Abstract
Fulminant hepatic failure (FHF) in humans produces a bleeding diathesis due in large part to a reduction in the biosynthesis of liver-derived coagulation factors. Remarkably, factor VIII procoagulant activity is elevated in most of these patients despite widespread liver cell death. FHF can be modeled in mice by administration of azoxymethane, the active ingredient found in cycad palm nuts. We compared the expression of factor VIII to other hepatic hemostatic factors in azoxymethane-induced murine FHF. Mice displayed dose-dependent decreases in all coagulation factor activities measured, including factors V, VII, VIII, and IX. At the highest dose of azoxymethane (50 μg/g body weight), factor VIII activity in plasma decreased by 98% within 36 hours after treatment, which was associated with an 80% reduction in hepatic factor VIII messenger RNA (mRNA). In contrast, factor VIII mRNA levels in spleen, kidney, and lung tissue of azoxymethane-treated mice were unchanged. Cellular damage in these mice appeared to be limited to hepatocytes as evident by histologic examination. This finding is supported by 2 observations. First, hepatic mRNA levels of von Willebrand factor, which is synthesized by liver sinusoidal endothelial cells but not hepatocytes, were unchanged. Second, von Willebrand factor was detected antigenically in liver sections of azoxymethane-treated mice by immunofluorescence. These results indicate that the contribution of the liver to factor VIII biosynthesis is not replaced or significantly supplemented by other tissues in this model of FHF.
Introduction
Despite more than 50 years of investigation, the nature of factor VIII biosynthesis in vivo remains poorly understood.1-4 Factor VIII messenger RNA (mRNA) is present in nearly every tissue examined, including liver, spleen, lymph node, heart, brain, lung, kidney, testes, muscle, and placenta.5-7 The liver clearly is an important site of synthesis because liver transplantation cures human and canine hemophilia A.8-10 However, the relative contribution of hepatocytes and liver sinusoidal endothelial cells remains uncertain.7 11-15 Furthermore, the contribution of extrahepatic biosynthesis of factor VIII in health and disease states is not known.
Paradoxically, there usually is a significant rise in plasma factor VIII activity in human fulminant hepatic failure (FHF).16-19 This increase can be more than 14 times normal levels and occurs simultaneously with profound decreases in the circulating levels of vitamin K–dependent and fibrinolytic plasma proteins.16,20,21 The analysis of this phenomenon has been hindered by the lack of a reproducible animal model of FHF. However, a murine model of FHF recently was developed using the hepatotoxin azoxymethane (AOM), which is found in cycad palm nuts on the island of Guam.22 AOM-treated mice develop hepatocellular necrosis, elevated liver transaminase and ammonia levels in blood and hepatic encephalopathy. They die within days, with death related to the dose of AOM. In this study, we examined the expression of factor VIII and several other hemostatic factors, including factors V, VII, and IX, and von Willebrand factor (VWF) in AOM-induced murine FHF.
Materials and methods
Materials
Male C57BL/6 mice (22-30 g body weight) were purchased from Charles River Laboratory (Wilmington, MA). AOM and TriReagent were purchased from Sigma (St Louis, MO). AOM was diluted in sterile saline to a working concentration of 10 mg/mL and stored in aliquots at −70°C. Citrated human factor V–, VII–, VIII–, and IX–deficient plasmas and normal pooled human plasma (FACT) were purchased from George King Biomedical (Overland Park, KA). Neoplastine was purchased from Diagnostica Stago (Asnieres, France). Automated activated partial thromboplastin time (aPTT) reagent was purchased from Organon Teknika (Durham, NC). Oligonucleotides were synthesized by Gibco BRL (Gaithersburg, MD).
Animal care and maintenance
Mice were fed rodent chow and water ad libitum and kept on a 12 hour-12 hour light-dark cycle. AOM was administered by intraperitoneal injection at doses of 0, 15, 30, or 50 μg/g body weight to 8- to 12-week-old mice. Blood was collected by tail bleed or terminal cardiac puncture into one-tenth volume 3.8% sterile trisodium citrate. Plasma was isolated from whole blood by centrifugation at 1800g for 15 minutes at 4°C and stored at −70°C until assayed. In all experiments saline-injected control mice were age matched with experimental mouse groups.
