Abstract
The phagocyte nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH) oxidase was functionally reconstituted in monkey kidney COS-7 cells by transfection of essential subunits, gp91phox, p22phox, p47phox, and p67phox. COS-7 cells express the essential small guanosine 5′-triphosphatase, Rac1. Transgenic COS-phox cells were capable of arachidonic acid–induced NADPH oxidase activity up to 80% of that of human neutrophils, and of phorbol myristate acetate (PMA)–induced activity up to 20% of that of neutrophils. Expression of all 4 phox components was required for enzyme activity, and enzyme activation was associated with membrane translocation of p47phox, p67phox, and Rac1. Expression of p47phox Ser303Ala/Ser304Ala or Ser379Ala phosphorylation-deficient mutants resulted in significantly impaired NAPDH oxidase activity, compared with expression of wild-type p47phox or the p47phox Ser303Glu/Ser304Glu phosphorylation mimic, suggesting that p47phoxphosphorylation contributes to enzyme activity in the COS system, as is the case in neutrophils. Hence, COS-phox cells should be useful as a new whole-cell model that is both capable of high-level superoxide production and readily amenable to genetic manipulation for investigation of NADPH oxidase function. PMA-elicited superoxide production in COS-phox cells was regulated by activation of protein kinase C (PKC) and Rac. Although COS-7 cells differ from human neutrophils in PKC isoform expression, transient expression of major neutrophil isoforms in COS-phox cells did not increase PMA-induced superoxide production, suggesting that endogenous isoforms were not rate limiting. Val204 in p67phox, previously shown to be required for NADPH oxidase activity under cell-free conditions, was found to be essential for superoxide production by intact COS-phox cells, on the basis of transfection studies using a p67phox(Val204Ala) mutant.
Introduction
The phagocyte nicotinamide adenine dinucleotide phosphate (reduced form) (NADPH) oxidase plays a central role in host defense and in the inflammatory response by catalyzing the transfer of electrons from NADPH to molecular oxygen, producing superoxide radicals.1,2 Superoxide is directly microbicidal and is also converted into reactive oxygen derivatives, which synergize with toxic granule proteins to facilitate microbial killing. Genetic deficiency of the NADPH oxidase results in chronic granulomatous disease, a syndrome characterized by recurrent, often life-threatening, infections and chronic granulomas.3However, although the NADPH oxidase is essential for host defense, excessive or inappropriate release of reactive oxidants has been implicated as a mediator of inflammatory tissue injury.4
The NADPH oxidase is a multicomponent enzyme whose activity requires assembly of cytosolic subunits p47phox, p67phox, and Rac with membrane-associated flavocytochrome b558, a heterodimer of gp91phox and p22phox.5 In resting neutrophils, p47phox and p67phox are present in a high-molecular-weight complex along with p40phox,6 which interacts with phosphorylated lipids7,8 but is not required for NADPH oxidase function. Membrane translocation in intact cells is dependent on phosphorylation of multiple serine residues in p47phox.1 This induces a conformational change in p47phox,9which reveals binding sites for the flavocytochrome subunits.10-14 Gene expression of gp91phox, p47phox, and p67phox is largely restricted to mature phagocytes. Genetic defects in any 1 of the 4 essential phoxcomponents, gp91phox, p22phox, p47phox, or p67phox, result in chronic granulomatous disease.3 Rac activation and membrane translocation occur independently of p47phox/p67phoxtranslocation15 and involve release from cytosolic-binding protein RhoGDI16 and exchange of bound guanosine 5′-diphosphate (GDP) for guanosine 5′-triphosphate (GTP). Rac-GTP interacts with numerous effector proteins, including p67phox.17 Hematopoietic-specific Rac2 is the predominant Rac isoform in human neutrophils, whereas Rac1 and Rac3 isoforms are widely expressed.18-20Decreased neutrophil NADPH oxidase activity has been reported in Rac2−/− mice21,22 and in a patient with recurrent bacterial infections who was found to express a dominant-negative form of Rac2.23,24 An additional small GTPase, Rap1A, copurifies with flavocytochromeb558,25 but its precise function is not known, and it is not necessary for cell-free reconstitution of oxidase activity.
How the cytosolic components regulate flavocytochrome activation is not completely understood, but evidence suggests that p47phox functions as a regulated adaptor protein, which enhances the binding of p67phoxto the flavocytochrome by 100-fold.26 The p47phox subunit is also essential for translocation of p67phox in whole cells, on the basis of studies of p47phox-deficient chronic granulomatous disease neutrophils.12,27 Association of p47phox and p67phox is mediated via reciprocal src homology–3 (SH-3) domain/proline-rich region interactions1,2 Cellular agonists induce phosphorylation of multiple serine residues along the C terminal quarter of p47phox inducing an activating conformational change.1 In particular, phosphorylation of residues 303, 304, and 328 is sufficient to disrupt an intramolecular SH-3 domain interaction, thereby allowing p47phox to interact with p22phox.13,14 Phosphorylation of Ser379 is also necessary for p47phox membrane translocation and NADPH oxidase activity in whole cells.28Evidence suggests that the oxidase can be activated through multiple signaling pathways, which are differentially regulated by various stimuli. Protein kinase C (PKC) has been implicated in virtually all of the signaling pathways that activate neutrophils.29 PKC can phosphorylate p47phox in vitro,30 and in cell-free oxidase assays phosphorylation by PKC alone is sufficient to induce an activating conformational change in p47phox, enabling membrane translocation and low-level superoxide production.31
Only the N-terminal 210 amino acids of p67phoxare necessary for flavocytochrome activation under cell-free conditions.32 The N-terminal 150 amino acids of p67phox contain 4 α-helical tetratricopeptide repeat (TPR) motifs, which create a binding site for Rac-GTP.33 Just distal to the TPR domain, amino acids 199 through 210 in p67phox have been postulated to form an activation domain that regulates electron transfer from NADPH to flavin adenine dinucleotide (FAD) in flavocytochrome b558.32,34 In cell-free superoxide assays, point substitutions within this activation domain reduce oxidase activity, and a truncated (1-210) p67phox Val204Ala mutant was completely inactive when added as a recombinant protein.32
Previous studies of NADPH oxidase assembly and activation in intact cells have used primary human or murine hematopoietic cells, or immortalized cell lines of hematopoietic lineage. Primary phagocytic cells, such as neutrophils, are not easily amenable to genetic modification. Expression of transgenes in immortalized cells of hematopoietic lineage35-40 have provided many useful insights. However, these cell lines suffer variously from limitations on the ready manipulability of different oxidase subunits, the ease of transfection, long-term expression of transfected proteins, and level of superoxide production. Cell-free assays of NADPH oxidase activity offer flexibility in the use of purified recombinant components. However, there are clear differences between oxidase activation in reconstituted cell-free assays compared with the physiologic environment of whole cells.1,41 For example, in the cell-free assay, in which oxidase assembly is triggered by the addition of anionic amphiphile, p47phoxphosphorylation is not required,42,43 nor are the SH-3 domains of p47phox and p67phox necessary.44 45
The aim of the current study was to develop a readily transfectable whole-cell system for investigating the NADPH oxidase by genetic approaches. Transgenic expression of recombinant oxidase subunits in monkey kidney COS-7 cells conferred high-level superoxide production that was activated by either phorbol ester or arachidonic acid (AA). Phorbol myristate acetate (PMA)–activated superoxide production in these cells appeared to be regulated by PKC and Rac, and was dependent on serine residues in p47phoxpreviously implicated as critical phosphorylation sites in intact cells. We also established that the p67phoxactivation domain, which is essential for superoxide production under cell-free conditions, was required for assembly of a functional NADPH oxidase in whole cells.
Materials and methods
Reagents and antibodies
Chemicals were purchased from Sigma (St Louis, MO) unless otherwise stated. Phosphate-buffered saline (PBS), penicillin/streptomycin, trypsin/EDTA, glycerol, geneticin (neomycin), Lipofectamine Plus, and Dulbecco modified Eagle low-glucose medium (DMEM) were purchased from Gibco BRL (Grand Island, NY).
Mouse monoclonal antibodies (Abs) for gp91phoxand p22phox were kindly provided by D. Roos and A. Verhoeven (Central Laboratory of the Netherlands Blood Transfusion Service, Amsterdam). Polyclonal rabbit serum raised against p67phox was provided by P. Heyworth (Scripps Research Institute, La Jolla, CA), and polyclonal rabbit serum against Rap1A was provided by M. Quinn (Montana State University, Bozeman). Polyclonal rabbit serum was raised against recombinant human Rac2, RhoGDI, and p47phox. The following antibodies were purchased: mouse monoclonal Ab against Rac1 (Upstate, Lake Placid, NY); isoform-specific rabbit polyclonal Abs for PKC isoforms α, βI, βII, δ, ε, γ, η, and ζ (Panvera, Madison, WI); rabbit polyclonal Abs for PKC isoforms ι and θ (Santa Cruz Biotechnology, Santa Cruz, CA); mouse monoclonal Ab against Myc-epitope tag 9E10 (Upstate). Recombinant human protein standards for PKC isoforms α, βI, βII, δ, ε, γ, and ζ were from Panvera.
Expression vectors
Mammalian expression vector pRK5 was purchased from Pharmingen (San Diego, CA), and pcDNA3.1 from Invitrogen (Carlsbad, CA). Full-length complementary DNA (cDNA) for p47phoxwas subcloned into pRK5 and into pEF-PGKhygro, which is similar to pEF-PGKneo,46 except that it contains a linked expression cassette for hygromycin rather than neomycin resistance. Full-length cDNA for human p67phox was subcloned into pEF-PGKpac.47 The p67phox(Val204Ala) DNA was generated by polymerase chain reaction (PCR) mutagenesis of wild-type p67phox cDNA. Wild-type p67phox and p67phox(Val204Ala) cDNAs were each subcloned upstream of a Myc-epitope tag in pcDNA3.1. The full-length cDNAs for human Rac1 and Rac2 were subcloned downstream of a Myc-epitope tag in pRK5. PCR mutagenesis was used to introduce a (T17N) mutation in each. The resulting cDNAs were sequenced in entirety. The full-length cDNA for bovine Rho-GDI was subcloned into pRK5. Bernard Babior (Scripps Research Institute) generously provided p47phox Ser303Ala/Ser304Ala; p47phox Ser303Glu/Ser304Glu; and p47phox Ser379Ala cDNAs in pEBOpBLL. Alexandra Newton (University of California San Diego) provided rat PKC-βII/pcDNA3 and rat PKC-α/pBK-CMV. Peter Parker (Imperial Cancer Research Fund, London, United Kingdom) provided Myc-tagged mouse PKC-δ/pEF-Link.
Cell culture
COS-7 cells were obtained from American Type Culture Collection (Manassas, VA) (CRL-1651) and grown in DMEM with 10% heat-inactivated fetal calf serum (FCS) (Hyclone, Logan, UT), 50 U/mL penicillin, and 50 μg/mL streptomycin. Adherent COS-7 cells were harvested by incubating with trypsin/EDTA for 5 minutes at 37°C. Following addition of DMEM/10%FCS to neutralize the trypsin, cells were pelleted by centrifugation at 4°C, washed in ice-cold PBS, repelleted, and rapidly resuspended in ice-cold PBS.
Transfection of COS-7 cell lines
COS-7 cells had previously been stably transfected with gp91phox cDNA in pEF-PGKpac and p22phox cDNA in pEF-PGKneo.47 The gp91phox/p22phox–transfected COS-7 cells were either transfected with p47phox/pEF-PGKhygro, transfected with p67phox/pEF-PGKhygro, or cotransfected at a 1:10 DNA ratio with p47phox/pEF-PGKhygro and p67phox/pEF-PGKpac. DOTAP (Boehringer Mannheim, Indianapolis, IN) was used to transfect 11 μg DNA per 100-mm plate of cells per the manufacturer's instructions. Clones were selected by limiting dilution in 0.2 mg/mL hygromycin (Calbiochem, La Jolla, CA), 1.8 mg/mL neomycin, and 1 μg/mL puromycin. Of 50 cotransfected clones screened by immunoblotting, 3 expressed all 4 phox components. The clone with the highest expression of p47phox and p67phox also had the highest rate of superoxide production and was selected for detailed analysis.
Lipofectamine Plus was used to transiently transfect 2 μg DNA per 60-mm plate of COS-7–derived cell lines. Cells were analyzed 21 to 24 hours after transfection. Transient transfection efficiency assayed by immunohistochemisty to detect expression of a Myc-epitope tag averaged 35%.
Neutrophil isolation
Neutrophils were isolated from heparinized venous blood donated by healthy adult volunteers, as described.48 Briefly, whole blood was subjected to dextran sedimentation, centrifugation over Isolymph (Gallard-Schlesinger Industries, Carle Place, NY), or Ficoll/Paque (Amersham Pharmacia, Piscataway, NJ), and to hypotonic lysis of contaminating red cells.
Measurement of NADPH oxidase activity
Superoxide production by whole COS-7 cell lines, harvested by brief trypsinization as described above, was measured in a quantitative kinetic assay on the basis of the superoxide dismutase–inhibitable reduction of cytochrome c. Assays were performed at 37°C by means of a Thermomax microplate reader and associated SOFTMAX Version 2.02 software (Molecular Devices, Sunnyvale, CA) as described previously.46 Briefly, cells were suspended (2.5 × 105 cells per well) in 250 μL PBS plus 0.5 mM MgCl2, 0.9 mM CaCl2, and 7.5 mM dextrose (PBSG) containing 75μM cytochrome c. Cells were activated by the addition of 0.4 μg/mL PMA, 100 μM AA, or a combination of 0.4 μg/mL PMA plus 60 μM AA. Samples containing 250 U superoxide dismutase, in addition to the reagents listed, were run in parallel. Superoxide production was quantified by means of an extinction coefficient of 21.1 mM−1cm−1 for cytochromec. The maximum rate of superoxide generation over a 3-minute interval was calculated by means of SOFTMAX software.
For detection of nitroblue tetrazolium (NBT) reduction, cells were incubated for 30 minutes at 37°C in PBSG containing NBT plus 0.4 μg/mL PMA. Cells were fixed with methanol and stained with 0.2% safrinin. A blinded observer randomly counted 200 cells at × 400 magnification to determine the percentage of NBT+ cells.
Analysis of protein expression
Cells were pelleted and resuspended in lysis buffer (20 mM Tris-HCl pH 8, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 20 μg/mL chymostatin, 2 mM phenylmethylsulfonyl fluoride [PMSF], 1 mM 4-(2-aminoethyl) benzenesulfonyl fluoride [AEBSF], and 10 μM leupeptin). Lysate was incubated on ice for 30 minutes and cleared by microcentrifuging (14 000 rpm, 2.5 minutes, 4°C). Protein concentration was quantified by means of the BCA Protein Assay (Pierce, Rockford, IL). With the use of the buffer system of Laemmli, samples were electrophoresed in sodium dodecyl sulfate–polyacrylamide gels (SDS-PAGE) followed by transfer to nitrocellulose membranes. Membranes were blocked in Tris-buffered saline with 0.5% Tween-20 (TBST) containing 6% powdered milk, exposed to primary antibodies diluted in TBST, exposed to horseradish peroxidase–conjugated secondary anti–immunoglobulin G, washed thoroughly, and visualized by enhanced chemiluminescence (ECL) (Amersham Pharmacia). Densitometric analysis of films was performed by means of Stratagene Eagle Eye II Still Video System and associated software (Stratagene, La Jolla, CA). Serial protein dilutions along with multiple exposures were analyzed to ensure that this analysis was performed in the linear range.
Measurement of translocation of cytosolic NADPH oxidase components
Cells were suspended at 4 × 107/mL in PBSG, warmed for 5 minutes at 37°C, and then stimulated with 0.4 μg/mL PMA for 10 minutes at 37°C, or with 100 μM AA for 5 minutes at 37°C. Control cells were incubated (10 minutes at 37°C) without stimulus. Cells were returned to ice and immediately diluted 10 × with ice-cold PBS, then centrifuged (1200 rpm, 6 minutes, 4°C). Cells were resuspended in 2 mL relaxation buffer (100 mM KCl, 10 mM Hepes, 3.5 mM MgCl2, 3 mM NaCl, 1.2 mM ethyleneglycotetraacetic acid [EGTA], 25 mM NaF, 5 mM Na3VO4, 1 mM p-nitrophenyl phosphate, 0.5 μM microcystin, 20 μg/mL chymostatin, 2 mM PMSF, 10 μM leupeptin, and 1 mM AEBSF), disrupted in a Dounce homogenizer (Kontes Glass, Vineland, NJ), then centrifuged (1300 rpm, 10 minutes, 4°C) to remove unbroken cells and nuclei. Supernatant was layered on a discontinuous 20%/38% sucrose gradient and centrifuged at 204 000g for 30 minutes (SW55 rotor; Beckman Instruments, Palo Alto, CA). The cytosol was removed from the top of the gradient, and the membrane fraction was collected at the 20%/38% interface. Fractions were electrophoresed, and the proteins analyzed as described above.
Analysis of PKC isoform expression
Cell fractionation was essentially as described.48Briefly, neutrophils or COS-7 cells were treated with 1 mM diisopropyl fluorophosphate for 5 minutes on ice, washed once with 10 vol ice-cold Hanks balanced salt solution, and resuspended at 2 × 108cells per milliliter in extraction buffer (50 mM Tris, pH 7.5, containing 2 mM EGTA, 1 mM PMSF, 1 μg/mL leupeptin, 10 μM benzamidine, 10 μM pepstatin, and 0.2 μg/mL aprotinin). Cells were disrupted by sonication on ice, and the resulting homogenate was centrifuged (800g, 10 minutes, 4°C). Neutrophil cytosolic, membrane and granule fractions were obtained from the 800gsupernatant after centrifugation over a 15%/40% discontinuous sucrose gradient at 4°C for 30 minutes at 150 000g (SW55 rotor). Neutrophil cytosol was removed from the top of the gradient, and the membrane fraction was collected at the 15%/40% interface. COS-7 cell cytosolic and membrane fractions were obtained from the supernatant and pellet, respectively, following centrifugation at 368 000gfor 30 minutes at 4°C. Samples were electrophoresed in 7% SDS-polyacrylamide gels and transferred electrophoretically to nitrocellulose. Blots were processed as previously described.48
Statistical analysis
Results were analyzed by means of InStat (GraphPad software, San Diego, CA). Treatments were compared with control by means of the 2-tailed paired Student t test or analysis of variance followed by the Student-Newman-Keuls multiple comparisons test, as indicated. P < .05 was considered significant. Data are expressed as mean ± SD.
Results
The phagocyte NADPH oxidase can be functionally reconstituted in heterologous COS-7 cells
We previously generated a COS-7 cell line that coexpressed gp91phox and p22phox from stable transgenes.47 The 7D5 monoclonal antibody, which is directed to an extracellular epitope of gp91phox,49-51 stained unpermeabilized COS-7 gp91phox/p22phox cells, and membranes prepared from these cells supported superoxide production in the presence of neutrophil cytosol.52 The p47phox and p67phox cDNAs were cotransfected into COS-7 gp91phox/p22phox cells to determine if superoxide production could be elicited in whole cells in this heterologous system. Of 50 clones screened by immunoblot analysis, 3 expressed all 4 phox components. One of the 3 clones exhibited ≈25% higher p47phox expression, ≈50% greater p67phox expression, and a higher rate of superoxide production (see below) than the other 2 clones, so it was selected for detailed analysis. The level of phoxprotein expression in this clone was estimated by densitometric analysis of immunoblots relative to serial dilutions of human neutrophil extract (Table 1). The transgenic COS-phox cells expressed the 4 phoxproteins at a level equivalent to or higher than human neutrophils on a per-cell–equivalent basis. However, the flavocytochrome subunits were less concentrated on the basis of per milligrams total protein in the COS-phox cells as compared with human neutrophils because the larger COS-7 cells contained approximately 8 times as much total protein per cell. Immunoblot analysis also showed that the COS-phox cells expressed endogenous Rac1 and Rap1A, but not hematopoietic-specific Rac2, as expected (Table 1).
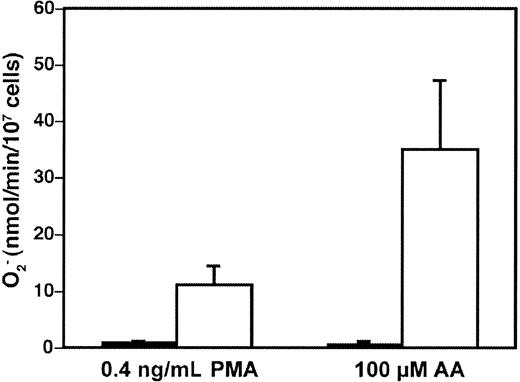
The transgenic COS-phox cells were capable of high-level NADPH oxidase activity upon activation by either the phorbol ester PMA (phorbol 12-myristate 13-acetate), or AA, 2 soluble stimuli commonly used to activate the neutrophil NADPH oxidase (Figure1). The PMA-elicited rate of superoxide production by the COS-phox cells was 11 ± 3.3 (n = 43) nmol/107 cells per minute (maximum velocity [Vmax]), which was constant over a PMA concentration range of 100 to 1000 ng/mL. This compared favorably both to a PMA-elicited Vmax of 47 ± 7.7 nmol/107 cells per minute (n = 5 from 4 different donors) for human neutrophils and to a Vmax of 35 ± 18 nmol/107 cells per minute (n = 29) reported for PLB-985 cells differentiated to a neutrophil phenotype.35 Reported rates of PMA-elicited superoxide production in human neutrophils have ranged as high as 117 nmol/107 cells per minute in various studies.53-55 The AA-elicited rate of superoxide production by the COS-phox cells was 35 ± 12 nmol/107 cells per minute (n = 11) (Vmax) at an optimal concentration of 100 μM. This was comparable to the rate of superoxide production by human neutrophils of 41 ± 9.7 nmol/107 cells per minute elicited by 100 μM AA, or 34 ± 11 nmol/107 cells per minute elicited by 80 μM AA (for both, n = 5 from 4 different donors). AA-elicited superoxide production of up to 105 ± 24 nmol/107cells per minute (Vmax) has previously been reported for human neutrophils with the use of 82 μM AA.54
High-level superoxide production in transgenic COS-phox cells.
Superoxide production by either wild-type COS-7 cells (▪) or transgenic COS-phox cells (■), following detachment from tissue-culture dishes and stimulation with either 0.4 μg/mL PMA or 100 μM AA, was determined by reduction of ferricytochromec. Data are expressed as mean ± SD; n ≥ 3.
High-level superoxide production in transgenic COS-phox cells.
Superoxide production by either wild-type COS-7 cells (▪) or transgenic COS-phox cells (■), following detachment from tissue-culture dishes and stimulation with either 0.4 μg/mL PMA or 100 μM AA, was determined by reduction of ferricytochromec. Data are expressed as mean ± SD; n ≥ 3.
Wild-type COS-7 cells have been reported to express an endogenous oxidase, genetically distinct from the phagocyte NADPH oxidase, which is capable of low-level superoxide production that may play a role in mitogenic signaling.56 57 However, as shown in Figure 1, any endogenous superoxide production in wild-type COS-7 cells was below the threshold of detection in the cytochrome c reduction assay. NADPH oxidase activity measurable by cytochrome creduction required expression of the 2 flavocytochrome subunits plus both cytosolic phox components, as COS-7 cell lines expressing only gp91phox/p22phox, only gp91phox/p22phox/p47phox, or only gp91phox/p22phox/p67phoxdid not produce significant levels of superoxide after either PMA or AA stimulation (data not shown).
The p47phoxserine residues at 303, 304, and 379 are necessary for optimal NADPH oxidase activation in COS-phox cells
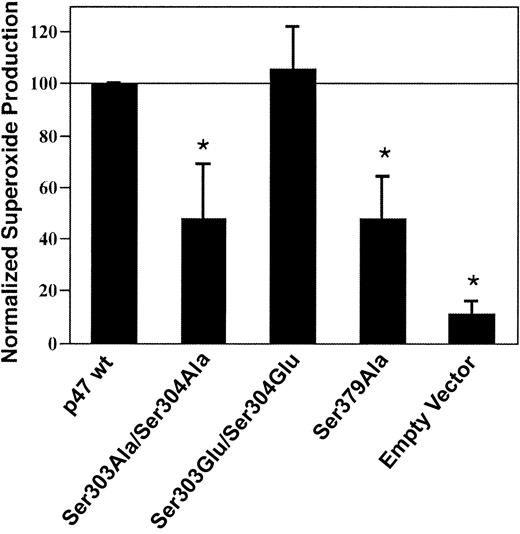
To ascertain whether phosphorylation of key p47phox serine residues 303, 304, and 379 contributed to NADPH oxidase activation, COS-7 gp91phox and p22phoxcells were transiently transfected with wild-type p67phox plus either empty vector, wild-type p47phox, or p47phoxmutants Ser303Ala/Ser304Ala, Ser303Glu/Ser304Glu, or Ser379Ala. The alanine substitutions are kinase insensitive, whereas the glutamate substitutions mimic phosphorylated residues. NADPH oxidase activity was significantly lower in COS cells expressing the kinase-insensitive p47phox Ser303Ala/Ser304Ala or Ser379Ala mutants compared with cells expressing wild-type p47phoxor the Ser303Glu/Ser304Glu phosphorylation mimic (Figure2), suggesting that phosphorylation of p47phox serine residues 303, 304, and 379 contributes to optimal NADPH oxidase activation in the COS cell system.
Effect of p47phox on serine residues 303, 304, and 379 NADPH oxidase activation in COS-phox cells.
COS cells stably expressing gp91phox and p22phox were transiently transfected with wild-type p67phox plus either wild-type p47phox (p47 wt) or p47phoxmutants Ser303Ala/Ser304Ala, Ser303Glu/Ser304Glu, or Ser379Ala, or empty pRK5 vector. Cells were analyzed 24 hours after transfection. Following stimulation with 0.4 μg/mL PMA, superoxide production was determined by reduction of ferricytochrome c. Measured rates of superoxide production were normalized to that of cells expressing wild-type p47phox, and data were also normalized on the basis of the level of expression of the mutants compared with wild-type p47phox expression. Relative protein expression levels compared with wild-type p47phox were 76 ± 22% for p47phox Ser303Ala/Ser304Ala, 82 ± 27% for p47phox Ser303Glu/Ser304Glu, and 90 ± 29% for p47phox Ser379Ala, as determined by densitometry performed on blots of whole-cell lysates following SDS–PAGE (not shown). Data represent mean ± SD; n = 3. *Superoxide production is significantly lower for cells transfected with p47phox Ser303Ala/Ser304Ala, p47phox Ser379Ala, or empty vector, compared with cells expressing wild-type p47phox or p47phox Ser303Glu/Ser304Glu (P < .01, Student-Newman-Keuls multiple-comparisons test). Superoxide production by the cells transfected with wild-type p47phox was 1.8 ± 0.3 nmol/107cells per minute.
Effect of p47phox on serine residues 303, 304, and 379 NADPH oxidase activation in COS-phox cells.
COS cells stably expressing gp91phox and p22phox were transiently transfected with wild-type p67phox plus either wild-type p47phox (p47 wt) or p47phoxmutants Ser303Ala/Ser304Ala, Ser303Glu/Ser304Glu, or Ser379Ala, or empty pRK5 vector. Cells were analyzed 24 hours after transfection. Following stimulation with 0.4 μg/mL PMA, superoxide production was determined by reduction of ferricytochrome c. Measured rates of superoxide production were normalized to that of cells expressing wild-type p47phox, and data were also normalized on the basis of the level of expression of the mutants compared with wild-type p47phox expression. Relative protein expression levels compared with wild-type p47phox were 76 ± 22% for p47phox Ser303Ala/Ser304Ala, 82 ± 27% for p47phox Ser303Glu/Ser304Glu, and 90 ± 29% for p47phox Ser379Ala, as determined by densitometry performed on blots of whole-cell lysates following SDS–PAGE (not shown). Data represent mean ± SD; n = 3. *Superoxide production is significantly lower for cells transfected with p47phox Ser303Ala/Ser304Ala, p47phox Ser379Ala, or empty vector, compared with cells expressing wild-type p47phox or p47phox Ser303Glu/Ser304Glu (P < .01, Student-Newman-Keuls multiple-comparisons test). Superoxide production by the cells transfected with wild-type p47phox was 1.8 ± 0.3 nmol/107cells per minute.
Both PMA and AA elicit membrane translocation of p47phox, p67phox, and Rac in COS-phox cells
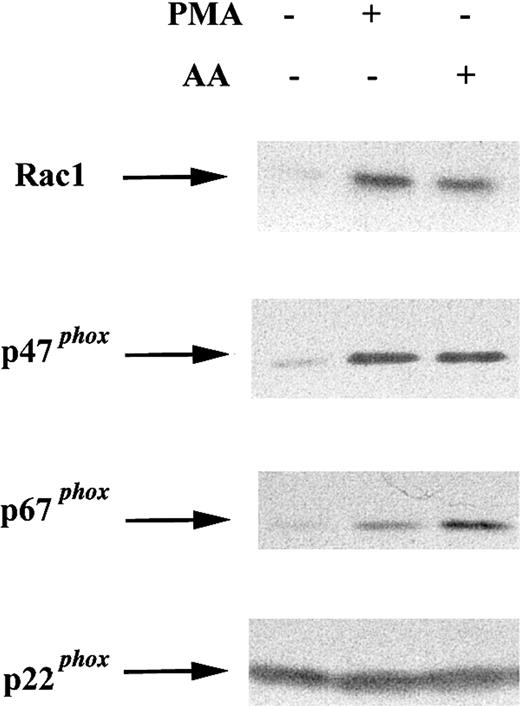
To determine whether COS-phox cells resemble neutrophils, in which cytosolic NADPH oxidase components translocate to the membrane in response to soluble agonists, membrane was extracted from cells following activation with 0.4 μg/mL PMA (10 minutes, 37°C), 100 μM AA (5 minutes, 37°C), or no stimulus (10 minutes, 37°C). Membrane fractions were subjected to SDS-PAGE and immunoblotting. Activation of COS-phox cells with either PMA or AA induced membrane translocation of Rac1, p47phox, and p67phox(Figure 3).
Effect of soluble agonists on membrane translocation of cytosolic NADPH oxidase components in COS-phoxcells.
COS-phox cells were activated with 0.4 μg PMA (10 minutes, 37°C) or 100 μM AA (5 minutes, 37°C), as indicated. Control cells were incubated for 10 minutes at 37°C with no stimulus. Cells were disrupted in a Dounce homogenizer and fractionated over a discontinuous 20%/38% sucrose gradient. The membrane fraction (6 μg) was separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies directed against NADPH oxidase subunits Rac1, p47phox, p67phox, and p22phox. Blots are representative of 2 independent experiments.
Effect of soluble agonists on membrane translocation of cytosolic NADPH oxidase components in COS-phoxcells.
COS-phox cells were activated with 0.4 μg PMA (10 minutes, 37°C) or 100 μM AA (5 minutes, 37°C), as indicated. Control cells were incubated for 10 minutes at 37°C with no stimulus. Cells were disrupted in a Dounce homogenizer and fractionated over a discontinuous 20%/38% sucrose gradient. The membrane fraction (6 μg) was separated by SDS-PAGE, transferred to nitrocellulose, and probed with antibodies directed against NADPH oxidase subunits Rac1, p47phox, p67phox, and p22phox. Blots are representative of 2 independent experiments.
Endogenous PKC isoforms are not rate limiting for PMA-induced NADPH oxidase activity in COS-phox cells
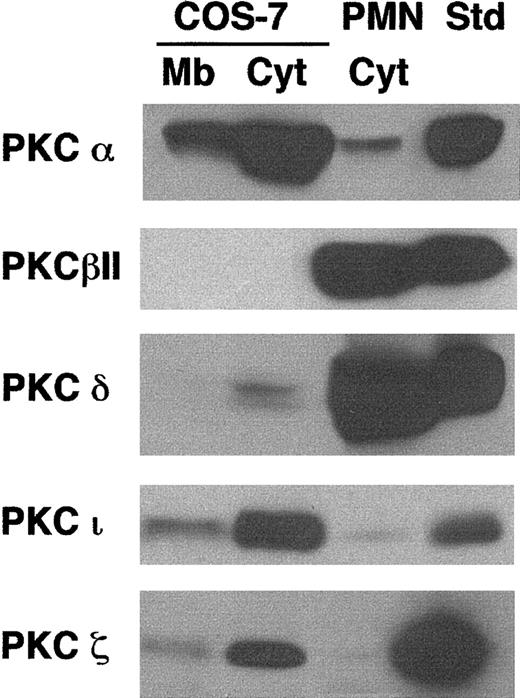
Preincubation of COS-phox cells with the PKC-selective inhibitor GF109203x (1 μM, 30 minutes, 37°C) reduced PMA-induced superoxide production by 89% ± 12% (n = 3), suggesting that PMA-elicited NADPH oxidase activation was mediated predominately through PKC signaling pathways. A variety of studies have suggested that conventional PKC isoforms α or β mediate PMA-induced NADPH oxidase assembly in neutrophils and monocytes.4 58-60 We compared PKC isoform expression in COS-7 cells to that in human neutrophils by using immunoblotting to examine expression of PKC isoforms α, βI, βII, δ, ε, γ, η, ι, θ, and ζ. The most substantial differences were found with PKC isoforms α, βII, δ, ι, and ζ (Figure 4). COS-7 cells expressed much more PKC-α, PKC-ι, and PKC-ζ than human neutrophils, but had undetectable levels of PKC-βII and low levels of PKC-δ, both of which were expressed at much higher levels in neutrophils. Both cell types expressed very low levels of PKC-βI; COS-7 cells also expressed low levels of PKC-γ (not shown). Isoforms ε, η, and θ did not appear to be significantly expressed in either cell type (not shown).
Comparison of PKC isoform expression in COS-7 cells and human neutrophils.
Membrane (Mb) and cytosolic (Cyt) fractions were isolated from whole-cell lysates of human neutrophils or transgenic COS-7 cells expressing gp91phox/p22phox. Cell fractions (100 μg), along with protein standards for various PKC isoforms (50 ng), were separated by SDS-PAGE, transferred to nitrocellulose, and probed with PKC isoform-specific antibodies. The standard for PKC-ι was 13μg A431 cell lysate (Upstate). Representative blots are shown for PKC isoforms α (n = 2), βII (n = 3), δ (n = 3), ζ (n = 3), and ι (n = 3). The cytosolic fraction from wild-type COS-7 cells was also evaluated and gave results similar to those from the COS-7 cells expressing gp91phox/p22phox(not shown).
Comparison of PKC isoform expression in COS-7 cells and human neutrophils.
Membrane (Mb) and cytosolic (Cyt) fractions were isolated from whole-cell lysates of human neutrophils or transgenic COS-7 cells expressing gp91phox/p22phox. Cell fractions (100 μg), along with protein standards for various PKC isoforms (50 ng), were separated by SDS-PAGE, transferred to nitrocellulose, and probed with PKC isoform-specific antibodies. The standard for PKC-ι was 13μg A431 cell lysate (Upstate). Representative blots are shown for PKC isoforms α (n = 2), βII (n = 3), δ (n = 3), ζ (n = 3), and ι (n = 3). The cytosolic fraction from wild-type COS-7 cells was also evaluated and gave results similar to those from the COS-7 cells expressing gp91phox/p22phox(not shown).
To determine whether PKC isoforms that are enriched in neutrophils would enhance NADPH oxidase activation in COS-phox cells, PKC-βII or δ cDNAs were expressed by transient transfection to levels that were more than 5 times their level of expression in human neutrophils (per milligram total protein) (data not shown). For comparison, PKC-α cDNA was also transiently expressed, increasing PKC-α expression in the COS-phoxcells by more than 3-fold (per milligram total protein). However, no significant difference in either PMA- or AA-elicited superoxide production was found between cells expressing any of the transgenic PKCs compared with cells transfected with empty vector (data not shown). These results suggest that the endogenous PKC isoform expression level and composition are not rate limiting for PMA-induced superoxide production by transgenic COS-phox cells.
Rac activation contributes to agonist-elicited superoxide production in COS-phox cells
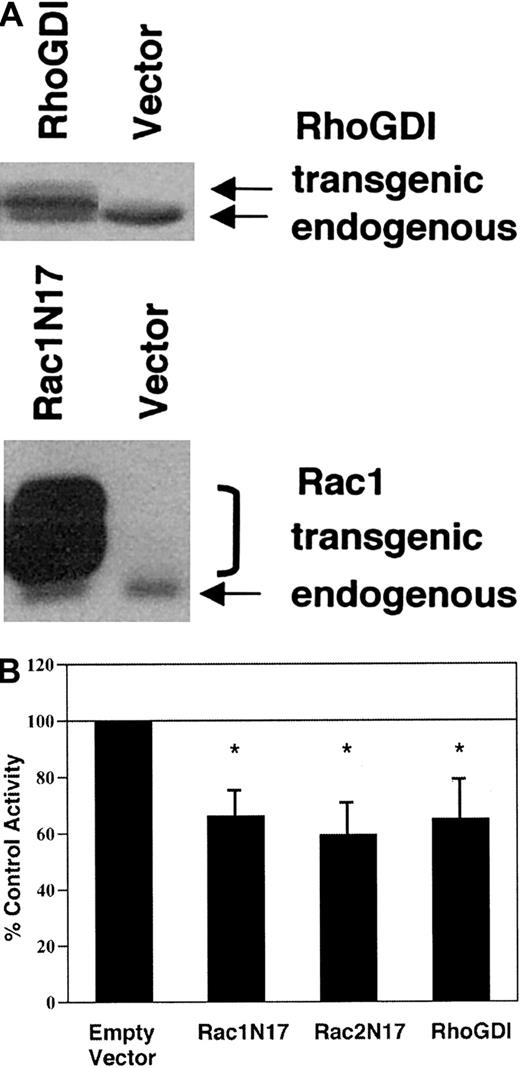
To investigate whether Rac activation plays a role in the regulation of NADPH oxidase activity in transgenic COS-phoxcells, we transiently expressed either dominant-negative RacN17 mutants or RhoGDI, a ubiquitously expressed Rho-family binding protein that sequesters Rac in the cytosol.16 Transient transfection efficiency under our conditions was approximately 35%, as assessed by immunohistochemistry to detect expression of Myc epitope–tagged transgenic protein. Relative to endogenous RhoGDI and Rac1, recombinant RhoGDI and, particularly, Myc epitope–tagged Rac1N17 were overexpressed (Figure 5A). Transgenic expression of Rac1N17, Rac2N17, or RhoGDI reduced PMA-elicited superoxide production by 34% to 41% as compared with empty vector–transfected control cells in the cytochrome creduction assay (Figure 5B). AA-elicited superoxide production was similarly inhibited (data not shown). Using NBT to detect PMA-elicited superoxide production by individual cells, we found that 69% ± 1.7% of empty vector–transfected control cells were NBT+, compared with 52% ± 1.9% of Rac1N17-transfected cells, 48% ± 15% of Rac2N17-transfected cells, and 48% ± 14% of RhoGDI-transfected cells (all P < .05 compared with empty vector–transfected control cells; n = 5). Thus, there were 25% to 31% fewer NBT+ cells in cell populations transfected with Rac1N17, Rac2N17, or RhoGDI compared with empty vector–transfected control cells; this is consistent with complete inhibition of superoxide production in most cells expressing dominant-negative Rac or RhoGDI. Immunohistochemistry confirmed that the majority of cells expressing the Myc-epitope tag were NBT−. A few Myc+ cells were NBT+, which may reflect cell-to-cell variability in expression of the dominant-negative Rac. Taken together, these results suggest that inhibition of Rac activation impaired agonist-elicited NADPH oxidase activity in transgenic COS-phox cells.
Effect of dominant-negative Rac or RhoGDI expression on agonist-elicited superoxide production by COS-phoxcells.
COS-phox cells were transiently transfected with expression vectors encoding dominant-negative Rac1(T17N), Rac2(T17N), or RhoGDI. Control cells were transfected with empty pRK5 vector. Cells were analyzed 21 hours after transfection. (A) Whole-cell lysates were separated by 12% SDS-PAGE, transferred to nitrocellulose, and probed with monoclonal Ab to Rac1 or polyclonal Ab to RhoGDI. Transgenic Rac1N17 migrated more slowly than endogenous Rac1 owing to an N-terminal Myc-epitope tag, and it migrated as a doublet owing to the presence of both isoprenylated and nonisoprenylated forms. Transgenic RhoGDI migrated more slowly than endogenous RhoGDI owing to 5 extra N terminal amino acids. Blots are representative of 4 or more independent assays. (B) Cells were stimulated with 0.4 μg/mL PMA and assayed for superoxide production by the ferricytochrome c reduction assay. The activity of the control, empty vector–transfected cells was 12.2 ± 2.8 nmol O2− per 107cells per minute. Bars represent mean ± SD; n ≥ 4. *P < .01 by paired t test.
Effect of dominant-negative Rac or RhoGDI expression on agonist-elicited superoxide production by COS-phoxcells.
COS-phox cells were transiently transfected with expression vectors encoding dominant-negative Rac1(T17N), Rac2(T17N), or RhoGDI. Control cells were transfected with empty pRK5 vector. Cells were analyzed 21 hours after transfection. (A) Whole-cell lysates were separated by 12% SDS-PAGE, transferred to nitrocellulose, and probed with monoclonal Ab to Rac1 or polyclonal Ab to RhoGDI. Transgenic Rac1N17 migrated more slowly than endogenous Rac1 owing to an N-terminal Myc-epitope tag, and it migrated as a doublet owing to the presence of both isoprenylated and nonisoprenylated forms. Transgenic RhoGDI migrated more slowly than endogenous RhoGDI owing to 5 extra N terminal amino acids. Blots are representative of 4 or more independent assays. (B) Cells were stimulated with 0.4 μg/mL PMA and assayed for superoxide production by the ferricytochrome c reduction assay. The activity of the control, empty vector–transfected cells was 12.2 ± 2.8 nmol O2− per 107cells per minute. Bars represent mean ± SD; n ≥ 4. *P < .01 by paired t test.
A p67phoxactivation domain is necessary for NADPH oxidase activation in whole cells
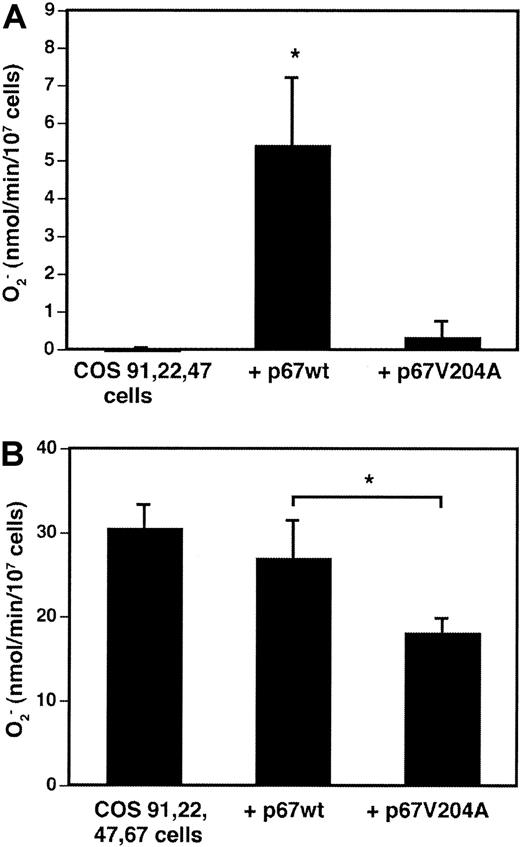
A p67phox derivative truncated at amino acid 210 and containing a Val204Ala substitution in a putative flavocytochrome “activation” domain was previously found to be completely inactive when added as a recombinant protein to a cell-free superoxide assay.32 The truncated p67phox Val204Ala mutant translocated to the membrane under these conditions and was a potent inhibitor of superoxide production in the presence of recombinant wild-type p67phox.32 To examine the function of full-length p67phox Val204Ala in whole cells, wild-type p67phox or the mutant Val204Ala derivative were transiently expressed in COS-phox cells. In COS-7 cells stably expressing gp91phox, p22phox, and p47phox, transient expression of wild-type p67phoxresulted in significant superoxide production upon PMA activation, whereas superoxide production was not detected with transient expression of p67phox Val204Ala (Figure6A). In the “complete” COS-phoxcells, which stably expressed wild-type p67phox in addition to gp91phox, p22phox, and p47phox, transient expression of additional wild-type p67phox did not significantly affect the rate of superoxide production (Figure 6B). In contrast, transient expression of p67phox Val204Ala significantly reduced superoxide production by an average of 30%, as compared with either mock-transfected control cells or wild-type p67phox-transfected cells (Figure 6B). This reduction was similar to the transfection efficiency, suggesting that expression of the Val204Ala mutant was a potent inhibitor of superoxide production in the transfected cell population. These results are consistent with those obtained in cell-free NADPH oxidase activation assays, which suggest that incorporation of p67phox Val204Ala into the oxidase complex results in an inactive enzyme.32
Effect of Val204Ala p67phox mutation on NADPH oxidase activation in whole cells.
(A) COS-7 cells stably expressing gp91phox, p22phox, and p47phox, but not p67phox, were transiently transfected with expression vectors encoding either wild-type p67phox or p67phox containing a Val204Ala point mutation. Control cells were mock transfected. At 21 hours after transfection, cells were harvested and stimulated with 0.4 μg/mL PMA plus 60 μM AA, and superoxide production was measured by the ferricytochrome creduction assay. Data are expressed as mean ± SD; n = 3. *P = .01, Student t test. (B) COS-phox cells stably expressing gp91phox, p22phox, p47phox, and p67phox were transiently transfected and tested as described above. Data are expressed as mean ± SD; n = 5. *P < .01, Student t test.
Effect of Val204Ala p67phox mutation on NADPH oxidase activation in whole cells.
(A) COS-7 cells stably expressing gp91phox, p22phox, and p47phox, but not p67phox, were transiently transfected with expression vectors encoding either wild-type p67phox or p67phox containing a Val204Ala point mutation. Control cells were mock transfected. At 21 hours after transfection, cells were harvested and stimulated with 0.4 μg/mL PMA plus 60 μM AA, and superoxide production was measured by the ferricytochrome creduction assay. Data are expressed as mean ± SD; n = 3. *P = .01, Student t test. (B) COS-phox cells stably expressing gp91phox, p22phox, p47phox, and p67phox were transiently transfected and tested as described above. Data are expressed as mean ± SD; n = 5. *P < .01, Student t test.
Discussion
In this study, we show that expression of all 4 essentialphox subunits can reconstitute high-level NADPH oxidase activity in a nonhematopoietic, easily transfectable cell line derived from monkey COS-7 cells. These COS-phox cells are a new whole-cell model that is readily amenable to genetic manipulation, providing the ability to easily express oxidase subunits and regulatory proteins, either transiently or stably, to examine the impact on NADPH oxidase function in intact cells. Many features of oxidase assembly cannot be recapitulated in cell-free oxidase assays, including the requirement for the SH-3 domains of p67phox or for p47phox phosphorylation, both of which are important for translocation to the membrane and oxidase activity in whole cells.1,41 Primary phagocytes are not readily transfectable, and functional analysis of mutant phoxproteins in cell lines with NADPH oxidase expression, such as PLB-985 or Epstein-Barr virus (EBV)–transformed B cells, are limited to genetically deficient cells that are also not efficiently transfected and, for B-cell lines, have low levels of superoxide production. The NADPH oxidase has also been reconstituted by transfection of hematopoietic but nonphagocytic K562 leukemia cells, which can then produce superoxide at 5% to 15% the rate of human neutrophils.40 61 The COS-phox cells represent the first functional reconstitution of the phagocyte NADPH oxidase in a nonhematopoietic cell line and can produce superoxide at up to 80% the rate of human neutrophils upon activation by either the phorbol ester PMA or AA. Hence, COS-7 cells contain upstream signaling components that can couple to transgenically expressed phox subunits to activate high levels of NADPH oxidase activity.
Whereas in human neutrophils, PMA and AA can induce similar rates of superoxide production, in COS-phox cells AA was a more potent stimulus, inducing more than 3-fold higher rates of superoxide production compared with PMA. This difference in agonist efficacy suggests some differences in signaling pathways that lead to oxidase activation in COS cells compared with human neutrophils. Nevertheless, the final assembled state of the oxidase in the COS-phoxsystem seems to simulate the native system because a large amount of superoxide is generated, expression of all 4 essential phoxcomponents is required, and oxidase activation is associated with membrane translocation of p47phox, p67phox, and Rac. Furthermore, p47phox kinase-insensitive mutants Ser303Ala/Ser304Ala or Ser379Ala are significantly deficient in NADPH oxidase activity in the COS-phox cells compared with wild-type p47phox or the Ser303Glu/Ser304Glu phosphorylation-mimic mutant. The Ser303Ala/Ser304Ala and Ser379Ala mutants are similarly deficient in supporting whole-cell NADPH oxidase activity in the EBV-transformed B cell or K562 heterologous systems, whereas they are fully active in cell-free assays.13,28 62The COS-phox system therefore should be a useful model for studying functional domains in NADPH oxidase subunits necessary for assembly of an active enzyme in intact cells.
In COS-phox cells, we found that the main effect of PMA on NAPDH oxidase activation was exerted through PKC, similar to phagocytic cells, where PKC inhibitors effectively abolish PMA-induced superoxide production.63,64 We investigated whether differences between endogenous PKC isoforms in COS-phox cells compared with neutrophils accounted for the lower rate of COS-phoxsuperoxide production elicited by PMA compared with arachidonate. COS-7 cells expressed much more PKC-α, PKC-ι, and PKC-ζ than human neutrophils but had undetectable levels of PKC-βII and low levels of PKC-δ, both of which were expressed at much higher levels in neutrophils. The α, β, and δ isoforms are diacylglycerol dependent, and therefore phorbol responsive. PKC-β has been implicated as an important regulator of PMA-induced p47phox phosphorylation and NADPH oxidase activation in neutrophils.58-60 However in monocytes, antisense depletion of PKC-βI and PKC-βII had no effect on NADPH oxidase activation and depletion of PKC-α was found to be significantly inhibitory, suggesting that different PKC isoforms may contribute to NADPH oxidase activation in different cell types.4 In COS-phox cells, we found that transient expression of the major neutrophil isoforms βII or δ did not significantly increase PMA-induced superoxide production, suggesting that endogenous PKC-α and/or PKC-δ are sufficient for PMA-induced NADPH oxidase activation in COS-phox cells and that these isoforms are not rate limiting.
Stimulation of COS-phox cells with either PMA or AA induced Rac translocation to the membrane. Furthermore, expression of transgenic RacN17 or RhoGDI significantly inhibited both PMA- and AA-induced superoxide production in COS-phox cells. These results suggest that Rac activation and membrane translocation are important for assembly of the active NADPH oxidase complex in whole COS cells. This is consistent with the requirement for Rac-GTP to achieve high-level superoxide-generating activity in cell-free systems derived from neutrophils or using highly purified proteins.65,66Rac-GTP binds directly to p67phox,17,33 and to a second site in the NADPH oxidase complex, possibly the flavocytochrome.67 Our results also support the finding that expression of D57N dominant-negative Rac2 is associated with reduced NADPH oxidase activity in human neutrophils.23,24 68
Capitalizing on the ease with which the phox components can be manipulated in COS-phox cells, we used this system to assess the importance of a putative activation domain in p67phox in a whole-cell environment. This p67phox activation domain (residues 199 through 210) was originally identified by testing the activity of recombinant p67phox C-terminal truncation mutants in cell-free oxidase assays. Whereas p67phoxtruncated at amino acid 210 supported NADPH oxidase activity at levels comparable to wild-type p67phox, p67phox truncated at amino acid 204 was inactive.32 Amino acid substitutions within the activation domain of truncated p67phox (1 through 210) revealed that a p67phox Val204Ala mutant was completely inactive in the cell-free oxidase assay.32 The p67phox Val204Ala mutant associated with the membrane fraction after cell-free activation and competed with wild-type p67phox for assembly into the NADPH oxidase complex.32 The p67phoxactivation domain was not involved in binding interactions with the other cytosolic factors and was postulated to bind directly to flavocytochrome b558.32 In vitro flavin reduction assays suggest that the p67phoxactivation domain regulates hydride/electron flow from NADPH to FAD in the flavocytochrome.34 Our results in the COS-phox system establish that substitution of alanine for valine at residue 204 in the p67phox activation domain also abrogates superoxide production when NADPH oxidase assembly is triggered in whole cells. Furthermore, coexpression of full-length p67phox Val204Ala mutant with wild-type p67phox inhibited superoxide production in the COS-phox cells. Similar results were obtained in cell-free NADPH oxidase activation assays, which suggest that p67phox Val204Ala is incorporated into the oxidase complex but results in an inactive enzyme.32
In conclusion, we functionally reconstituted the phagocyte NADPH oxidase in nonhematopoietic COS-7 cells by stable transfection of 4 essential phagocyte oxidase components, gp91phox, p22phox, p47phox, and p67phox. This whole-cell model system was capable of high-level superoxide production in response to phorbol ester or AA. The COS-phoxsystem resembled neutrophils in that superoxide production was dependent on Rac activation and the presence of all 4 phoxsubunits and was associated with membrane translocation of the cytosolic subunits. Serines 303, 304, and 379 in p47phox, previously shown to be important for activation of the NADPH oxidase in whole cells, were also required for optimal superoxide production by COS-phox cells. Using this model system, we established that the p67phoxactivation domain, which is essential for superoxide production under cell-free conditions, was required for assembly of a functional NADPH oxidase in whole cells. Thus the COS-phox cells represent a new, readily transfectable whole-cell system for studying the phagocyte NADPH oxidase by genetic approaches.
We thank Nancy Pech for assistance with NBT assays, Dianne Greene for assistance with identification of PKC isoforms, Teri Mallory and Shari Upchurch for expert help in manuscript preparation, and David Skalnik for helpful discussions.
Supported by National Institutes of Health grants RO1HL45635 (M.C.D.), 2RO1AI-22564 (L.C.M.), CA84138 (J.D.L.), AI35947 (U.G.K.), and GM37696 (U.G.K.). The Wells Center for Pediatric Research is a Center for Excellence in Molecular Hematology funded by National Institutes of Health grant P50DK49218.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mary C. Dinauer, Cancer Research Institute R4, Indiana University School of Medicine, 1044 W Walnut St, Rm 402A, Indianapolis, IN 46202; e-mail: mdinauer@iupui.edu.