More than a decade ago it was demonstrated that neutrophil activation in plasma results in the time-dependent formation of lipid hydroperoxides through an unknown, ascorbate-sensitive pathway. It is now shown that the mechanism involves myeloperoxidase (MPO)-dependent use of multiple low-molecular–weight substrates in plasma, generating diffusible oxidant species. Addition of activated human neutrophils (from healthy subjects) to plasma (50%, vol/vol) resulted in the peroxidation of endogenous plasma lipids by catalase-, heme poison-, and ascorbate-sensitive pathways, as assessed by high-performance liquid chromatography (HPLC) with on-line electrospray ionization tandem mass spectrometric analysis of free and lipid-bound 9-HETE and 9-HODE. In marked contrast, neutrophils isolated from multiple subjects with MPO deficiency failed to initiate peroxidation of plasma lipids, but they did so after supplementation with isolated human MPO. MPO-dependent use of a low-molecular–weight substrate(s) in plasma for initiating lipid peroxidation was illustrated by demonstrating that the filtrate of plasma (10-kd MWt cutoff) could supply components required for low-density lipoprotein lipid peroxidation in the presence of MPO and H2O2. Subsequent HPLC fractionation of plasma filtrate (10-kd MWt cutoff) by sequential column chromatography identified nitrite, tyrosine, and thiocyanate as major endogenous substrates and 17β-estradiol as a novel minor endogenous substrate in plasma for MPO in promoting peroxidation of plasma lipids. These results strongly suggest that the MPO–H2O2system of human leukocytes serves as a physiological mechanism for initiating lipid peroxidation in vivo.
Introduction
The peroxidation of lipids and the consequent generation of bioactive lipid oxidation products are believed to play important roles in the pathogenesis of atherosclerosis and other inflammatory processes.1-5 Lipoxygenase, cyclooxygenase, and cytochrome P450 are considered the primary enzymatic participants in these events.6-10 These enzymes are expressed in leukocytes and catalyze the direct insertion of molecular oxygen (O2) into polyenoic fatty acids, forming hydroperoxides and other advanced oxidation products.8 Whether alternative chemical pathways contribute to the oxidation of lipoproteins and lipids in complex biologic matrices, such as plasma, has not yet been fully defined.
We and others have proposed that another potential pathway for initiating lipid peroxidation in vivo may involve myeloperoxidase (MPO), a heme protein present in neutrophils, monocytes, and certain subpopulations of tissue macrophages.11-13 On phagocyte activation in peripheral tissues and fluids, MPO is secreted into the extracellular milieu and into the phagolysosome, where it uses hydrogen peroxide (H2O2) generated during a respiratory burst as a cosubstrate. Activated intermediates, Compounds 1 and 2, are sequentially formed that generate reactive oxidants and diffusible radical species through 2- and 1-electron oxidation reactions, respectively.5,14 At plasma levels of halides, chloride (Cl−) is a major cosubstrate for MPO and the cytotoxic oxidant hypochlorous acid (HOCl) is produced.15 In addition to halides16 and the pseudohalide thiocyanate (SCN−),17,18 various organic and inorganic components found in plasma have been suggested to potentially serve as naturally occurring substrates for MPO. These include, but are not limited to, estrogens,19 catecholamines,20tyrosine,21 nitrite (NO),22 ascorbate,23NADPH,24 serotonin,25 and nitric oxide (nitrogen monoxide, NO).26 The relative contribution of peroxidation of these substrates to the overall activity of MPO in vivo is unknown. The products they form and the reactions they initiate may serve significant biologic functions. For example, NO, a major end-product of NO metabolism, and tyrosine undergo MPO-catalyzed 1-electron oxidation reactions generating nitrogen dioxide (• NO2)22,27 and tyrosyl radical,21 respectively. These diffusible oxidant species have been shown to initiate lipid peroxidation in model systems using isolated lipoproteins or lipid vesicles as targets.11-13 Moreover, protein oxidation products that can be formed by MPO-generated tyrosyl radical or• NO2, such as dityrosine28and nitrotyrosine,22,29,30 have been observed in human atherosclerotic lesions31-33 and other inflammatory sites at which lipid peroxidation products and MPO are also found.34-36 Although in vitro studies examining individual potential substrates for MPO in isolation have suggested their potential role in promoting lipid oxidation, the actual substrates used by MPO in complex biologic tissues and fluids in vivo have not yet been determined. Indeed, a role for MPO in the oxidation of low-density lipoprotein (LDL) and in the initiation of lipid peroxidation has recently been questioned by several investigators. Noguchi et al37 examined the capacity of leukocytes isolated from wild-type and MPO knockout mice to promote the oxidation of LDL in model systems ex vivo and observed only modest differences in the parameters of lipid oxidation monitored.37 It has also recently been suggested that MPO-catalyzed oxidation of LDL is inhibited, rather than promoted, by the presence of NO, particularly when focusing on protein oxidation products.38 Moreover, an anti-oxidant rather than a pro-oxidant function for MPO-generated tyrosine oxidation products and LDL oxidation has been proposed.39,40 It has also been suggested by some investigators that HOCl generated by MPO can promote the oxidation of lipoprotein lipids and the formation of hydroperoxides,41 whereas other studies have not supported these observations.11,30 Finally, recent studies have noted species differences between murine and human leukocytes with respect to MPO and the generation of reactive oxidant species.42-45
In the current study we sought to definitively establish whether human leukocytes use MPO for catalyzing the oxidation of lipids in complex biologic matrices, such as in plasma, where numerous competing cosubstrates for the enzyme are present. We also sought to identify chemically the component(s) in plasma that serve as preferred substrates for the MPO–H2O2 system of leukocytes for the initiation of lipid peroxidation. In studies using leukocytes isolated from healthy and MPO-deficient subjects, in combination with HPLC with on-line electrospray ionization tandem mass spectrometry (LC/ESI/MS/MS), we now show that human neutrophils use MPO to initiate lipid peroxidation in whole plasma through multiple distinct diffusible substrates.
Materials and methods
Chemicals and reagents
Hanks balanced salt solution (HBSS) was purchased from Gibco BRL (Grand Island, NY). Free fatty acids were purchased from Cayman Chemical (Ann Arbor, MI). Organic solvents were obtained from Fisher Scientific (Pittsburgh, PA). All other reagents were purchased from Sigma Chemical (St Louis, MO) unless otherwise indicated.
General procedures
All buffers were passed over a Chelex-100 resin column (Bio-Rad, Hercules, CA) and were supplemented with diethylenetriamine pentaacetic acid (DTPA) to remove potential contaminant transition metal ions that might catalyze LDL oxidation during incubation. Protein content was determined by the Markwell-modified Lowry protein assay with bovine serum albumin as standard.46 The concentration of reagent H2O2 was assayed spectrophotometrically (ε240 = 39.4 M−1cm−1).47 Production of H2O2 by glucose and glucose oxidase (GO, EC1.1.3.4) was determined by the oxidation of Fe(II) and the formation of an Fe(III)–thiocyanate complex.22 Peroxidase activity in isolated leukocytes was evaluated by determining the rate of guaiacol oxidation.48 HOCl production was quantified by the taurine chloramine method.49 Superoxide (O2• −) production by activated human neutrophils was measured as the superoxide dismutase (SOD)–inhibitable reduction of ferricytochrome c.50 In situ staining of native gels for peroxidase activity was performed as described.51 All protocols were in accordance with institutional guidelines on research involving human subjects of either the Cleveland Clinic Foundation or the University of Iowa and were approved by their respective institutional review boards. Informed consent was provided according to the Declaration of Helsinki.
Data are presented as mean ± SD. Comparisons between control and tested groups were made using nonparametric analysis, andP < .05 was considered significant between 2 groups.
MPO and lipoprotein isolation
MPO (donor: hydrogen peroxide, oxidoreductase, EC 1.11.1.7) was isolated and characterized as described.28,51 Purity of isolated MPO was established by demonstrating R/Z ≥ 0.85 (A430/A280), sodium dodecyl sulfate–polyacrylamide gel electrophoresis analysis with Coomassie blue staining, and in-gel tetramethylbenzidine peroxidase staining to confirm no eosinophil peroxidase contamination.51 Purified MPO was stored in 50% glycerol at −20°C. Enzyme concentration was determined spectrophotometrically (ε430 = 170 000 M−1cm−1).52 LDL was isolated from fresh plasma by sequential ultracentrifugation as a 1.019 < d < 1.063 g/mL fraction, and dialysis was performed in sealed jars under argon atmosphere.53 Final preparations were kept in 50 mM sodium phosphate (pH 7.0), 100 μM DTPA, and were stored under N2 until use. LDL concentrations are expressed per milligram LDL protein.
Human neutrophil preparations
Human neutrophils were isolated from whole blood obtained from healthy and MPO-deficient subjects, as described.54Neutrophil preparations were suspended in HBSS (Mg2+-, Ca2+-, phenol-, and bicarbonate-free, pH 7.0) and were used immediately for experiments. Molecular characterization of 2 of the MPO-deficient subjects have been reported in previous publications.55 56
Lipid peroxidation reaction
Isolated human neutrophils (106/mL) were incubated at 37°C with either 50% (vol/vol) normal human plasma or isolated human LDL (0.2 mg/mL) under air in HBSS supplemented with 100 μM DTPA. Neutrophils were activated by adding 200 nM phorbol myristate acetate (PMA) and were maintained in suspension by gentle mixing every 5 minutes. After 2 hours, reactions were stopped by immersion in ice or water bath, centrifugation at 4°C, and immediate addition of 50 μM butylated hydroxytoluene (BHT) and 300 nM catalase to the supernatant. Lipid peroxidation products in the supernatant were then rapidly assayed as described below.
Reactions with isolated MPO were typically performed at 37°C in sodium phosphate buffer (20 mM, pH 7.0) supplemented with 100 μM DTPA using 30 nM MPO, 1 mM glucose (G), and 20 ng/mL GO. Under this condition, a constant flux of H2O2 (0.18 μM/min) was generated by the glucose–glucose oxidase (G/GO) system. Unless otherwise stated, reactions were terminated by immersion in ice or water bath and the addition of 50 μM BHT and 300 nM catalase to the reaction mixture.
Lipid extraction and sample preparation
Lipids were extracted and prepared for mass spectrometry analysis under argon or nitrogen atmosphere at all steps. First, hydroperoxides in the reaction mixture were reduced to their corresponding hydroxides by adding SnCl2 (1 mM final). A known amount of deuterated internal standard, 12-HETE-d8 (Cayman Chemical), was added to the sample, and plasma lipids were extracted by adding a mixture of 1 M acetic acid–2-isopropanol–hexane (2/20/30, vol/vol/vol) at a ratio of 5 mL organic solvent mix:1 mL plasma. After vortexing and centrifugation, lipids were extracted to the hexane layer. Plasma was re-extracted by the addition of an equal volume of hexane, followed by vortexing and centrifugation. Cholesteryl ester hydroperoxides (CE-H(P)ODEs) were analyzed as their stable SnCl2- reduced hydroxide forms by drying of the combined hexane extracts under N2, reconstituting samples with 200 μL 2-isopropanol–acetonitrile–water (44/54/2, vol/vol/vol) and storage at −80°C under argon until analysis. For the assay of free fatty acids and their oxidation products, total lipids (phospholipids, cholesterol esters, triglycerides) were dried under N2 and resuspended in 1.5 mL 2-isopropanol, and fatty acids were released by base hydrolysis with 1.5 mL 1 M NaOH at 60°C for 30 minutes under argon. Hydrolyzed samples were acidified to pH 3.0 with 2 M HCl, and fatty acids were extracted twice with 5 mL hexane. The combined hexane layers were dried under N2, resuspended in 100 μL methanol, and stored under argon at −80°C until analysis by LC/ESI/MS/MS, as described below.
HPLC fractionation of plasma filtrate
To study the role played by low-molecular–weight compounds in plasma as substrates for MPO in the promotion of lipid peroxidation, whole plasma from healthy donors was filtered through a 10-kd MWt cutoff filter (Centriprep YM-10; Millipore, Bedford, MA) by centrifugation. The filtrate of plasma was used either directly or after fractionation by HPLC. Reverse-phase HPLC fractionation was performed using a Beckman C-18 column (4.6 × 250 mm, 5 μm OD; Beckman Instruments, Fullerton, CA). Separation of low-molecular–weight compounds in plasma filtrate (0.5 mL) was carried out at a 1.0 mL/min flow rate with 100% mobile phase A (water containing 0.1% acetic acid) over 10 minutes, followed by a linear gradient generated with 100% mobile phase B (methanol containing 0.1% acetic acid) over 10 minutes, followed by 100% mobile phase B over 5 minutes. Effluent was collected as 1-mL fractions, dried under N2, and resuspended in buffer (0.1 mL) for analysis. Fractionation of plasma filtrate (0.5 mL) by strong anion exchange HPLC (SAX-HPLC) was performed on a SPHERIS HPLC column (4.6 × 250 mm, 5 μm SAX; Phase Separations, Norwalk, CT). The separation of low-molecular–weight compounds in plasma filtrate was carried out at the flow rate of 0.9 mL/min under isocratic conditions using 45 mM ammonium acetate buffer (pH 4.0) as mobile phase. Effluent was collected as 1-mL fractions, dried under N2, and resuspended in buffer (0.1 mL) for analysis.
Mass spectrometry
LC/ESI/MS/MS was used to quantify free radical-dependent oxidation products of arachidonic acid 9-H(P)ETE and linoleic acid 9-H(P)ODE. Immediately before analysis, 1 vol H2O was added to 5 vol methanol-suspended sample, which was then passed through a 0.22-μm filter (Millipore). Sample (20 μL) was injected onto a Prodigy C-18 column (1 × 250 mm, 5 μm OD, 100 A; Phenomenex, Rancho Palos Verdes, CA) at a flow rate of 50 μL/min. Separation was performed under isocratic conditions using 95% methanol in water as the mobile phase. In each analysis, the entirety of the HPLC column effluent was introduced onto a Quattro II triple quandrupole MS (Micromass). Analyses were performed using electrospray ionization in negative-ion mode with multiple reaction monitoring of parent and characteristic daughter ions specific for the isomers monitored. Transitions monitored were mass-to-charge ratio (m/z) 295→171 for 9-HODE, m/z 319→151 for 9-HETE, and m/z 327→184 for 12-HETE-d8. N2 was used as the curtain gas in the electrospray interface. Internal standard 12-HETE-d8 was used to calculate extraction efficiencies (greater than 80% for all analyses). External calibration curves constructed with authentic standards were used to quantify 9-HETE and 9-HODE.
Reverse-phase HPLC quantification of CE-H(P)ODEs
Samples (100 μL) reconstituted in methanol (without base hydrolysis) were injected onto a Beckman C-18 column (4.6 × 250 mm, 5 μm OD; Beckman Instruments). Lipids were separated using an isocratic solvent system composed of 2-isopropanol–acetonitrile–water (44/54/2, vol/vol/vol) at a flow rate of 1.5 mL/min. CE-H(P)ODEs were quantified as their stable hydroxide forms by UV detection at 234 nm using CE-9-HODE (Cayman Chemical) for generation of an external calibration curve.
Results
Neutrophils isolated from MPO-deficient subjects fail to initiate lipid peroxidation in plasma but do so after the addition of isolated MPO
More than a decade ago, Frei et al57 made the seminal observation that neutrophil activation in plasma results in the time-dependent formation of lipid hydroperoxides. The precise mechanism involved was not identified but was characterized by its sensitivity to ascorbate, which had to be depleted before lipid oxidation products were formed.57 To test the hypothesis that MPO might serve as the enzymatic catalyst for leukocyte-dependent peroxidation of plasma lipids, we compared neutrophils from healthy and MPO-deficient subjects. Lack of functional MPO activity was confirmed by the absence of HOCl production after leukocyte activation (Table1) and the absence of an MPO activity band within native gels of leukocyte detergent extracts after in-gel tetramethylbenzidine peroxidase staining (data not shown). MPO-deficient neutrophils displayed enhanced agonist-dependent O2• − generation relative to comparably treated normal neutrophils (Table 1), as previously reported.58-60 Neutrophils isolated from healthy and MPO-deficient subjects failed to generate detectable levels of NO or NO in a 2-hour period.
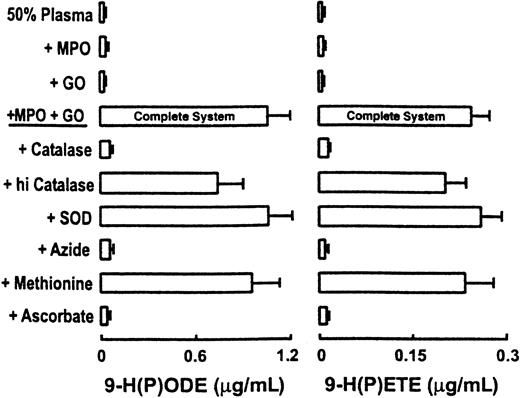
To determine the role of MPO in promoting lipid oxidation in plasma exposed to activated neutrophils, we next incubated cells with whole plasma (50%, vol/vol) and physiological levels of Cl−(100 mM final). Phagocytes were activated with PMA, and the formation of 9-H(P)ODE and 9-H(P)ETE, specific oxidation products of linoleic and arachidonic acids, respectively, was determined by LC/ESI/MS/MS. Normal neutrophils generated significant levels of 9-H(P)ODE and 9-(H)PETE in plasma after cell activation by PMA (Figure1). In stark contrast, MPO-deficient neutrophils failed to generate significant levels of lipid peroxidation products after stimulation with PMA, despite their enhanced capacity to produce O2• −. Addition of catalytic amounts of MPO restored the capacity of MPO-deficient neutrophils to initiate the peroxidation of endogenous plasma lipids (Figure 1).
MPO-deficient neutrophils fail to initiate lipid peroxidation in plasma.
Neutrophils (1 × 106/mL) isolated from healthy and MPO-deficient subjects were incubated at 37°C in HBSS supplemented with DTPA (100 μM, pH 7.0) and fresh human plasma (50%, vol/vol). Cells were activated by the addition of phorbol myristate acetate (PMA, 200 nM) and incubated for 2 hours (Complete System). The contents of 9-H(P)ODE and 9-H(P)ETE formed within endogenous plasma lipids were then determined by LC/ESI/MS/MS as described in “Materials and methods.” Where indicated, human MPO (30 nM) was added to reaction mixtures. Data represent the mean ± SD of triplicate determinations. Each bar within a cluster for a given condition represents results obtained from independent experiments performed with neutrophil preparations from a distinct donor. PMN(MPO+), neutrophils isolated from healthy subjects; PMN(MPO−), neutrophils isolated from MPO-deficient subjects.
MPO-deficient neutrophils fail to initiate lipid peroxidation in plasma.
Neutrophils (1 × 106/mL) isolated from healthy and MPO-deficient subjects were incubated at 37°C in HBSS supplemented with DTPA (100 μM, pH 7.0) and fresh human plasma (50%, vol/vol). Cells were activated by the addition of phorbol myristate acetate (PMA, 200 nM) and incubated for 2 hours (Complete System). The contents of 9-H(P)ODE and 9-H(P)ETE formed within endogenous plasma lipids were then determined by LC/ESI/MS/MS as described in “Materials and methods.” Where indicated, human MPO (30 nM) was added to reaction mixtures. Data represent the mean ± SD of triplicate determinations. Each bar within a cluster for a given condition represents results obtained from independent experiments performed with neutrophil preparations from a distinct donor. PMN(MPO+), neutrophils isolated from healthy subjects; PMN(MPO−), neutrophils isolated from MPO-deficient subjects.
Characterization and reaction requirements for peroxidation of endogenous plasma lipids by activated human neutrophils and isolated human MPO
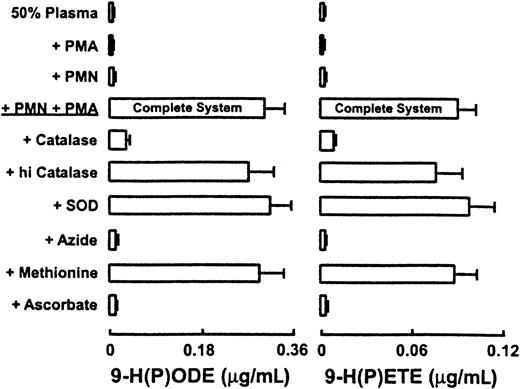
Addition of catalase, but not heat-inactivated catalase, to cell mixtures resulted in the near complete ablation of lipid peroxidation in plasma, strongly suggesting a critical role for H2O2 in the cell-dependent reaction (Figure2). Incubation of reaction mixtures with SOD failed to attenuate the oxidation of plasma lipids (Figure 2). In contrast, addition of heme poisons (eg, azide, cyanide) and water-soluble antioxidant ascorbate resulted in complete inhibition of neutrophil-dependent peroxidation of plasma lipids. Finally, addition of HOCl scavengers, such as dithiothreitol and the thioether methionine, failed to attenuate neutrophil-dependent peroxidation of endogenous plasma lipids, assessed by quantification of 9-H(P)ODE and 9-H(P)ETE (Figure 2).
Characterization of neutrophil-dependent initiation of lipid peroxidation of endogenous plasma lipids.
Neutrophils (1 × 106/mL) isolated from healthy subjects (PMN) were incubated at 37°C in HBSS supplemented with DTPA (100 μM, pH 7.0) and fresh human plasma (50%, vol/vol). Cells were activated by the addition of phorbol myristate acetate (200 nM) and then incubated for 2 hours (Complete System). The contents of 9-H(P)ODE and 9-H(P)ETE formed within endogenous plasma lipids were then determined by LC/ESI/MS/MS as described in “Materials and methods.” Additions or deletions to the Complete System were as indicated. Final concentrations of additions to the Complete System were 30 nM human MPO, 1 mM NaN3, 300 nM catalase, 300 nM heat-inactivated catalase, 100 μM methionine, 100 μM ascorbate, and 10 μg/mL SOD. Data represent the mean ± SD of 3 independent experiments.
Characterization of neutrophil-dependent initiation of lipid peroxidation of endogenous plasma lipids.
Neutrophils (1 × 106/mL) isolated from healthy subjects (PMN) were incubated at 37°C in HBSS supplemented with DTPA (100 μM, pH 7.0) and fresh human plasma (50%, vol/vol). Cells were activated by the addition of phorbol myristate acetate (200 nM) and then incubated for 2 hours (Complete System). The contents of 9-H(P)ODE and 9-H(P)ETE formed within endogenous plasma lipids were then determined by LC/ESI/MS/MS as described in “Materials and methods.” Additions or deletions to the Complete System were as indicated. Final concentrations of additions to the Complete System were 30 nM human MPO, 1 mM NaN3, 300 nM catalase, 300 nM heat-inactivated catalase, 100 μM methionine, 100 μM ascorbate, and 10 μg/mL SOD. Data represent the mean ± SD of 3 independent experiments.
Results thus far presented strongly suggest that neutrophils use the MPO–H2O2 system to generate reactive species distinct from chlorinating intermediates as the primary oxidants for initiation of lipid peroxidation in plasma. To confirm a physiological role for MPO, we next added purified human MPO and an H2O2-generating system (G/GO) to plasma and monitored the formation of specific oxidation products by LC/ESI/MS/MS analysis. Formation of 9-H(P)ODE and 9-H(P)ETE occurred readily and had an absolute requirement for the presence of MPO and the H2O2-generating system (Figure3). Lipid oxidation was again inhibited by catalase, azide, or ascorbate but was not affected by the addition of SOD or methionine (Figure 3). Collectively, these results strongly support a pivotal role for the MPO–H2O2 system of leukocytes as a primary mechanism for initiating lipid peroxidation in complex biologic tissues and fluids such as plasma.
Characterization of MPO-dependent initiation of lipid peroxidation of endogenous plasma lipids.
Fresh human plasma (50%, vol/vol) was incubated with isolated human MPO (30 nM) at 37°C in HBSS supplemented with DTPA (100 μM, pH 7.0) and an H2O2-generating system composed of G/GO for 12 hours (Complete System). Under this condition, a continuous flux of H2O2 is formed at 10 μM/h. The contents of 9-H(P)ODE and 9-H(P)ETE formed within endogenous plasma lipids were then determined by LC/ESI/MS/MS as described in “Materials and methods.” Additions or deletions to the Complete System were as indicated. Final concentrations of additions to the Complete System were 1 mM NaN3, 300 nM catalase, 300 nM heat-inactivated catalase, 200 nM SOD, 100 μM methionine, and 100 μM ascorbate. Data represent the mean ± SD of 3 independent experiments.
Characterization of MPO-dependent initiation of lipid peroxidation of endogenous plasma lipids.
Fresh human plasma (50%, vol/vol) was incubated with isolated human MPO (30 nM) at 37°C in HBSS supplemented with DTPA (100 μM, pH 7.0) and an H2O2-generating system composed of G/GO for 12 hours (Complete System). Under this condition, a continuous flux of H2O2 is formed at 10 μM/h. The contents of 9-H(P)ODE and 9-H(P)ETE formed within endogenous plasma lipids were then determined by LC/ESI/MS/MS as described in “Materials and methods.” Additions or deletions to the Complete System were as indicated. Final concentrations of additions to the Complete System were 1 mM NaN3, 300 nM catalase, 300 nM heat-inactivated catalase, 200 nM SOD, 100 μM methionine, and 100 μM ascorbate. Data represent the mean ± SD of 3 independent experiments.
Endogenous low-molecular–weight substances in plasma serve as cosubstrates for the MPO-catalyzed initiation of lipid peroxidation in whole plasma
The active site of MPO sits at the base of a deep and narrow heme pocket inaccessible to compounds significantly larger than a dipeptide.61 Thus, the ability of isolated MPO and of an H2O2-generating system to initiate lipid peroxidation in plasma is consistent with low-molecular–weight compounds in plasma serving as cosubstrates for MPO to generate diffusible species capable of conveying oxidizing equivalents from the heme group to distant targets, such as plasma lipoproteins. To test this hypothesis, isolated human LDL was incubated with MPO and an H2O2-generating system to generate a physiological flux of H2O2. In the absence of other cosubstrates, no significant oxidation of lipoprotein lipids was observed (Figure 4). In contrast, the addition of low-molecular–weight constituents recovered from plasma that had been filtered through a 10-kd MWt cutoff filter reconstituted the capacity of the MPO–H2O2 system to promote lipid peroxidation (Figure 4, left). In a parallel set of experiments, either plasma or dialyzed plasma was exposed to the MPO–H2O2 system, and the extent of lipid peroxidation was determined. Lipid peroxidation occurred in plasma, but not in dialyzed plasma, exposed to the MPO–H2O2 system (Figure 4, right), suggesting that MPO used low-molecular–weight substrates within plasma to initiate peroxidation of plasma lipids. Consistent with this observation, the subsequent addition of plasma filtrate to reaction mixtures using dialyzed plasma as the target for oxidation restored the ability of the MPO–H2O2 system to promote lipid peroxidation (Figure 4, right).
MPO uses endogenous low-molecular–weight/dialyzable substrates in plasma to initiate lipid peroxidation.
(left) LDL (0.2 mg/mL) was incubated with isolated human MPO (30 nM) and an H2O2-generating system composed of G/GO for 12 hours at 37°C in HBSS supplemented with DTPA (100 μM, pH 7.0) as in Figure 3. Where indicated, the filtrate of plasma (Fp), the low-molecular–weight constituents derived from plasma filtered through a 10-kd MWt cutoff filter, was added (50%, vol/vol) to the MPO–LDL reaction mixtures. Isolated MPO, an H2O2-generating system (G/GO) and LDL were added to Fp (Complete System). The content of 9-H(P)ETE formed within endogenous plasma lipids was then determined by LC/ESI/MS/MS as described in “Materials and methods.” (right) Plasma (P) or dialyzed plasma (DP) was incubated with isolated MPO and an H2O2-generating system (G/GO) under conditions similar to those described above. The content of 9-H(P)ETE formed within endogenous plasma lipids was then determined by LC/ESI/MS/MS as described in “Materials and methods.” Where indicated, DP and P were added.
MPO uses endogenous low-molecular–weight/dialyzable substrates in plasma to initiate lipid peroxidation.
(left) LDL (0.2 mg/mL) was incubated with isolated human MPO (30 nM) and an H2O2-generating system composed of G/GO for 12 hours at 37°C in HBSS supplemented with DTPA (100 μM, pH 7.0) as in Figure 3. Where indicated, the filtrate of plasma (Fp), the low-molecular–weight constituents derived from plasma filtered through a 10-kd MWt cutoff filter, was added (50%, vol/vol) to the MPO–LDL reaction mixtures. Isolated MPO, an H2O2-generating system (G/GO) and LDL were added to Fp (Complete System). The content of 9-H(P)ETE formed within endogenous plasma lipids was then determined by LC/ESI/MS/MS as described in “Materials and methods.” (right) Plasma (P) or dialyzed plasma (DP) was incubated with isolated MPO and an H2O2-generating system (G/GO) under conditions similar to those described above. The content of 9-H(P)ETE formed within endogenous plasma lipids was then determined by LC/ESI/MS/MS as described in “Materials and methods.” Where indicated, DP and P were added.
HPLC fractionation and identification of low-molecular–weight substrates in plasma used by MPO for initiation of lipid peroxidation
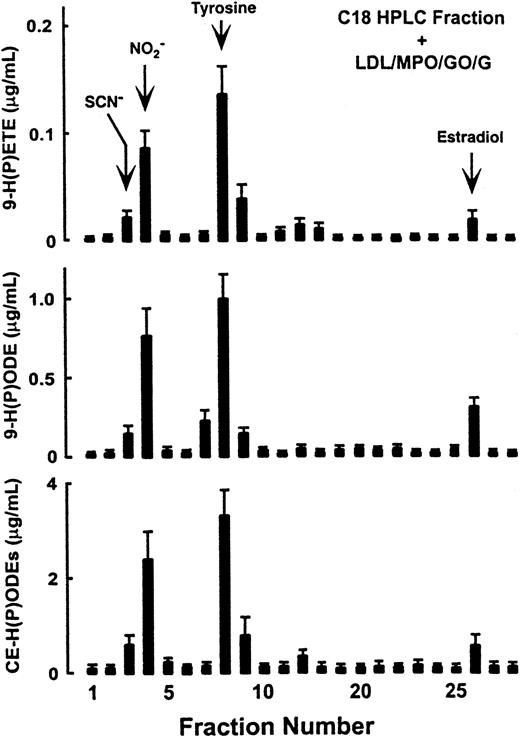
To identify the component(s) within the filtrate of plasma that served as physiological cosubstrates for MPO and that promoted peroxidation of plasma lipids, plasma filtrate was fractionated on a reverse-phase HPLC column, and the ability of each fraction to provide substrates for MPO-dependent oxidation of LDL surface and core lipids was determined (Figure 5). Comparisons with the retention times for potential candidate substrates for MPO suggested that the early eluting substrate(s) in fraction 3 comigrated with low-molecular–weight organic anions and SCN−, fraction 4 with NO, fraction 8 with tyrosine and fraction 26 with estradiol (Figure 5). HPLC with on-line electrospray ionization tandem mass spectrometry analysis (positive ion mode) demonstrated that fraction 8 was composed almost exclusively of an analyte with m/z 182.08—the m/z anticipated for the molecular cation of tyrosine (data not shown). The high salt content of fractions 3 and 4 prevented analysis by LC/ESI/MS/MS.
Reverse-phase HPLC fractionation and identification of low-molecular–weight components in plasma used by MPO to initiate lipid peroxidation.
Plasma was filtered through a 10-kd MWt cutoff filter. The filtrate of plasma containing the low-molecular–weight components was then fractionated by reverse-phase HPLC as described in “Materials and methods.” Each column fraction was dried under N2, reconstituted in 50 mM sodium phosphate buffer (pH 7.0), and incubated with a lipid source (LDL, 0.2 mg/mL), isolated human MPO (30 nM), and an H2O2-generating system (G/GO) (10 μM/h flux of H2O2). After incubation at 37°C for 12 hours, the contents of 9-HETE, 9-HODE, and CE-HODEs were then determined as described in “Materials and methods.” Retention times of some compounds described as MPO substrates in vitro include: Cl− and Br−, fraction 2 (F2); SCN−, F3; NO, F4; ascorbic acid, F5; tyrosine, F8; 6-hydroxy-dopamine, F19; serotonin, F20; catecholamines, F18-23; estradiols, F26.
Reverse-phase HPLC fractionation and identification of low-molecular–weight components in plasma used by MPO to initiate lipid peroxidation.
Plasma was filtered through a 10-kd MWt cutoff filter. The filtrate of plasma containing the low-molecular–weight components was then fractionated by reverse-phase HPLC as described in “Materials and methods.” Each column fraction was dried under N2, reconstituted in 50 mM sodium phosphate buffer (pH 7.0), and incubated with a lipid source (LDL, 0.2 mg/mL), isolated human MPO (30 nM), and an H2O2-generating system (G/GO) (10 μM/h flux of H2O2). After incubation at 37°C for 12 hours, the contents of 9-HETE, 9-HODE, and CE-HODEs were then determined as described in “Materials and methods.” Retention times of some compounds described as MPO substrates in vitro include: Cl− and Br−, fraction 2 (F2); SCN−, F3; NO, F4; ascorbic acid, F5; tyrosine, F8; 6-hydroxy-dopamine, F19; serotonin, F20; catecholamines, F18-23; estradiols, F26.
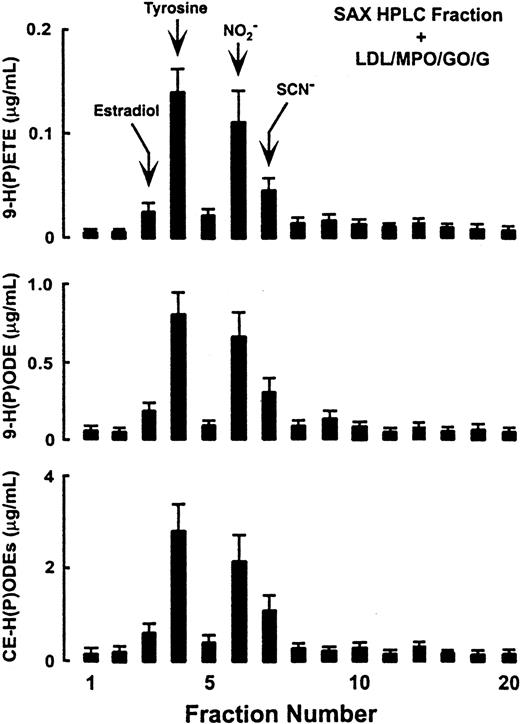
To further identify and confirm the cosubstrates of MPO in plasma that support the initiation of lipid peroxidation by the enzyme, plasma filtrate was fractionated by alternative column chromatographies (ion exchange, straight phase). Under every chromatography system examined, 4 compounds (tyrosine, NO, SCN−, and 17β-estradiol) comigrated with fractions that reconstituted the capacity of MPO–H2O2 systems to initiate lipid peroxidation. Results of fractionation on a strong anion exchange column are shown in Figure 6. Consistent with prior results using reverse-phase HPLC (Figure 5), 17β-estradiol, tyrosine, NO, and SCN−coeluted with the major fractions that supported MPO-dependent initiation of lipid peroxidation (Figure 6).
Strong anion exchange HPLC fractionation and identification of low-molecular–weight components in plasma used by MPO to initiate lipid peroxidation.
Plasma was filtered through a 10-kd MWt cutoff filter. The filtrate containing the low-molecular–weight components was then fractionated by HPLC using a strong anion exchange column as described in “Materials and methods.” Each fraction was then assessed for its capacity to provide cosubstrate for the MPO–H2O2 system and initiate LDL lipid peroxidation as in Figure 5. Retention times of some compounds described as MPO substrates in vitro include: serotonin, estradiols, Cl−, fraction 3 (F3); tyrosine, F4; ascorbic acid, F5; NO, F6; SCN−, F7.
Strong anion exchange HPLC fractionation and identification of low-molecular–weight components in plasma used by MPO to initiate lipid peroxidation.
Plasma was filtered through a 10-kd MWt cutoff filter. The filtrate containing the low-molecular–weight components was then fractionated by HPLC using a strong anion exchange column as described in “Materials and methods.” Each fraction was then assessed for its capacity to provide cosubstrate for the MPO–H2O2 system and initiate LDL lipid peroxidation as in Figure 5. Retention times of some compounds described as MPO substrates in vitro include: serotonin, estradiols, Cl−, fraction 3 (F3); tyrosine, F4; ascorbic acid, F5; NO, F6; SCN−, F7.
Confirmation that MPO uses physiologically relevant levels of nitrite, SCN−, and tyrosine as cosubstrates to promote peroxidation of lipids in plasma and demonstration that 17β-estradiol can be used by MPO to initiate LDL lipid oxidation under certain conditions
Levels of NO in the plasma of healthy human subjects measure approximately 3.6 to 5 μM, but they may reach 50 μM at sites of inflammation.22 The concentration of free tyrosine in plasma typically varies between 44 μM and 72 μM in healthy fasting subjects.62 Each has been previously shown to be used by MPO as substrate to initiate lipid oxidation in simple model systems,11 13 but neither has been reported to initiate MPO-dependent oxidation of endogenous lipids in plasma. To assess a potential role for NO and tyrosine as cosubstrates involved in lipid oxidation by the MPO–H2O2 system, we incubated physiologically relevant levels of each with dialyzed plasma as a lipid source (50%, vol/vol) and plasma levels of Cl− (100 mM). As shown in Figure 7, even in the presence of the competing cosubstrate Cl−, biologically relevant concentrations of NO and tyrosine were effectively used by the MPO–H2O2 system to promote peroxidation of endogenous plasma lipids. Similar dose-dependent effects of NO and tyrosine as substrates were noted for MPO-mediated peroxidation of core LDL lipids in buffer containing plasma levels of halides (Figure 8, left). Addition of both NO and tyrosine resulted in lipid peroxidation, but at a level lower than that observed with either substrate alone (not shown).
MPO uses NO and tyrosine as substrates to promote peroxidation of endogenous plasma lipids under physiologically relevant conditions.
Isolated MPO (30 nM) and an H2O2-generating system (G/GO) (10 μM/h flux of H2O2) were incubated with dialyzed plasma (50%, vol/vol) and the indicated concentrations of NO and tyrosine in 50 mM sodium phosphate buffer (pH 7.0), supplemented with 100 μM DTPA and 100 mM NaCl. After incubation at 37°C for 12 hours, the contents of 9-H(P)ODE and 9-H(P)ETE were then determined as described in “Materials and methods.”
MPO uses NO and tyrosine as substrates to promote peroxidation of endogenous plasma lipids under physiologically relevant conditions.
Isolated MPO (30 nM) and an H2O2-generating system (G/GO) (10 μM/h flux of H2O2) were incubated with dialyzed plasma (50%, vol/vol) and the indicated concentrations of NO and tyrosine in 50 mM sodium phosphate buffer (pH 7.0), supplemented with 100 μM DTPA and 100 mM NaCl. After incubation at 37°C for 12 hours, the contents of 9-H(P)ODE and 9-H(P)ETE were then determined as described in “Materials and methods.”
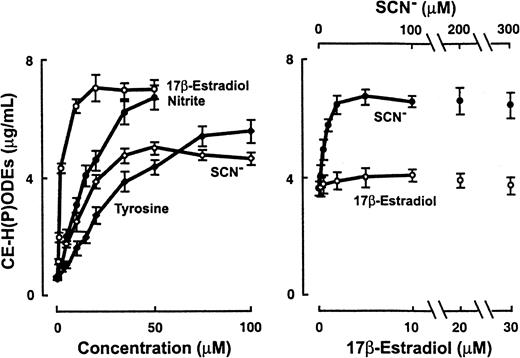
MPO-initiated oxidation of LDL lipids in the presence of multiple competing cosubstrates.
(left) Isolated MPO (30 nM) and an H2O2-generating system (G/GO) (10 μM/h flux of H2O2) were incubated with LDL (0.2 mg/mL) and the indicated concentrations of either SCN−, 17β-estradiol, NO, or tyrosine in 50 mM sodium phosphate buffer (pH 7.0), supplemented with 100 μM DTPA and 100 mM NaCl. After incubation at 37°C for 12 hours, the content of CE-H(P)ODE was then determined as described in “Materials and methods.” (right) Isolated MPO (30 nM), an H2O2-generating system (G/GO) (10 μM/h flux of H2O2), and physiological levels of Cl− (100 mM), Br− (100 μM), NO (5 μM), tyrosine (100 μM), and LDL (0.2 mg/mL) were collectively incubated with the indicated concentrations of SCN− and 17β-estradiol in 50 mM sodium phosphate buffer (pH 7.0), supplemented with 100 μM DTPA. After incubation at 37°C for 12 hours, the content of CE-H(P)ODE was then determined as described in “Materials and methods.”
MPO-initiated oxidation of LDL lipids in the presence of multiple competing cosubstrates.
(left) Isolated MPO (30 nM) and an H2O2-generating system (G/GO) (10 μM/h flux of H2O2) were incubated with LDL (0.2 mg/mL) and the indicated concentrations of either SCN−, 17β-estradiol, NO, or tyrosine in 50 mM sodium phosphate buffer (pH 7.0), supplemented with 100 μM DTPA and 100 mM NaCl. After incubation at 37°C for 12 hours, the content of CE-H(P)ODE was then determined as described in “Materials and methods.” (right) Isolated MPO (30 nM), an H2O2-generating system (G/GO) (10 μM/h flux of H2O2), and physiological levels of Cl− (100 mM), Br− (100 μM), NO (5 μM), tyrosine (100 μM), and LDL (0.2 mg/mL) were collectively incubated with the indicated concentrations of SCN− and 17β-estradiol in 50 mM sodium phosphate buffer (pH 7.0), supplemented with 100 μM DTPA. After incubation at 37°C for 12 hours, the content of CE-H(P)ODE was then determined as described in “Materials and methods.”
Although studies have shown that SCN− and 17β-estradiol may serve as reducing substrates for peroxidases in vitro, the capacity of MPO to use either compound for initiating lipid oxidation has not yet been reported. SCN− levels in plasma from a healthy subject range from 17 to 69 μM and may reach more than 200 μM in the plasma of smokers.62 Normal levels of total estrogens in serum are reported to be 37 to 184 pM in men and 18 to 1266 pM in women, and they can reach significantly higher levels with supplementation and pregnancy.62 When 17β-estradiol or SCN− was incubated with MPO, an H2O2-generating system and LDL in media containing plasma levels of Cl− lipid peroxidation was initiated in a dose-dependent fashion (Figure 8, left). When all cosubstrates were present at physiological levels, tyrosine and NO augmented MPO–H2O2-dependent initiation of lipid oxidation under all conditions examined (data not shown). With physiologically relevant concentrations, SCN−, but not 17β-estradiol, was able to further promote lipid peroxidation in the presence of multiple competing cosubstrates for MPO, including Cl−, Br−, NO, and tyrosine (Figure 8, right).
Discussion
MPO is the single most abundant protein in neutrophils, but the precise substrates used and the biochemical reactions mediated by this enzyme in vivo are still not fully defined. MPO uses Cl−in vivo to generate microbicidal and chlorinating oxidants, as confirmed by the detection of chlorinated products at sites of inflammation in mice45 and humans.63Furthermore, selective defects in host defenses (eg, against fungal and yeast pathogens) are noted in mice and humans deficient in MPO activity.45,64,65 However, a complex array of potential reactions in addition to those involving halogenating oxidants have been identified for MPO in model systems.14-30 Numerous low-molecular–weight organic and inorganic substances found in biologic matrices may serve as cosubstrates for MPO catalysis in isolation in vitro, generating reactive oxidants and diffusible radical species (reviewed in Podrez et al5 and Kettle and Winterbourn14). The relative importance of these species and their contributions to reactions that occur in vivo is uncertain.
Although lipid peroxidation and lipid-derived signaling molecule formation are believed to be critical in atherosclerosis and other inflammatory disorders, the pathways responsible for these processes in vivo are not fully established. Leukocyte activation in whole plasma has long been appreciated as a physiological mechanism for promoting peroxidation of endogenous plasma lipids.57 However, the enzymatic participant(s) and the reactive intermediates involved in leukocyte-mediated lipid oxidation within complex matrices such as plasma have not been directly defined. Results of the current study definitively identify MPO as a major enzymatic catalyst for promoting lipid oxidation by activated human neutrophils in plasma. Furthermore, the MPO–H2O2 system uses low-molecular–weight components in plasma distinct from Cl− for initiating lipid peroxidation at sites of inflammation, including tyrosine, NO, SCN−, and perhaps 17β-estradiol.
Recent studies using leukocytes isolated from wild-type and MPO knockout mice report modest differences in their capacity to promote lipoprotein lipid oxidation and inhibition in lipid oxidation by the addition of SOD.37 Two major species differences between murine and human neutrophils include the significant generation of NO by mouse but not human neutrophils and the 6- to 10-fold decrease in MPO content found in murine neutrophils compared with that observed in humans.30,42,43 Moreover, mixed leukocyte preparations rather than isolated neutrophils were used in the studies with MPO knockout mice.37 Whether these factors contributed to the limited role reported for MPO by the elicited murine leukocytes and the sensitivity to SOD remains to be established. Neutrophils from multiple unrelated MPO-deficient humans all failed to promote peroxidation of endogenous lipids in plasma unless supplemented with catalytic levels of purified MPO. Catalase, peroxidase inhibitors, and ascorbate, but not SOD, inhibited leukocyte-dependent peroxidation of plasma lipids, consistent with the MPO–H2O2 system as the responsible mechanism. The current results thus strongly support a major physiological role for MPO in initiating lipid peroxidation by activated human leukocytes and suggest that a function of the enzyme at sites of inflammation may be to generate lipid oxidation products with biologic activity.
Four compounds in human plasma could support MPO-dependent peroxidation of LDL lipids in the presence of physiological levels of Cl−: NO, tyrosine, SCN−, and 17β-estradiol. Tyrosine and NO have been implicated in the modification of lipoproteins by the MPO system of leukocytes in prior studies.11-13 However, as far as we are aware, a role for either SCN− or 17β-estradiol in the initiation of lipid oxidation by peroxidases has not yet been reported. The addition of tyrosine and NO to the MPO–H2O2 system modestly inhibited MPO-dependent lipid oxidation compared with that observed with either NO or tyrosine alone, potentially reflecting the loss of intermediates (• NO2 and tyrosyl radical) that promote lipid peroxidation through radical–radical coupling reactions, generating nitrotyrosine.66
Cigarette smoking is a risk factor for cardiovascular disease, and plasma levels of SCN− in smokers may be increased 2 to 3 times more than that observed in nonsmokers.67,68 A positive correlation between serum SCN− levels and the formation of advanced atherosclerotic plaques within coronary arteries has been noted.67,69 The current discovery of SCN− as a physiological reducing substrate for MPO that promotes LDL lipid oxidation suggests a potential mechanism for this correlation. In recent studies, hypothiocyanate was identified as a proximate oxidant that accumulates in buffer during oxidation of SCN− by eosinophil peroxidase, a related leukocyte peroxidase.70 It is unclear how this 2 e−oxidation product of SCN− would initiate lipid oxidation. Indeed, the current studies suggest that MPO also generates the 1-electron oxidation product, thiocyanyl radical (• SCN), or that the addition of SCN− to MPO–H2O2 systems promotes the formation of a protein radical species on MPO that is accessible to lipid targets. It is interesting that van Dalen and Kettle71 recently demonstrated the formation of a radical species after MPO-catalyzed oxidation of SCN−. Further examination of the mechanisms of how SCN− oxidation by MPO leads to the initiation of lipid oxidation are warranted because it is tempting to speculate that this pathway potentially contributes to the enhanced risk for cardiovascular disease noted in smokers.
Identification of 17β-estradiol as a potential substrate for MPO in plasma that can initiate lipid oxidation was unanticipated. The current studies suggest, however, that 17β-estradiol does not likely play a significant role in lipid oxidation mediated by MPO in vivo unless localized consumption of alternative competing cosubstrates, such as tyrosine, SCN−, and NO has occurred, and supraphysiological levels are achieved, such as through supplementation. It is nonetheless interesting that elevated levels of 17β-estradiol in serum have been reported in subjects with acute myocardial infarction and septic shock.72,73 Although many studies have suggested that estrogens may serve as antioxidant cardioprotectants,74 recent studies involving more than 3000 women demonstrated that estrogen replacement did not reduce, but possibly enhanced, the overall rate of coronary events in women with established coronary artery disease.74-76
Taken together, our results highlight the probable contribution of MPO in promoting lipid oxidation at sites of inflammation. The development of peroxidase inhibitors as novel anti-inflammatory agents thus merits consideration. The sensitivity of leukocyte- and MPO-dependent oxidation of plasma lipids to ascorbate also has implications for the choice of anti-oxidant regimen one considers. In studies using whole plasma, small unilamellar vesicles, or LDL as targets, ascorbate inhibits MPO-mediated peroxidation of lipids far more effectively than that observed with α-tocopherol (R.Z. and S.L.H., unpublished results, January 2000). Finally, the capacity of the MPO–H2O2 system of leukocytes to form bioactive lipid oxidation products in plasma suggests that MPO plays a broad role in inflammation biology and host defenses. Indeed, we recently demonstrated that exposure of LDL to the MPO–H2O2 system of activated monocytes in media containing NO converts the lipoprotein to a high uptake form capable of promoting cholesterol accumulation and foam cell formation.12 Oxidized lipid(s) were shown to serve as major constituent(s) that facilitated recognition of the modified lipoprotein by the macrophage scavenger receptor CD36.12,77 This receptor has recently been implicated in foam cell formation and atherosclerosis.77 78 Thus, MPO-dependent peroxidation of cell membranes and lipoproteins may serve as a physiological mechanism to promote the uptake of modified and senescent cellular constituents by CD36+ cells.
Results of the first large study evaluating nearly 100 MPO-deficient subjects compared with a control population were recently reported.79 In addition to a modestly increased frequency of infectious diseases, MPO deficiency was associated with a decreased incidence of cardiovascular events.79 We have demonstrated a strong positive correlation between the level of MPO per leukocyte and the risk for atherosclerosis in subjects with angiographically defined coronary artery disease status.80 The current results provide further evidence for a potential mechanism contributing to the many links between MPO and cardiovascular disease.
We thank Dave Schmitt for technical assistance. Mass spectrometry experiments were performed at the Cleveland Clinic Foundation Mass Spectrometry Resource within the Center for Cardiovascular Diagnostics and Prevention.
Supported by National Institutes of Health grants HL62526 and HL61878 (S.L.H.) and a Merit Review Grant from the Veterans Administration (W.M.N.). R.Z. is supported by a postdoctoral fellowship from the American Heart Association. Z.S. is the recipient of a Jane Coffin Childs Memorial Fund for Medical Research Fellowship.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Stanley L. Hazen, Cleveland Clinic Foundation, Lerner Research Institute, Dept of Cell Biology, 9500 Euclid Ave, NC-10, Cleveland, OH 44195; e-mail: hazens@ccf.org.