Histology and immunohistochemistry
Organs designated for histology were harvested and immediately placed in 10% neutral phosphate-buffered formalin (Fisher Scientific, Pittsburgh, PA) and incubated at 4°C overnight for fixation. Tissue processing and sectioning were performed by the Department of Animal Resources at Emory University. Tissue sections 5 to 6 μm thick were stained with hematoxylin and eosin and visualized by light microscopy.
Portions of livers designated for immunofluorescence were harvested and immediately placed in liquid nitrogen for 1 to 2 minutes. Tissue-Tek OCT (Sakura, Torrance, CA) was applied to tissues prior to sectioning. Sections 6 μm thick were cut at −20°C, equilibrated to room temperature, and fixed with acetone. All subsequent incubation steps were performed in a humidified chamber. Successive blocking steps included addition of avidin and biotin blockade (Vector Kit, Vector Laboratories, Burlingame, CA) for 15 minutes each, 15 ng/mL Fc blockade (BD Pharmingen, San Diego, CA) for 15 minutes, and 4% normal goat serum blockade (Vector Laboratories) for 30 minutes. Incubation with primary polyclonal rabbit IgG antihuman VWF (Dako, Carpinteria, CA; 1:100 dilution) proceeded for 1 hour followed by a 15-minute incubation with biotinylated secondary goat antirabbit IgG (Vector Laboratories; 1:200 dilution). Between each antibody application, slides were washed thoroughly with phosphate-buffered saline (PBS). Incubation with avidin-conjugated fluorescein isothiocyanate (FITC; 1:50 dilution; Vector Laboratories) was performed for 15 minutes. Slides were then washed with PBS and mounted using aqueous media (Lerner Laboratories, Pittsburgh, PA).
Coagulation factor assays
Clotting assays were performed using a ST art 4 BIO Coagulation Instrument (Diagnostica Stago). For factor V and factor VII assays, 50 μL factor V- or factor VII-deficient plasma and 5 μL citrated mouse plasma were dispensed into a prewarmed cuvette at 37°C. After a 60-second incubation, 100 μL neoplastine was added and the clotting time was measured. For assays of factors VIII and IX, 50 μL factor VIII– or factor IX–deficient plasma, 5 μL citrated mouse plasma, and 50 μL aPTT were dispensed into a prewarmed cuvette at 37°C. After a 180-second incubation, 50 μL 0.02 M CaCl2 was added to initiate the clotting time. Clotting times were compared to a standard curve prepared using pooled normal human plasma using linear regression analysis of clotting time versus the logarithm of activity. The coagulation factor activities stated by the manufacturer of the standard plasma were used. The activities of factors V, VII, VIII, and IX in plasmas of mice obtained 72 hours after injection of control saline were 11.1 ± 2.1, 4.65 ± 0.73, 3.09 ± 0.89, and 0.93 ± 0.16 U/mL (mean ± sample SD), respectively.
RNA analysis
Tissues for RNA analysis were harvested, immediately placed in RNAlater (Ambion, Austin, TX), and stored at 4°C until use. RNA extraction was performed using TriReagent following the manufacturer's protocol. RNA integrity was confirmed by an A260/A280 ratio of more than 1.7 and ethidium bromide visualization following agarose gel electrophoresis. Competitive reverse transcription–polymerase chain reaction (RT-PCR) analysis was performed as described previously11 with the following changes. Factor VIII competitor complementary (RNA) was constructed by PCR amplification of total liver complementary DNA (cDNA) using the following primers: (1) loop-out sense primer, 5′-ctgggtaccgggccctGGGACCTTTACTTTATGG//CCATATAACATTTACCCTCA-3′, corresponding to murine factor VIII cDNA5nucleotides 1402-1490 (in caps) with the deletion of nucleotides 1421-1469, where the underlined region designates the ApaI restriction site and the // designates the position of the deletion, and (2) antisense primer, 5′-CACCGCGGTGGCGGCCGCATAGACCATTTTGTGTTTGAA-3′, corresponding to nucleotides 1884-1923, where the underlined region contains a NotI restriction site. The PCR product containing the loop-out region was cloned into the pBluescript II KS-phagemid (Stratagene, La Jolla, CA) using the vectorApaI/NotI restriction sites. The VWF competitor RNA was constructed by PCR amplification of total liver cDNA using the following primers based on the sequence of murine VWF exon 2823 using the human full-length pre-pro-VWF cDNA numbering system24: (1) loop-out sense primer, 5′-ccggaattccggCAGATCCGCCTCATCGAGAAGC AGG//AGCTACCTCTGTGACCTTGC-3′, corresponding to nucleotides 4369-4481 (in caps), where the underlined region designates the EcoRI restriction site and the // designates the position of the deletion, and (2) antisense primer, 5′-CGCGGATCCGCGGCCGCCCTGGTAGCGGATCTC-3′, corresponding to nucleotides 4798-4830, where the underlined region designates aBamHI restriction site. The VWF loop-out PCR product was cloned as described above using the EcoRI/BamHI restriction sites. cRNA molecules were generated by in vitro transcription using T3 RNA polymerase. Transcripts were purified using RNeasy spin columns (Qiagen, Valencia, CA) and quantitated spectrophotometrically.
Competitive RT-PCR was performed using the One-Step RT-PCR Kit (Qiagen) following the manufacturer's protocol. Two hundred nanograms total RNA was used along with serial dilutions of competitor cRNA in a final reaction volume of 50 μL. The sequences of the sense and antisense factor VIII PCR primers used were 5′-TGGGACCTTTACTTTATGG-3′ and 5′-AAAAACATAGCCATTGATGCTGTG-3′, respectively. The sequences of the VWF PCR sense and antisense primers were 5′-GAGATCCGCTACCAGGGCGGC-3′ and 5′-GCCGCCCTGGTAGCGGATCTC-3′, respectively. PCR reactions were performed in a Hybaid OmniGene thermocycler. The conditions for factor VIII DNA amplification were 55°C, 35 minutes (1 cycle); 95°C, 5 minutes (1 cycle); 95°C, 1 minute, 58°C, 1 minute, 72°C, 1 minute (35 cycles); 72°C, 10 minutes (1 cycle); and 30°C, 30 seconds (1 cycle). The conditions for VWF amplification were 55°C, 35 minutes (1 cycle); 95°C, 15 minutes (1 cycle); 95°C, 1 minute, 68°C, 1 minute, 72°C, 1 minute (35 cycles); 72°C, 10 minutes and 30°C, 30 seconds (1 cycle). PCR products were visualized by agarose gel electrophoresis and quantitated densitometrically as described previously.11
Results
Induction of FHF in mice
Eight- to 12-week-old male C57BL/6 mice received intraperitoneal injections of 0, 15, 30, and 50 μg/g body weight AOM (saline control, AOM-15, AOM-30, and AOM-50, respectively). Mice in the AOM-30 and AOM-50 groups became lethargic within 8 hours, indicative of stage II hepatic encephalopathy. In contrast, AOM-15 mice maintained normal activity. All AOM-30 and AOM-50 mice developed coma (stage IV hepatic encephalopathy) and died, whereas AOM-15 mice remained asymptomatic until 72 hours after injection, at which time they were killed. The average postinjection survival times in the AOM-30 and AOM-50 groups were 45 and 33 hours, respectively. These results are consistent with those of the original report of AOM-induced murine FHF, in which 20 μg/g body weight of AOM was not toxic, but 50 μg/g body weight was uniformly fatal.22
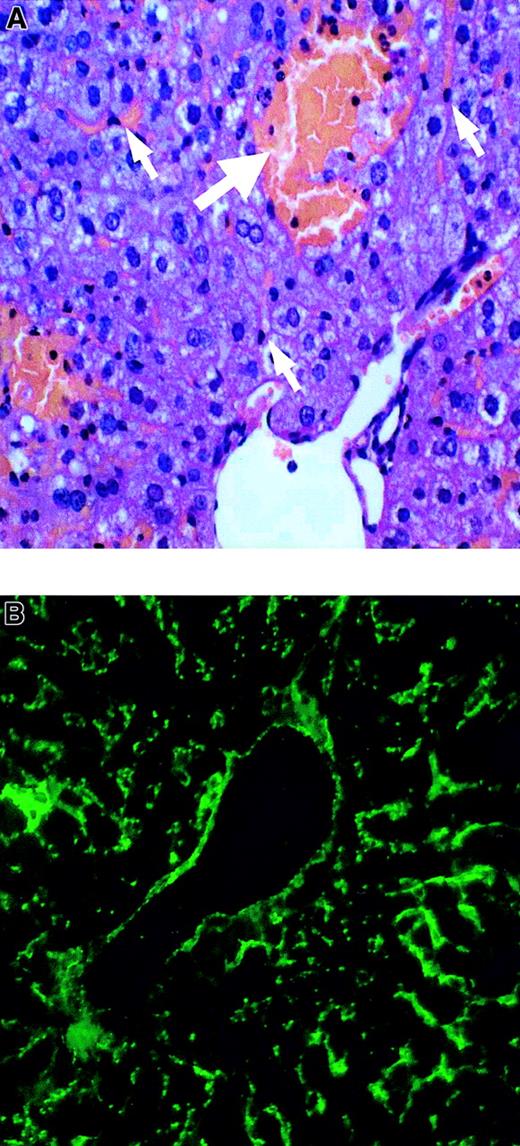
Liver, spleen, kidney, and lung tissues from the control and AOM groups were evaluated histologically. Liver sections from the AOM-30 and AOM-50 groups displayed moderate and severe multifocal necrosis, respectively, with apparent sparing of liver sinusoidal endothelial cells (Figure 1A). Hepatocellular necrosis was not evident in the AOM-15 and saline control tissues. Spleen, kidney, and lung tissues from mice in all groups appeared normal (not shown). VWF protein was detected in control and AOM-50 mice by immunofluorescence. AOM-50 mice displayed intense staining for VWF antigen within the vascular and sinusoidal endothelium (Figure 1B). This result also is consistent with the proposal that AOM is specifically toxic to hepatocytes.22
Liver histology and VWF staining in AOM-induced FHF.
(A) Paraffin section AOM-50 mouse liver stained with hematoxylin and eosin and visualized by light microscopy. White arrows denote liver sinusoidal endothelial cells. Severe necrosis is identified by the bolded arrow. (B) Frozen section of AOM-50 mouse liver revealing staining of VWF in large vessel and sinusoidal endothelium by FITC immunofluorescence. Original magnification × 200 in panels A and B.
Liver histology and VWF staining in AOM-induced FHF.
(A) Paraffin section AOM-50 mouse liver stained with hematoxylin and eosin and visualized by light microscopy. White arrows denote liver sinusoidal endothelial cells. Severe necrosis is identified by the bolded arrow. (B) Frozen section of AOM-50 mouse liver revealing staining of VWF in large vessel and sinusoidal endothelium by FITC immunofluorescence. Original magnification × 200 in panels A and B.
Levels of plasma factors V, VII, and IX in AOM-treated mice
The synthesis of the vitamin K–dependent coagulation proteins, which include prothrombin, factors VII, IX, and X, protein C, protein S, and protein Z, occurs in hepatocytes. Factor V is expressed in hepatocytes and also in megakaryocytes and possibly certain types of endothelial cells.25 Compared to control mice, plasma levels of factors V, VII, and IX were normal or slightly increased in the AOM-15 group, but were markedly decreased in the AOM-30 and AOM-50 groups (Table 1). Levels of factors V and VII were less than 2% of control, whereas the reduction in factor IX was not as severe. This may be due to a longer circulatory life of factor IX compared to other coagulation factors.26 The data correlate well with the hepatic necrosis observed in the treatment groups. The decrease in activity of these factors mimics the presentation of human FHF.
Plasma coagulation factor levels after AOM treatment
| Group . | Time (h) . | Factor V (%)* . | Factor VII (%)* . | Factor IX (%)* . |
|---|---|---|---|---|
| AOM-15 | 72 | 99.9 ± 9.5 (5) | 134 ± 35 (5) | 133 ± 18 (5) |
| AOM-30 | 48 | 2.4 ± 0.3 (2) | 10.1 ± 6.2 (2) | 29.0 ± 5.0 (2) |
| AOM-50 | 36 | 0.59 ± 0.05 (3) | 1.6 ± 0.2 (3) | 11.3 ± 2.9 (3) |
| Group . | Time (h) . | Factor V (%)* . | Factor VII (%)* . | Factor IX (%)* . |
|---|---|---|---|---|
| AOM-15 | 72 | 99.9 ± 9.5 (5) | 134 ± 35 (5) | 133 ± 18 (5) |
| AOM-30 | 48 | 2.4 ± 0.3 (2) | 10.1 ± 6.2 (2) | 29.0 ± 5.0 (2) |
| AOM-50 | 36 | 0.59 ± 0.05 (3) | 1.6 ± 0.2 (3) | 11.3 ± 2.9 (3) |
Percent procoagulant activity (mean ± sample SD with the number of mice in parenthesis) relative to saline-injected control mice at 72 hours.
Plasma factor VIII levels in AOM-treated mice
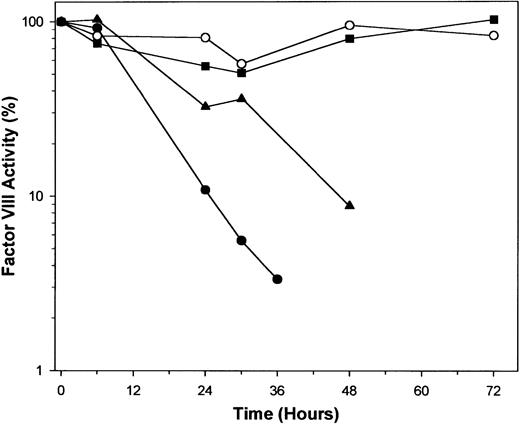
Activity of factor VIII decreased with time in a dose-dependent fashion (Figure 2). In the AOM-30 and AOM-50 groups, there was a progressive decline to less than 10% of initial levels until death. In the AOM-15 group, activity of factor VIII appeared to decrease during the first 30 hours, but returned to normal by the end of the experiment. An equal volume of normal mouse plasma completely corrected the clotting defect in plasma from AOM-treated mice, excluding the possibility that these results were due to a nonspecific inhibitor of the aPTT-based factor VIII assay or a specific inhibitor of factor VIII (data not shown).
Factor VIII procoagulant activity in control and AOM-treated mice.
Plasmas were obtained by tail snip bleeding in control (open circles), AOM-15 (squares), AOM-30 (triangles), and AOM-50 (closed circles) groups and factor VIII was measured as described in “Materials and methods.” Each data point represents the mean value of 5 to 7 mice, except in the AOM-30 group at 48 hours (n = 3) and the AOM-50 group at 36 hours (n = 3), which had lower numbers due to death.
Factor VIII procoagulant activity in control and AOM-treated mice.
Plasmas were obtained by tail snip bleeding in control (open circles), AOM-15 (squares), AOM-30 (triangles), and AOM-50 (closed circles) groups and factor VIII was measured as described in “Materials and methods.” Each data point represents the mean value of 5 to 7 mice, except in the AOM-30 group at 48 hours (n = 3) and the AOM-50 group at 36 hours (n = 3), which had lower numbers due to death.
Steady-state factor VIII hepatic mRNA levels in AOM-treated mice
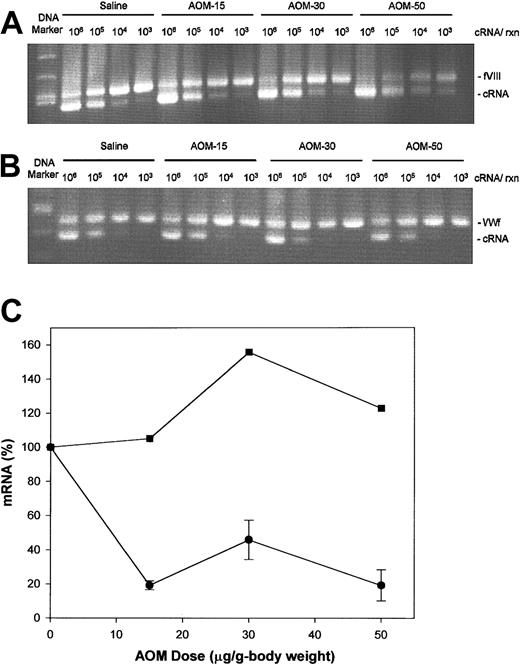
Competitive RT-PCR analysis for factor VIII mRNA was performed on total liver RNA from control and AOM-treated groups (Figure 3A). As a control, competitive RT-PCR of VWF mRNA, which is synthesized in liver sinusoidal endothelial cells but not hepatocytes, also was done (Figure 3B). In this method,11 27-29 known amounts of synthetic competitor cRNA, corresponding to the amplified region except for a small looped-out segment, is added to the total RNA sample. The cDNA produced from the cRNA is a competitive inhibitor in the PCR reaction and produces a band with slightly greater electrophoretic mobility. The point of equivalence, where the amount of each PCR product is equal, yields the desired number of copies of mRNA. The point of equivalence was determined by linear regression of the log ratio of cRNA/mRNA band intensity versus log cRNA concentration. The factor VIII mRNA levels were significantly decreased in the AOM-15, AOM-30, and AOM-50 groups (P = .034, .028, and .023, respectively, ttest) to approximately 20% of control values (Figure 3C). In contrast, VWF mRNA levels were not changed at any dose of AOM, indicating that vascular and sinusoidal endothelial cells remain functionally intact in AOM-induced FHF. The factor VIII mRNA levels in spleen, kidney, or lung tissue were not changed in any of the AOM groups (data not shown).
Hepatic factor VIII and VWF mRNA levels in AOM-treated mice.
Competitive RT-PCR analysis of factor VIII (A) and VWF (B) mRNA was performed on total liver RNA from mice killed at 36 (AOM-50), 48 (AOM-30), or 72 hours (AOM-15 and saline controls), as described in “Materials and methods.” The number of cRNA copies added to the reaction is shown above the gel. DNA markers are 700, 600, 517, and 500 base pair (bp) in panel A and 517, 500, 400, and 300 bp in panel B. (C) Quantitation of competitive RT-PCR data for factor VIII (circles) and VWF (squares). Bands derived from mRNA and cRNA were stained with ethidium bromide and quantitated densitometrically. For determination of factor VIII mRNA levels, the number of mice in the control, AOM-15, AOM-30, and AOM-50 groups was 3, 3, 2, and 2, respectively. Data are expressed as mean ± sample SD.
Hepatic factor VIII and VWF mRNA levels in AOM-treated mice.
Competitive RT-PCR analysis of factor VIII (A) and VWF (B) mRNA was performed on total liver RNA from mice killed at 36 (AOM-50), 48 (AOM-30), or 72 hours (AOM-15 and saline controls), as described in “Materials and methods.” The number of cRNA copies added to the reaction is shown above the gel. DNA markers are 700, 600, 517, and 500 base pair (bp) in panel A and 517, 500, 400, and 300 bp in panel B. (C) Quantitation of competitive RT-PCR data for factor VIII (circles) and VWF (squares). Bands derived from mRNA and cRNA were stained with ethidium bromide and quantitated densitometrically. For determination of factor VIII mRNA levels, the number of mice in the control, AOM-15, AOM-30, and AOM-50 groups was 3, 3, 2, and 2, respectively. Data are expressed as mean ± sample SD.
Discussion
Several interesting and apparently disparate observations have been made related to the biosynthesis of factor VIII. Liver transplantation in human and canine hemophilia A results in an increase in factor VIII levels to normal, and thus cures the bleeding diathesis.8-10 However, transplantation of liver from hemophilia A dogs to normal dogs does not produce hemophilia A.10 These findings indicate that the liver is sufficient but not necessary for factor VIII synthesis. However, extrahepatic sites of hemostatically significant factor VIII synthesis have not been identified conclusively. Human and canine hemophilia A are not cured by bone marrow or kidney transplantation.10,30-32 Spleen transplantation in hemophilia A dogs has yielded conflicting results, probably due to poor graft survival.33-35 Transplantation of normal spleen cells into patients with hemophilia A reportedly produced factor VIII levels over 30% of normal for up to 4 months,36,37 but these results have not been confirmed. Additionally, spleen transplantation in humans did not produce increased factor VIII levels in another study.31 The spleen is not necessary for production of normal factor VIII levels because splenectomy is not associated with a decrease in levels.
FHF, defined as hepatic failure with stage III (deep somnolence) or IV (coma) encephalopathy that develops in less than 8 weeks in the absence of pre-existing liver disease, is associated with increased factor VIII levels in humans.16-19,38 In contrast, all other hepatic coagulation and fibrinolytic factors, including fibrinogen; prothrombin; factors V, VII, IX, X, XI, XII, and XIII; prekallikrein; high-molecular-weight kininogen; protein C; plasminogen; antithrombin III; and α2-antiplasmin are decreased in FHF.39 Elevated levels of factor VIII also have been observed in cirrhosis in some,38,40,41 but not all,42 studies.
Human FHF usually is due to acetaminophen (paracetamol) overdose or viral hepatitis. Viral hepatitis is associated with higher elevations of factor VIII than acetaminophen toxicity, possibly due to the longer disease course in viral hepatitis.17 However, in 8 cases of FHF due to Amanita phalloides mushroom poisoning, factor VIII levels were normal, whereas levels of prothrombin, factors V, VII, IX, and X, and plasminogen were severely reduced.43 Thus, within the clinical setting of FHF, different mechanisms of liver injury have differential effects on factor VIII expression.
FHF is a setting that could provide valuable insights into the control of factor VIII expression. However, it has been difficult to develop animal models of FHF.44 Acetaminophen and carbon tetrachloride, which reliably produce FHF in humans, have inconsistent effects in mice and other animals. Additionally, there is no widely used model of FHF due to viral hepatitis. Galactosamine, which has been used as a hepatotoxin in rabbits, dogs, rats, and mice, produces variable results, is not a cause of FHF in humans, and produces a different histologic pattern of injury than other FHF syndromes.22,44 45
The recent report that AOM produces FHF in mice prompted us to investigate its effect on the synthesis of factor VIII and other coagulation factors. We found that AOM reproducibly produced hepatic necrosis, FHF, and death in C57BL/6 mice in a dose-dependent manner similar to the original report.22 At the highest doses of AOM used (30 and 50 μg/g body weight), there was a dramatic drop in factor VIII levels, as well as factors V, VII, and IX (Table 1). The decrease in factor VIII was associated with a decrease in factor VIII mRNA in liver (Figure 3A,C), but not kidney, lung, or spleen. In contrast, hepatic VWF mRNA and antigen levels did not decrease in AOM-mediated FHF (Figure 3B,C). Because VWF is synthesized by endothelial cells, including liver sinusoidal endothelium, but not hepatocytes,11 46 the results indicate that AOM produces selective necrosis of hepatocytes and spares liver sinusoidal endothelial cells and extrahepatic tissues.
Although factor VIII levels in the AOM-15 group decreased only transiently, there was a decrease in hepatic factor VIII mRNA levels in this group similar to that observed in the AOM-30 and AOM-50 groups (Figure 3C). This indicates that defects in factor VIII synthesis at the translational or posttranslational level are a major cause of the decrease in factor VIII levels in the AOM-30 and AOM-50 groups. Alternatively, local extracellular degradation of newly synthesized factor VIII, perhaps by proteolysis associated with necrosis or apoptosis possibly is an important factor.
The decrease in factor VIII levels in this model is in contrast to the elevated factor VIII levels that are induced by acetaminophen or viral hepatitis in humans. In these settings, extracellular signals could be generated in response to liver injury that promote extrahepatic or liver sinusoidal endothelial cell factor VIII synthesis. The mechanism of the injury produced by AOM in mice, which is unknown,22may differ qualitatively or in degree of severity such that these signals are not produced, which may be species specific or a general property of AOM-induced liver injury. Further investigation into the pathophysiology of AOM-induced liver injury will be necessary to address this issue.
We wish to thank Dr Dirck Dillehay in the Division of Animal Resources at Emory University for his evaluation of the histologic specimens.
Supported by grant R01-HL40921 from the National Institutes of Health.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Pete Lollar, Winship Cancer Institute, 1639 Pierce Dr, Rm 1003, Woodruff Memorial Bldg, Emory University, Atlanta, GA 30322; e-mail: jlollar@emory.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal