transfected with the human CD40L were used for stimulation of malignant B cells.29 Malignant B cells were cultured on irradiated CD40L-transfected fibroblasts in IMDM supplemented with 10% human serum, 50 μg/mL human transferrin, 5 μg/mL human insulin, 15 μg/mL gentamicin, 2.4 mg/mL HEPES, and 2.9 mg/mL l-glutamine, and addition of 5 ng/mL rhIL-4 for 3 days. Before stimulation of T cells, CD40L-activated CLL cells were irradiated with 32 Gy.
Purified CD3+ T cells (2 × 106) from healthy donors or CLL patients were cultured in RPMI with 10% human serum, 50 U/mL penicillin, 50 μg/mL streptomycin, 15 μg/mL gentamicin (Life Technologies), 2.4 mg/mL HEPES, and 2.9 mg/mL l-glutamine. After stimulation with DCs or CD40L-activated CLL cells, the following cytokines were added to T-cell cultures: 1 ng/mL rhIL-7 (Pepro Tech, Rocky Hill, NJ) was added on day 0, rhIL-2 (kind gift from Dr J. Ritz, Dana-Farber Cancer Institute) was added on days 1 (50 U/mL) and 4 (25 U/mL). T cells were restimulated every week with stimulator cells in a ratio ranging from 1:4 to 1:10. Proliferation and cytotoxicity assays were performed 3 days after the fourth stimulation.
Immunophenotyping
Immunophenotyping was performed by fluorescence-activated cell sorter analysis to verify isolation and purification steps as well as to investigate activation markers and expression of specific receptors on T and B cells. The following mAbs conjugated with FITC or phycoerythrin (PE) were used: mouse IgG control, CD1c, CD3, CD4, CD5, CD8, CD19, CD20, CD21, CD23, CD28, CD54, CD56, CD70, CD80, CD83, CD86, CD95, CD152, CD154, HLA-ABC, HLA-DR, anti-κ, anti-λ (Dako, Carpinteria, CA); CD2 (Coulter, Fullerton, CA); and CD40, CD45RA, CD45RO, CD58 (BD Pharmingen, Franklin Lakes, NJ). The following chemokine receptors were investigated using mAbs against CCR2, CCR3, CCR5, CXCR1, CXCR2, CXCR3, CXCR4, and CXCR5 (R & D Systems). For investigation of chemokine receptor expression on circulating T cells freshly purified peripheral blood mononuclear cells from patients with CLL and healthy donors were analyzed under exactly identical circumstances. In case of nonconjugated antibodies a secondary staining was performed with FITC-conjugated goat anti mouse/rat IgG antibody (Jackson, West Grove, PA).
Mixed lymphocyte reaction
Irradiated (32 Gy) unstimulated CLL cells were used as stimulator cells in a 72-hour thymidine incorporation assay. Briefly, 5 × 104 cultured T cells were used in triplicate and stimulated with stimulator cells in a ratio of 1:1 to 1:4 in a 96-well microtiter U-bottom culture plate. After 72 hours, cells were pulsed with 0.5 μCi 3H-thymidine (0.0185 MBq; NEN, Boston, MA), cultured for another 12 hours, and harvested onto glass fiber filters (Wallac, Turku, Finland). 3H-thymidine incorporation was measured using a Wallac Microbeta Plus Scintillation Counter.
Blocking experiments
Experiments for blocking of proliferation were performed using murine mAbs against MHC class I and class II. W6/32 (kind gift of Dr K. Anderson, Dana-Farber Cancer Institute) was used to block MHC class I and CR3/43 (Dako) to block MHC class II molecules.
Cytotoxicity assay
A standard chromium (51Cr) release assay was performed.37 Unstimulated CLL cells, CD40L-activated CLL cells, and K562 cells were labeled with 100 μCi 51Cr (3.7 MBq; NEN) and seeded in 96-well U-bottom microtiter plates at a concentration of 2.5 × 103 in triplicates. Effector cells were added in a ratio of 1:3, 1:10, and 1:30 and cocultured for 4 hours at 37°C in a 5% CO2 humidified atmosphere. After 4 hours the supernatants were harvested and the released 51Cr was measured in a γ-Counter (Wallac). Spontaneous release was determined by incubation of target cells in medium alone and maximum release was determined by resuspending the wells with 2% Triton X-100. Specific lysis was determined for each individual experiment as follows: specific lysis (%) = (experimental Cr51release − spontaneous 51Cr release)/maximum51Cr release − spontaneous 51Cr release) × 100.
Statistical analysis
Differences between experimental groups were analyzed using the Wilcoxon 2-sample test.
Results
Activation and proliferation of autologous and allogeneic T cells with CD40-activated CLL cells or DCs pulsed with apoptotic bodies of CLL cells as APCs
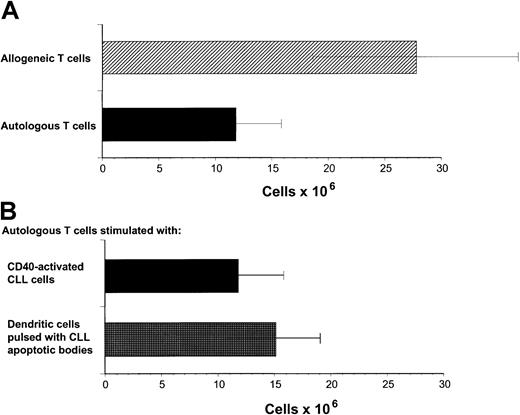
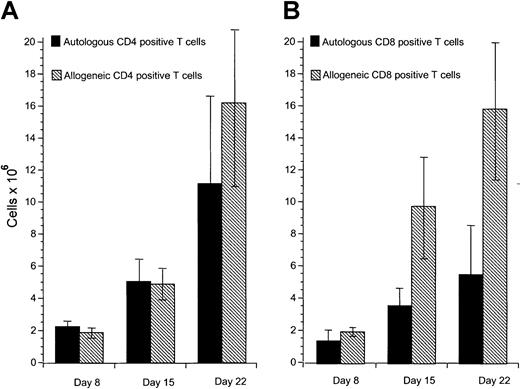
Our previous studies have demonstrated that cytotoxic T-cell responses can be generated against immunoglobulin-derived peptides expressed in CLL cells.21 22 However, cytotoxicity induced against autologous CLL cells in this system was weak. Other tumor-associated antigens are likely also to be presented on CLL cells but do not naturally induce effective antitumor responses. Because these antigens are not yet characterized, it is not possible to use DCs to present these antigens. We sought to investigate specific cellular responses against such naturally presented antigens in CLL. Because CD40 activation is an effective mechanism to increase APC function of malignant B cells including CLL cells, we investigated the proliferative potential of allogeneic and autologous T cells stimulated with CD40-activated CLL cells. Proliferation of allogeneic T cells was enhanced compared to autologous T cells in all experiments performed in response to CD40-activated CLL cells (Figure1A). DCs have been shown to be effective in inducing autologous antitumor responses against malignant cells in several tumor models. We therefore used DCs pulsed with apoptotic bodies of autologous CLL cells as described in “Patients, methods, and materials.” After 28 days of culture, T-cell proliferation was equivalent in response to pulsed DCs compared to CD40-activated CLL cells as stimulator cells (Figure 1B). Immunophenotypic investigation of T cells after stimulation with CD40-activated CLL cells showed no significant difference in proliferation of CD4+ T cells after 8 to 22 days in the autologous compared to the allogeneic setting (Figure2A). In contrast, reduced proliferation of CD8+ T cells was observed in the autologous compared to the allogeneic setting within this period (Figure 2B).
Proliferation of autologous and allogeneic T cells in response to CD40-activated CLL cells.
(A) Proliferation of autologous and allogeneic T cells in response to weekly stimulations with CD40-activated CLL cells after 29 days of T-cell culture. Isolated T cells (2 × 106) were plated on day 0. The results represent the mean ± SD of experiments performed on 11 patients. Control T cells that were not stimulated did not survive in culture for this period. (B) Proliferation of autologous T cells in response to CD40-CLL cells compared with DCs pulsed with apoptotic bodies. Proliferation of autologous T cells in response to weekly stimulations with CD40-activated CLL cells or pulsed DCs after 29 days of T-cell culture. Isolated T cells (2 × 106) were plated on day 0. The results represent the mean ± SD of experiments performed on 11 (CD40-activated CLL cells as APCs) and 4 (pulsed DCs as APCs) patients.
Proliferation of autologous and allogeneic T cells in response to CD40-activated CLL cells.
(A) Proliferation of autologous and allogeneic T cells in response to weekly stimulations with CD40-activated CLL cells after 29 days of T-cell culture. Isolated T cells (2 × 106) were plated on day 0. The results represent the mean ± SD of experiments performed on 11 patients. Control T cells that were not stimulated did not survive in culture for this period. (B) Proliferation of autologous T cells in response to CD40-CLL cells compared with DCs pulsed with apoptotic bodies. Proliferation of autologous T cells in response to weekly stimulations with CD40-activated CLL cells or pulsed DCs after 29 days of T-cell culture. Isolated T cells (2 × 106) were plated on day 0. The results represent the mean ± SD of experiments performed on 11 (CD40-activated CLL cells as APCs) and 4 (pulsed DCs as APCs) patients.
Proliferation of autologous and allogeneic T cells in response to CD40-activated CLL cells.
(A) Proliferation of CD4+ autologous and allogeneic T cells in response to CD40-activated CLL cells after 1 (day 8), 2 (day 15), and 3 (day 22) stimulations with CD40-activated CLL cells. Mean and SD are shown. Control T cells that were not stimulated did not survive in culture for this period. (B) Proliferation of CD8+ autologous and allogeneic T cells in response to CD40-activated CLL cells after 1 (day 8), 2 (day 15), and 3 (day 22) stimulations with CD40-activated CLL cells. Mean and SD are shown. Control T cells that were not stimulated did not survive in culture for this period. In each case data represent the mean of at least 3 separate donor cells tested.
Proliferation of autologous and allogeneic T cells in response to CD40-activated CLL cells.
(A) Proliferation of CD4+ autologous and allogeneic T cells in response to CD40-activated CLL cells after 1 (day 8), 2 (day 15), and 3 (day 22) stimulations with CD40-activated CLL cells. Mean and SD are shown. Control T cells that were not stimulated did not survive in culture for this period. (B) Proliferation of CD8+ autologous and allogeneic T cells in response to CD40-activated CLL cells after 1 (day 8), 2 (day 15), and 3 (day 22) stimulations with CD40-activated CLL cells. Mean and SD are shown. Control T cells that were not stimulated did not survive in culture for this period. In each case data represent the mean of at least 3 separate donor cells tested.
Specific proliferation and cytotoxicity of activated T cells in response to unstimulated CLL cells
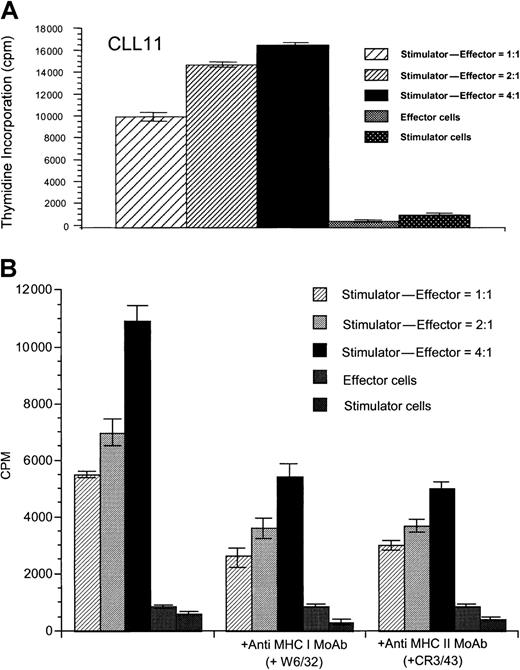
To determine whether T-cell lines generated after stimulation with CD40-activated CLL cells could proliferate in response to the native, unstimulated CLL cells, T cells were restimulated weekly 4 times with CD40-activated CLL cells. After 4 weekly stimulations with CD40-activated CLL cells, T cells proliferated specifically in response to unstimulated CLL cells in 3 of 5 allogeneic cases tested (data not shown) and in 5 of 7 autologous cases (Figure3A). T-cell lines generated against DCs pulsed with apoptotic bodies of CLL cells also demonstrated specific proliferation in response to unstimulated CLL cells in 3 of 4 autologous samples tested (data not shown), demonstrating that DCs in CLL patients must be capable of presenting antigens from the apoptotic CLL cells. Therefore, although unstimulated CLL cells cannot initiate a proliferative T-cell response, once these T cells have been generated, we here demonstrate that unstimulated CLL cells can function to induce proliferation of the established T-cell lines. Addition of either anti-MHC class I or anti-MHC class II mAbs partially reduced specific proliferation against the unstimulated CLL cells, suggesting that antigens are presented by both MHC class I and class II pathways (Figure 3B). Although allogeneic cytotoxic T cells that induced specific killing of CD40-activated or unstimulated CLL cells (Figure4) could be generated in 4 of 6 experiments performed, cytotoxic immune responses against CD40-activated CLL cells could be generated in this manner in only 1 of 9 autologous patient samples tested (data not shown). Comparable results were obtained after initial stimulation with DCs pulsed with autologous CLL apoptotic bodies, because cytotoxic immune responses could be generated only weakly in 1 of 4 different patient samples tested (data not shown). Addition of anti-CD80 mAb resulted in greatly reduced proliferation and cytotoxicity in both the autologous and allogeneic system (P < .01; data not shown), suggesting that the CD86/CD28 interaction could not substitute for CD28/CD80-mediated costimulation in this system. Although addition of anti-CD86 mAb partially reduced proliferation (P < .05) of both autologous and allogeneic T cells, cytotoxicity of the resulting allogeneic T cells against CLL cells was equal or even enhanced in 5 of 5 experiments performed (data not shown). Addition of IL-12 or mAbs against IL-10 or transforming growth factor β (TGF-β) did not enhance cytotoxic immune responses in the autologous system (data not shown).
Specific proliferation of autologous T cells in response to unstimulated CLL cells.
(A) Specific proliferation of autologous T cells in response to unstimulated CLL cells after stimulation with CD40-activated CLL cells is shown for patient CLL 11 and is representative of results seen in 5 of 7 experiments performed. (B) Proliferative response of autologous T cells is inhibited by addition of antibodies against MHC class I (W6/32) and MHC class II molecules (CR3/43). Results are shown for patient CLL 6 and are representative of 3 similar experiments performed. Thymidine incorporation was determined in cpm ± SD of triplicate determinations.
Specific proliferation of autologous T cells in response to unstimulated CLL cells.
(A) Specific proliferation of autologous T cells in response to unstimulated CLL cells after stimulation with CD40-activated CLL cells is shown for patient CLL 11 and is representative of results seen in 5 of 7 experiments performed. (B) Proliferative response of autologous T cells is inhibited by addition of antibodies against MHC class I (W6/32) and MHC class II molecules (CR3/43). Results are shown for patient CLL 6 and are representative of 3 similar experiments performed. Thymidine incorporation was determined in cpm ± SD of triplicate determinations.
Cytotoxic responses against CLL cells of allogeneic T cells.
After stimulation of allogeneic T cells from healthy donors with CD40-activated CLL cells, cytotoxic immune responses are shown in response to unstimulated (closed circles) and CD40-activated CLL cells (closed diamonds) and K562 cells as controls (open circles). Spontaneous release was determined by incubation of target cells in medium alone and maximum release was determined by resuspending the wells with 2% Triton X-100. Specific lysis was determined for each individual experiment as follows: specific lysis (%) = (experimental51Cr release − spontaneous 51Cr release)/maximum 51Cr release − spontaneous51Cr)release)] × 100 with an effector-target (E/T) ratio of 1:3, 1:10, and 1:30. Error bars represent SD. Results are representative of 4 of 6 experiments performed.
Cytotoxic responses against CLL cells of allogeneic T cells.
After stimulation of allogeneic T cells from healthy donors with CD40-activated CLL cells, cytotoxic immune responses are shown in response to unstimulated (closed circles) and CD40-activated CLL cells (closed diamonds) and K562 cells as controls (open circles). Spontaneous release was determined by incubation of target cells in medium alone and maximum release was determined by resuspending the wells with 2% Triton X-100. Specific lysis was determined for each individual experiment as follows: specific lysis (%) = (experimental51Cr release − spontaneous 51Cr release)/maximum 51Cr release − spontaneous51Cr)release)] × 100 with an effector-target (E/T) ratio of 1:3, 1:10, and 1:30. Error bars represent SD. Results are representative of 4 of 6 experiments performed.
Chemokine receptor expression on unstimulated and stimulated autologous and allogeneic T cells
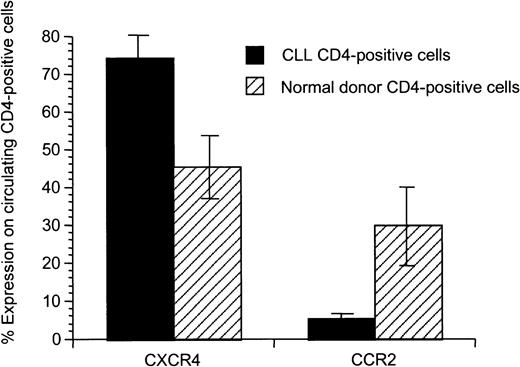
We hypothesized that there might be differences in the T-cell populations and activation state of autologous compared to allogeneic healthy T cells prior to priming. We saw no association between T-cell number or the CD4 and CD8 immunophenotype and the observed proliferative and cytotoxic responses (data not shown). To further investigate the T-cell populations, we analyzed chemokine receptor expression on CD4+ T cells of CLL patients compared to healthy donors and observed an altered chemokine receptor expression (Figure 5). CD4+ T cells from CLL patients exhibited enhanced expression of CXCR4 (P = .02) and decreased expression of CCR2 (P = .02).
Significant difference of expression of CXCR4 and CCR2 on CD4+ peripheral T cells from patients with CLL compared to healthy donors.
Mean and SD for 8 patients with CLL and 5 healthy donors are shown.
Significant difference of expression of CXCR4 and CCR2 on CD4+ peripheral T cells from patients with CLL compared to healthy donors.
Mean and SD for 8 patients with CLL and 5 healthy donors are shown.
Cytotoxic response after stimulation with pulsed DCs of T cells from patients with CLL against unstimulated and CD40-activated CLL cells
Given that we observed alterations in the chemokine receptor expression profile on T cells from patients with CLL compared to healthy donors, we further questioned whether basic defects reside in the T cells of patients with CLL or potentially in immunomodulatory effects of T cells seen in the autologous setting. We therefore examined whether T cells from patients with previously untreated CLL are capable of killing allogeneic CLL cells. Three patients with CLL underwent leukopheresis and from these preparations T cells were stimulated with DCs pulsed with apoptotic bodies of allogeneic CLL cells (Table 2). The resulting T-cell lines were then examined for their ability to kill the CD40-activated or unstimulated CLL target cells of the same patient from whom the apoptotic CLL cell bodies were generated. Under these circumstances, T cells from patients with CLL induced specific cytotoxicity in all 3 experiments performed using CD40-activated CLL cells as targets and killing of unstimulated CLL cells was induced in 2 of the 3 cases examined (Table 2). Again there was enhanced killing of CD40-activated CLL cells compared to unmodified CLL cells. These data suggest that T cells from patients with CLL are capable of killing allogeneic CLL cells and further that DCs from patients with CLL are capable of providing sufficient antigen presentation to initiate this response. They further demonstrate that T cells from patients with CLL are not globally defective in their cytotoxic function and that the generalized defects in granzyme-perforin or death ligand pathways are unlikely to be solely responsible for the observed absence of autologous cytotoxicity. However, these experiments do not rule out the possibility that in the autologous setting tolerance may occur that is specific for naturally presented peptides expressed on the autologous tumor cells.
Cytotoxic responses of allogeneic T cells of patients with CLL
| APC DCs . | Effector T cells . | Pulsed apoptotic cells . | Target cells . | Lysis, % . | |||
|---|---|---|---|---|---|---|---|
| Unstimulated CLL cells . | CD40-CLL cells . | K562 . | Unrelated CLL cells . | ||||
| CLL9 | CLL9 | CLL16 | CLL16 | 14 | 80 | 2 | 6 |
| CLL8 | CLL8 | CLL17 | CLL17 | 0 | 52 | 3 | 6 |
| CLL6 | CLL6 | CLL17 | CLL17 | 47 | 72 | 3 | 3 |
| APC DCs . | Effector T cells . | Pulsed apoptotic cells . | Target cells . | Lysis, % . | |||
|---|---|---|---|---|---|---|---|
| Unstimulated CLL cells . | CD40-CLL cells . | K562 . | Unrelated CLL cells . | ||||
| CLL9 | CLL9 | CLL16 | CLL16 | 14 | 80 | 2 | 6 |
| CLL8 | CLL8 | CLL17 | CLL17 | 0 | 52 | 3 | 6 |
| CLL6 | CLL6 | CLL17 | CLL17 | 47 | 72 | 3 | 3 |
Specific cytotoxicity of T cells against unstimulated and CD40-activated CLL cells after 4 stimulations with autologous DCs pulsed with apoptotic bodies of allogeneic CLL cells. The percent of lysis represents the mean of triplicates with an effector-target ratio of 1:30. SD was less than ± 10% in all experiments shown.
Discussion
The aim of this study was to investigate further the nature of immune responses of autologous and allogeneic T cells against CLL cells. Autologous cytotoxic T cells can be generated against immunoglobulin-derived peptides presented by APCs. The resulting T-cell lines kill unmodified CLL cells, demonstrating that CLL cells must be capable of presenting this tumor antigen. To examine whether T cells could be primed against naturally processed and presented tumor antigens by CD40-activated CLL cells and by DCs pulsed with apoptotic bodies of CLL cells, autologous and allogeneic T cells were stimulated with CD40-activated CLL cells and proliferative and cytotoxic responses against CD40-activated and unmodified CLL cells were examined. Whereas there was no difference in proliferation of CD4+autologous and allogeneic T cells, we observed reduced proliferation of autologous CD8+T cells. In most cases we were able to generate T-cell lines that exhibited specific proliferation in response to unmodified CLL cells, an observation that has not previously been reported. Proliferation of the autologous T-cell lines against unmodified CLL cells could be partially blocked by either MHC class I or class II mAbs, suggesting that both class I– and class II–restricted CLL-associated antigens are being presented and recognized by autologous T cells. Cytotoxic immune responses could be generated against autologous CD40-activated and unmodified CLL cells in only 2 patients, suggesting that these specifically proliferating autologous T cells do not provide sufficient T-cell help for induction of cytotoxic T-cell responses. Autologous DCs pulsed with apoptotic bodies of allogeneic CLL cells can present allogeneic CLL cell peptides and induced cytotoxic T-cell responses, suggesting that T cells of patients with CLL are functionally able to generate cytotoxic immune responses against allogeneic CLL cells. The low autologous cytotoxic responses might also be due to primary or secondary alterations in the effector populations, in keeping with differences in chemokine receptor expression on T cells from patients with CLL and healthy donors. Therefore, the interaction in vivo between autologous CLL cells, APCs, and T cells might favor induction of tolerance rather than induction of active antitumor responses.
Several reasons might be responsible for the low capacity to induce cytotoxic T-cell responses against autologous CLL cells. CLL cells fail to express or express weakly, critical adhesion and costimulatory molecules and CD154 has been shown to be an effective tool to up-regulate costimulatory molecules on the surface of CLL cells.24-26 We have previously demonstrated that killing of CLL cells by cytotoxic T cells generated against immunoglobulin-derived peptides presented by APCs was increased by either addition of exogenous peptide or by CD40 activation of the CLL target cells.21 This suggests that inadequate antigen presentation as well as costimulation contribute to the low levels of cytotoxicity observed against native CLL cells. In the present study, cytotoxic immune responses against CD40-activated CLL cells were always higher than against unmodified CLL cells. Addition of blocking mAbs against CD80 and CD86 reduced proliferative and cytotoxic immune responses, further demonstrating that adequate costimulation enhances T-cell effector function as well as being necessary for induction of T-cell–mediated immune responses. However, in contrast to other B-cell malignancies, enhancement of costimulation by CD40 activation or by presentation of whole tumor cells by DCs does not appear to be sufficient to induce autologous cytotoxic T-cell responses in CLL and additional factors probably play a role in regulating this response.
Tolerance induced by low differences for self-/non–self-discrimination, deletion of potentially self-reactive T cells due to thymic expression of the self-protein, or induction of peripheral T-cell tolerance in the tumor-bearing host may be responsible for T-cell unresponsiveness in CLL and the difficulty of initiating antitumor responses against naturally presented antigens. The superiority of peptide-pulsed APCs in inducing cytotoxic responses compared to CD40-activated CLL cells or pulsed DCs could be explained by the fact that antigen-derived peptides with low natural immunogenicity might represent subdominant epitopes normally ignored in the presence of tolerogenic dominant epitopes naturally presented by CD40-activated CLL cells and pulsed CCs.38 Dominant epitopes presented by autologous APCs might activate CD4 immunoregulatory cells responsible for induction of tolerance. Thus, peptides naturally presented by autologous APCs in vivo might not be ideal to induce autologous cytotoxic immune responses.39Alteration of the peripheral T-cell pool possibly induced in vivo by CLL cells might be involved in the reduced capability to induce cytotoxic T-cell responses against autologous CLL cells. We observed significantly increased CXCR4 expression and decreased CCR2 expression on T cells in patients with CLL compared to healthy donors, suggesting alterations in the circulating T-cell pool in patients with CLL. CXCR4, which has significantly enhanced expression on peripheral T cells of patients with CLL compared to healthy donors, is highly expressed on naı̈ve T cells compared to memory cells.40 Peripheral cytokines seem to regulate its expression as IL-2 and TGFβ-1 induce up-regulation of CXCR4 on naı̈ve T cells.40,41 CCR2 is down-regulated on T cells in CLL and it has been shown that CCR2 knockout mice have delayed T-cell priming and IFN-γ production against Mycobacterium tuberculosis.42 Thus, mechanisms responsible for modulation of chemokine receptor expression on T cells in CLL patients may have an impact on immunosuppression and reduced autologous antitumor effects seen in these patients. In our experiments, addition of exogenous IL-12 or mAbs against IL-10 or TGF-β did not enhance cytotoxic immune responses in the autologous system (data not shown).
In conclusion, these data further demonstrate that specific allogeneic and autologous proliferative T-cell responses can be generated against CLL cells. However, the role of these specific proliferating T cells after stimulation with autologous APCs is not clear and does not result in enhancement of cytotoxicity. These results need to be considered in the development of novel immunotherapeutic strategies in this disease. Future studies are needed to identify and further stimulate the antitumor effector cells and to prevent or reverse induction of tolerance.
We thank David Zahrieh and Donna Neuberg for statistical analysis and Peter Varney for assistance.
Supported by a grant to J.G.G. from the National Cancer Institute (CA 81534) and by the Grayce B. Kerr Foundation. A.M.K. and M.W. were supported by grants from the Deutsche Krebshilfe.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
John G. Gribben, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: john_gribben@dfci.harvard.edu.

![Fig. 4. Cytotoxic responses against CLL cells of allogeneic T cells. / After stimulation of allogeneic T cells from healthy donors with CD40-activated CLL cells, cytotoxic immune responses are shown in response to unstimulated (closed circles) and CD40-activated CLL cells (closed diamonds) and K562 cells as controls (open circles). Spontaneous release was determined by incubation of target cells in medium alone and maximum release was determined by resuspending the wells with 2% Triton X-100. Specific lysis was determined for each individual experiment as follows: specific lysis (%) = (experimental51Cr release − spontaneous 51Cr release)/maximum 51Cr release − spontaneous51Cr)release)] × 100 with an effector-target (E/T) ratio of 1:3, 1:10, and 1:30. Error bars represent SD. Results are representative of 4 of 6 experiments performed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/100/1/10.1182_blood.v100.1.167/7/m_h81322774004.jpeg?Expires=1769120621&Signature=yLPaePhPs1kZENJdKE5BhHc-q1TpQ3850ljVYXdw5I8ETsafQMRLTackdCng~sn5EgV5ZdF8i5Pgf2yjiwE6BReqKZ-C64YeRZ-M7LvPv29-y2CdaQ2utbmdgpx3~cDj3ykG89VZc-LW1X7PLEOzwjp5Ewc9PJiJXM9AwyhN5sopFdec9Kw1J6hgrMYI4EXu1bguRmYSlDlpN65KVKucb5IWTxQvFD9WeMlvu~~GPu9OrDbjT22eOULaR1usCyUic~tqBgkvBrH2G3PnzQb8NLFwZqOYZS2BxE5gtv8GvzLGEDi8REu1BrougYJjRwD9X94d3naH34N4N3kSHsvp0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal