Abstract
Campath-1H (alemtuzumab) is the most effective monoclonal antibody in single-agent use in B-cell chronic lymphocytic leukemia (CLL) with reported response rates of 33% to 70%. Combination therapy is now the conventional treatment for most hematologic malignancies. Monoclonal antibody treatments may sensitize tumor cells to subsequent chemotherapy. We report the combination of Campath-1H with fludarabine in patients with CLL refractory to each agent used singly. Six patients who had received a median of 8 courses of fludarabine (range, 4-10 courses) and 16 weeks of Campath-1H (range, 8-32 weeks) were treated. Five patients responded, including one who had a complete response by National Cancer Institute criteria. The responses observed were better in each patient than responses after each agent used singly. Complete morphologic bone marrow responses were seen in 3 patients, including eradication of disease measured by sensitive flow cytometry in 2. Campath-1H combined with fludarabine is a highly promising novel therapy for refractory CLL.
Introduction
Patients with chronic lymphocytic leukemia (CLL) resistant to purine analogues have a median survival of 10 months.1 Campath-1H (alemtuzumab) can lead to durable responses in up to 59%2-4 of refractory cases. Patients who are resistant to chemotherapy and fail to respond to Campath-1H therapy have an overall survival of 9 months (B.K., personal observation, 2000), and currently no effective therapy for these patients is available. Evidence suggests that monoclonal antibodies may sensitize malignant cells to chemotherapy.5 6 We therefore treated 6 patients, all men, previously refractory both to fludarabine and to Campath-1H, with a novel combination of intravenous Campath-1H and fludarabine.
Study design
All patients had progressive B-cell CLL with heavy marrow involvement and significant lymph node disease. Patients were enrolled in a prospective multicenter clinical trial to receive single-agent Campath-1H for disease refractory to purine analogues. Approval was obtained from the Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki. Patients who tolerated single-agent Campath-1H but who failed to attain a satisfactory response subsequently consented to receive Campath-1H and fludarabine in combination. Patients were excluded if there was active infection or in the presence of profound cytopenia (neutrophils < 1.0 × 109/L or platelets < 50 × 109/L). Treatment was continued to maximum response, with a minimum planned duration of 8 weeks (2 cycles). All received cotrimoxazole and acyclovir prophylaxis, and were prospectively monitored for cytomegalovirus (CMV) reactivation. Patients were assessed for response (National Cancer Institute criteria9) by marrow and whole body computerized tomography (CT) 2 months after completing therapy. Sensitive 4-color flow cytometry for residual disease (MRD flow),7,8 which detects fewer than 0.05% lymphocytes with a clonal B-CLL phenotype in a polyclonal background,8 was performed on marrow samples before treatment, every 4 weeks during treatment, and 2 months after treatment. Patients who were already receiving Campath-1H at the time of the addition of fludarabine continued at a dose level of 30 mg intravenously 3 times a week. Patients who were recommencing Campath-1H as part of combined therapy received an escalating dose, starting at 3 mg. Fludarabine 25 mg/m2 was infused intravenously for 3 days every 28 days.
Results and discussion
Unique Patient Number 1 (UPN1) presented at the age of 48 in 1987. In 1997, he received 4 courses of fludarabine followed by combination chemotherapy (cyclophosphamide, hydroxydaunomycin, Oncovin, prednisone [CHOP]), but had persistent lymphadenopathy and diffusely infiltrated marrow. In 1998, he achieved a complete remission after 12 weeks of Campath-1H. His disease progressed in February 2000 and he was retreated with 12 weeks of intravenous Campath-1H but failed to respond, and then continued with Campath-1H together with 2 cycles of simultaneous fludarabine (Table 1). He achieved a partial remission, had peripheral blood stem cells (PBSCs) harvested, and underwent an autologous peripheral blood stem cell transplantation (PBSCT) following cyclophosphamide and total body irradiation (Cyclo/TBI). Eight months after autograft he has no detectable disease.
UPN2 presented at the age of 50 in 1992. He did not achieve a response to 10 courses of fludarabine in 1996, and thus received 12 weeks of Campath-1H. He proceeded to a BCNV, Etoposide, ARA-C, Melphalan (BEAM) autograft in May 1997. Sixteen months later his disease progressed, but failed to respond to a further 20 weeks of Campath-1H. He had bulky lymphadenopathy and heavy marrow infiltration. His disease did not respond to Campath-1H and fludarabine in combination (2 cycles). He remains alive 12 months after combined therapy.
UPN3 presented at the age of 40 in 1992. He received 6 cycles of fludarabine in 1995 followed by 6 cycles of CHOP. He had persisting heavy marrow infiltration and massive lymphadenopathy. From November 1999 he received 16 weeks of Campath-1H during which time his lymphadenopathy progressed. He then continued on 30 mg Campath-1H with the addition of fludarabine for 4 cycles of therapy and attained a complete remission. PBSCs were harvested and he underwent PBSCT with Cyclo/TBI conditioning 2 months after combined therapy. He remains in clinical complete remission 11 months after autograft.
UPN4 presented at the age of 38 in 1997. After 8 cycles of fludarabine in December 1997, he had persistent cervical lymphadenopathy and a peripheral lymphocytosis. In November 1998, after 6 courses of CHOP he had persistent lymphadenopathy and became dependent on transfusions. After failing to respond to 13 weeks of Campath-1H monotherapy he continued Campath-1H with 2 cycles of combined fludarabine. He achieved a partial remission (Table 1) but with cytopenia, which recovered after treatment with methylprednisolone. Sixteen months after therapy his disease is progressing with recurrent lymphadenopathy but he remains untreated.
UPN5 presented at the age of 67 in 1995. He received a single course of fludarabine in June 1996, which led to prolonged neutropenia and an aspergillus pneumonia. In June 1998, he began 8 weeks of Campath-1H. Following this he had persistent 8-cm splenomegaly and 4-cm nodal disease, and his marrow biopsy remained heavily infiltrated. In September 1999, after 8 further cycles of fludarabine, he had persistent disease (Table 1). Six weeks later he started 3 cycles of combined Campath-1H and fludarabine, attaining a partial response. Campath-1H was interrupted at week 8 because of pseudomonas bronchopneumonia, which occurred during neutropenia; Campath-1H was recommenced after 5 days at the same dose of 30 mg. He remained well for 15 months when he was admitted with recurrent bronchopneumonia and died of overwhelming sepsis. Peripheral blood counts were normal. He had a spleen palpable at 4 cm and low-volume lymphadenopathy.
UPN6 presented at the age of 44 in 1995. In 1998, his CLL failed to respond to 6 cycles of fludarabine, which was combined with cyclophosphamide for the final 3 courses. In December 1999, he received 3 cycles of CHOP without response. In May 2000, he completed 12 weeks of intravenous Campath-1H. There was no improvement in his 1-cm lymphadenopathy or in his bone marrow appearances. In September 2000, the marrow contained 53% clonal B cells. He then began 3 cycles of combined Campath-1H and fludarabine attaining a partial remission (Table 1). Eight months after therapy he remains well and untreated with a peripheral lymphocytosis of 13.7 × 109/L.
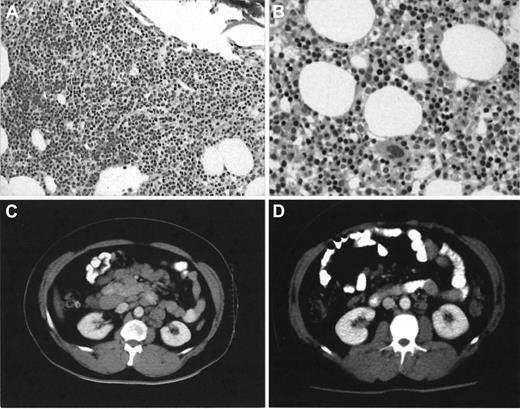
Five of 6 patients with advanced stage B-CLL who failed to respond to fludarabine or to Campath-1H when administered as single agents responded significantly better (1 complete and 4 partial responses) to both agents used in combination. Five patients are alive at a median follow-up of 12 months (range of follow-up, 8-16 months). Marrow trephine appearances normalized in 3 patients (UPN1, 3, and 5). Two of these patients (UPN1 and 5) had no detectable disease by MRD flow. Two other responders (UPN4 and 6) had hypocellular trephines with 0.8% and 0.35% clonal B cells detected. Complete resolution of massive lymphadenopathy occurred in UPN3 (Figure1). Toxicity during combined therapy was acceptable with only one patient (UPN5) requiring hospital admission with pseudomonas bronchopneumonia during neutropenia. No interruption occurred in any other patient. Two neutropenic patients responded to granulocyte colony-stimulating factor and no patients required platelet transfusions. No CMV reactivation occurred. Two patients (UPN1 and 3) have successfully undergone autologous PBSCT with stem cells collected after combined therapy.
Bone marrow trephine and CT scan appearances in patient UPN3 before and after combined Campath-1H and fludarabine therapy.
Note heavy infiltrate (A) × 10 by small lymphocytes observed after 16 weeks of single-agent Campath-1H has completely cleared (B) × 20 with normal erythropoiesis evident after combined Campath-1H and fludarabine. Stained with hematoxylin-eosin. The CT scan (D) shows almost complete radiologic resolution of his previously massive lymphadenopathy (C).
Bone marrow trephine and CT scan appearances in patient UPN3 before and after combined Campath-1H and fludarabine therapy.
Note heavy infiltrate (A) × 10 by small lymphocytes observed after 16 weeks of single-agent Campath-1H has completely cleared (B) × 20 with normal erythropoiesis evident after combined Campath-1H and fludarabine. Stained with hematoxylin-eosin. The CT scan (D) shows almost complete radiologic resolution of his previously massive lymphadenopathy (C).
We have shown Campath-1H and fludarabine in combination can increase the proportion of responses, including complete remissions, with acceptable toxicity. It appears that the 2 agents act synergistically, allowing enhanced targeting by antibody to previously inaccessible lymph node disease. This pilot study will be extended to a phase 2 clinical trial. The combination of Campath-1H and fludarabine appears to be effective and may represent a significant step forward in the therapy of CLL.
Supported in part by the Yorkshire Cancer Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Ben Kennedy, Haematological Malignancy Diagnostic Service, Institute of Pathology, Algernon Firth Bldg, University of Leeds, Leeds LS2 9JT, West Yorkshire, United Kingdom; e-mail:medbk@leeds.ac.uk.