TEL/platelet-derived growth factor receptor β (PDGFβR) is the protein product of the t(5;12) translocation in chronic myelomonocytic leukemia. TEL/PDGFβR transforms interleukin-3 (IL-3)–dependent Ba/F3 and 32D cells to IL-3 independence and induces a murine myeloproliferative disease in a bone marrow transplantation model of leukemogenesis. The fusion protein encodes a constitutively activated, cytoplasmic tyrosine kinase that activates multiple signal transduction pathways. To identify the signaling pathways that are necessary for transformation by TEL/PDGFβR, transformed Ba/F3 and 32D cells were studied. TEL/PDGFβR activates the kinase activity of phosphatidylinositol-3 (PI3) kinase and stimulates phosphorylation of its downstream substrates, including Akt and p70S6 kinase. Activation of this pathway requires the kinase activity of TEL/PDGFβR and is inhibited by the PDGFβR inhibitor, STI571. Furthermore, inhibition of PI3 kinase with the pharmacologic inhibitor, LY294002, inhibits growth of the transformed cells. Treated cells arrest in the G1 phase of the cell cycle within 16 hours but do not undergo apoptosis. To study the mechanism of cell cycle arrest by LY294002, the activity of the cdk4 complex, which regulates the transit of cells from the G1 to S phase in hematopoietic cells, was examined. Both STI571 and LY294002 lead to a decrease in the activity of cdk4 kinase activity and a decrease in expression of both Cyclin D2 and Cyclin E within several hours. These studies demonstrate the presence of a signaling pathway from TEL/PDGFβR to PI3 kinase and subsequently to regulation of the cdk4 kinase complex. Activation of this pathway is necessary for transformation by TEL/PDGFβR.
Introduction
Tyrosine kinase fusion proteins are the products of a growing family of oncogenes associated with both solid tumors and hematologic malignancies.1 The best known tyrosine kinase fusion protein is the BCR/ABL tyrosine kinase that results from a t(9;22) translocation in patients with chronic myeloid leukemia.2 Previous work has demonstrated that BCR/ABL activates multiple signal transduction pathways, including the phosphatidylinositol-3 (PI3) kinase pathway and that transformation by BCR/ABL requires activation of PI3 kinase.3-6 To determine if tyrosine kinase fusion proteins share common mechanisms of transformation or if each functions in unique ways, we have chosen to study the TEL/platelet-derived growth factor receptor β (PDGFβR) fusion protein that results from the t(5;12) translocation in patients with chronic myelomonocytic leukemia.7
TEL/PDGFβR contains the amino-terminal 154 amino acids of TEL fused to the transmembrane and cytoplasmic domains of the PDGFβR.TEL is a member of the ETS family of transcription factors and has been described as a common site of rearrangement in multiple forms of leukemia.8-10 Structurally, wild-type TEL contains a 5′ oligomerization domain, designated the PNT domain; this domain is retained in the fusion protein and is essential for the transforming activity of TEL/PDGFβR as demonstrated by us and others.11,12 Evidence suggests that the PNT domain may cause multimerization of the fusion protein, not simple dimerization.13 TEL has been reported to induce G1 arrest in vitro14 and to be required for yolk sac angiogenesis according to murine knockout experiments.15 PDGFβR is a well-characterized plasma membrane receptor with endogenous tyrosine kinase activity that is autophosphorylated in response to binding of dimeric PDGF ligand.16 In the fusion protein there is retention of the transmembrane domain and the complete tyrosine kinase domain of PDGFβR. An intact kinase activity is necessary for transforming activity.17 The protein retains multiple tyrosine sites that act as binding sites for SH2-containing signaling molecules in the wild type PDGFβR. Furthermore, immunolocalization of TEL/PDGFβR has demonstrated that the protein is located primarily in the cytosol, retaining neither the nuclear localization of TEL or the plasma membrane localization of PDGFβR.12 Thus, initial models of transformation by TEL/PDGFβR have suggested that the protein is constitutively oligomerized through the TEL PNT domain, leading to constitutive activation of the kinase activity of the 3′ PDGFβR kinase domain and activation of critical signaling pathways. However, the signaling pathways that are necessary for transformation remain undefined.
Several signaling pathways have been identified as being activated by TEL/PDGFβR. In transformed cell lines, TEL/PDGFβR is known to associate with or cause phosphorylation of phospholipase C (PLCγ1), SHP2, and JNK.11,18,19 In addition, we have recently shown that TEL/PDGFβR activates STAT1 and STAT5 but have been unable to demonstrate a necessity for STAT activation in transformation by TEL/PDGFβR20 (R. Dierova, M. Carroll, unpublished data, 2000). TEL/PDGFβR also up-regulates expression of the oncogene c-myc.18 Finally, TEL/PDGFβR, like BCR/ABL, has been reported to form a complex with the p85 subunit of PI3 kinase.11 However, no previous studies have looked at the role of PI3 kinase activation in TEL/PDGFβR-mediated transformation. PI3 kinases are important mediators of cell survival, mitogenesis, cytoskeletal modeling, and metabolic control.21 PI3 kinase catalyzes the phosphorylation of phosphatidylinositol (PtdIns) lipids on the D3 hydroxy group generating products such as PtdIns(3,4)P2 and PtdIns(3,4,5)P3.22 These phosphorylated lipids in turn can modulate the localization and activation of a number of proteins. The serine kinase Akt/PKB is one such downstream target that is reported to be necessary for mediating cell survival and growth. Other targets include p70S6 kinase that regulates the activity of ribosomes and NFκB. Reports have demonstrated a direct role of the PI3 kinase/Akt pathway in regulation of the cell cycle in both fibroblasts and hematopoietic cells.23 24
Here, we report that TEL/PDGFβR activates the kinase activity of PI3 kinase and leads to phosphorylation of a number of downstream mediators of PI3 kinase. Inhibition of PI3 kinase with the pharmacologic inhibitor, LY294002,25 26 leads to an arrest of cells in the G1 phase of the cell cycle. We demonstrate that this growth arrest is associated with decreased activation of cdk4 kinase, a key regulator of the G1 to S phase progression in these cells. Down-regulation of cdk4 kinase activity is associated with a decrease in expression of Cyclin D2. These studies demonstrate the presence of a signaling pathway from TEL/PDGFβR to PI3 kinase and subsequently to regulation of the cdk4 kinase complex that is required for transit through the G1/S cell cycle boundary in response to TEL/PDGFβR.
Materials and methods
Reagents
Ba/F3 cells have been well described.27 Cells were maintained in RPMI 1640 with 10% fetal calf serum. Parental cell lines were maintained in 0.5 ng/mL recombinant murine interleukin-3 (IL-3; R&D Systems, Minneapolis, MN). Ba/F3-TEL/PDGFβR cells have been previously described.11 The 32D cells28were infected with retrovirus encoding TEL/PDGFβR and selected for growth in the absence of IL-3. LY294002 was purchased from Calbiochem (San Diego, CA), prepared in dimethyl sulfoxide (DMSO), and used within 2 months after purchase. Dilutions were made such that maximum concentration of DMSO in final solution was less than 0.1%. STI571 (previously CGP57 148B29,30) was a kind gift of Novartis Pharmaceuticals (Basel, Switzerland). U012631 32 was purchased from Promega Corporation. Rapamycin was purchased from Sigma Chemicals.
Growth curves and cell cycle analysis
Subconfluent cells (2 to 5 × 105/mL) were counted and replated at indicated concentrations. Viable cells were counted daily by using trypan blue exclusion. Cell cycle analysis was performed by using propidium iodide. Briefly, cells were pelleted by centrifugation and fixed with 70% EtOH diluted with phosphate-buffered saline. Cells were treated briefly with RNase A (180 μg/mL; Sigma) at 37°C and then stained with 3 μg/mL propidium iodide. Cells were analyzed for DNA content by using a Becton Dickinson FACStar with data collected on FL2 and analyzed with the use of the ModFit program.
Western blotting
Cells were washed once in phosphate-buffered saline and lysed in lysis buffer (150 mM NaCl, 50 mM Tris-HCl, 1% Triton-X 100 plus protease and phosphatase inhibitors). Lysates were clarified by centrifugation and protein quantitated by using a modified Bradford reagent (Bio-Rad Laboratories, Hercules, CA). Lysates (100 μg) were loaded on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), proteins were separated by electrophoresis and blotted onto nitrocellulose or polyvinylidene diflouride. Blots were blocked in 5% dry milk in TBST (0.1% Tween-20, 0.01 M Tris-HCl, pH 7.6, 150 mM NaCl) or 5% bovine serum albumin (Sigma), rinsed in TBST for 5 seconds and incubated for 2 hours at room temperature in primary antibody. Blots were washed and incubated with horseradish peroxidase–conjugated secondary antibody and developed with enhanced chemiluminescence according to the manufacturer's directions (Amersham Biosciences, Piscataway, NJ). Antibodies used were as follows: phospho-Akt and phospho p70S6 kinase, Akt, p70S6 kinase (New England Biolabs, Beverly, MA), p21, p27, cdk4, and Cyclin antibodies (Santa Cruz Biotechnology, Santa Cruz, CA).
Immune complex protein kinase assay
Pellets from cells were lysed with lysis buffer; 10 mM Tris pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton-X 100, 0.1% SDS, 10 μg/mL aprotinin, 10 μg/mL leupeptin, 1 μg/mL pepstatin, 5 mM sodium orthovanadate, 50 mM NaF, 50 mM Na-pyrophosphate, and 150 μM phenylmethylsulfonyl fluoride. Total protein concentration in each sample was determined by using a micro BCA method (Bio-Rad) according to the manufacturer's instructions. We then transferred a 100-μg cell extract to Microfuge tube (total volume in 500 μL IP buffer). Immunoprecipitations were carried out by incubation overnight at 4°C with 2.5 μg rabbit polyclonal cdk4 antibody (Santa Cruz Biotechnology), followed by incubation for 4 hours with 25 μL protein A-agarose beads (Amersham). Precipitated protein pellets were washed 3 times with ice-cold kinase buffer (without Triton-X 100) and then resuspended in 20 μL ice-cold kinase buffer, 50 mM HEPES (pH 7.5), 80 mM β-glycerophosphate, 2.5 mM ethyleneglycol bis (b-aminoethyl ether)–N, N, N′, N′-tetraacetic acid (EGTA), 10 mM MgCl2, 1 mM dithiothreitol, 2.5 mM phenylmethylsulfonyl fluoride, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 10 mM cyclic adenosine monophosphate–dependent protein kinase-inhibitory peptide (Sigma). A total of 12 μL reaction mix containing 10 μCi (0.37 MBq) (γ-32P)adenosine triphosphate (ATP; approximately 3000 Ci/mmol [approximately 1.11 × 1014Bq/mmol]; Amersham), 25 μM unlabeled ATP, and 200 ng glutathione-S-transferase retinoblastoma (GST-Rb) protein as substrate (Santa Cruz Biotechnology) were added to each sample and incubated at 30°C for 15 minutes. Kinase reactions were stopped by the addition of an equal volume of 2× SDS sample buffer (4% SDS, 150 mM Tris-HCl [pH 6.8], 20% glycerol, 0.02% bromophenol blue, and 2 mM sodium vanadate) and by boiling for 5 minutes. Proteins were separated by electrophoresis in 10% SDS-PAGE, gels were dried and then autoradiographed. Radioactivity was quantified by using a Molecular Dynamics Storm 860 phosphorimager.
PI3 kinase assays
Cells were lysed in RIPA buffer at 4°C for 30 minutes. The debris was separated by centrifugation at 12 000g for 20 minutes at 4°C. Protein concentration was estimated in the cleared supernatant, and 1000 μg protein (total volume in 500 μL IP buffer) was used for immunoprecipitation. Immunoprecipitations were incubated overnight at 4°C with 2.5 μg PI3 kinase (p85) antibodies (Upstate Biotechnology, Lake Placid, NY), followed by incubation for 4 hours with 25 μL protein A-agarose beads. Precipitated protein pellets were washed 3 times with ice-cold kinase lysis buffer and 3 times with PI3 kinase buffer and then resuspended in 20 mL ice-cold kinase buffer (40 mM HEPES [pH 7.5], 2 mM EGTA, 6 mM MgCl2, 1 mM dithiothreitol, 2.5 mM phenylmethylsulfonyl fluoride, 5 mM NaCl, 0.2 mM EDTA, and 10 μM unlabeled ATP). Lipid mix (20 μL) was added (10 μg PI [Matreya, State College, PA] containing 0.5% wt/vol cholic acid, freshly prepared by sonication for 5 minutes on ice), and samples were vortexed and incubated at 30°C for 5 minutes. Then, an additional 40 μL reaction mix was added containing 10 μCi (0.37 MBq) (γ-32P)ATP (approximately 3000 Ci/mmol [approximately 1.11 × 1014 Bq/mmol]; Amersham) and incubated at 30°C for another 15 minutes. The reaction was stopped by addition of a mixture of chloroform-methanol (1:1) and 1.5 N HCl (40 μL). The reaction was then extracted with 160 μL mixture of chloroform-methanol at a ratio of 60:100, and appropriate washes were performed. The extracted reaction product was combined and dried in a vacuum centrifuge, and the residue was dissolved in 35 μL CHCl3/MeOH 2:1 vol/vol, separated by thin-layer chromatography, and developed with CHCl3/MeOH/NH4OH/H2O (129:114:15:21). The dried thin-layer chromatography plates were exposed on a phosphorimager screen, and the amount of PI3-phosphate produced was quantified by using a Molecular Dynamics Storm 860 phosphorimager.
Results
TEL/PDGFβR transforms 32D cells
We have previously shown that TEL/PDGFβR transforms IL-3–dependent Ba/F3 cells to IL-3 independence.11 To develop a confirmatory system, we have used 32D cells, another IL-3–dependent murine myeloid cell line.28 The 32D cells were infected by using the pMSCV retroviral construct encoding TEL/PDGFβR complementary DNA or a control vector expressing neomycin resistance.33 Cells were selected for growth in the absence of IL-3. Cells infected with the TEL/PDGFβR-expressing construct grew in the absence of IL-3, but vector control cells did not (data not shown). Expression of TEL/PDGFβR was confirmed by Western blotting (data not shown). All experiments below were repeated in both Ba/F3-TEL/PDGFβR and 32D-TEL/PDGFβR cells.
TEL/PDGFβR activates PI3 kinase
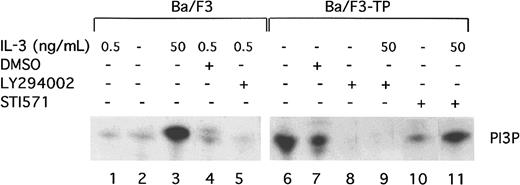
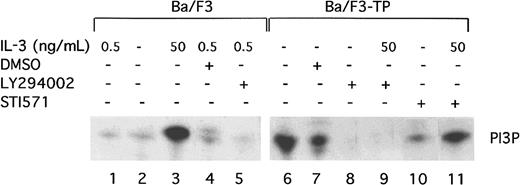
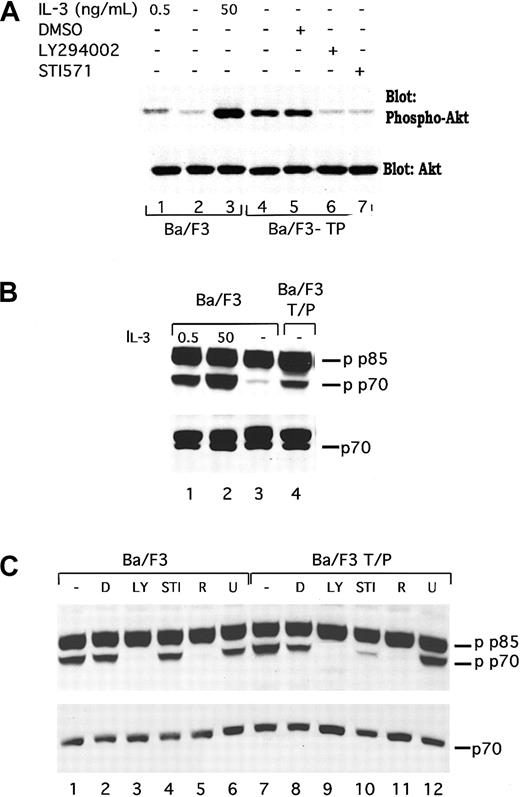
TEL/PDGFβR has been previously reported to associate with the p85 subunit of PI3 kinase.11 To show that TEL/PDGFβR activates the kinase activity of PI3 kinase, PI kinase assays were performed in transformed and untransformed Ba/F3 cells. As previously described, short-term, high-dose stimulation of parental Ba/F3 cells with IL-3 (50 ng/mL for 10 to 15 minutes) activates PI3 kinase (Figure1, lane 3). The activity is blocked by the PI3 kinase inhibitor, LY294002, as expected (Figure 1, lane 5). Activation of PI3 kinase in cells constitutively growing in 0.5 ng/mL IL-3 is present but low (Figure 1, lane 1). In contrast, Ba/F3-TEL/PDGFβR cells growing in log phase show high levels of PI3 kinase activity (Figure 1, lane 6). This activity is also blocked by LY294002 (Figure 1, lane 8). To demonstrate that activation of PI3 kinase depends on the kinase activity of TEL/PDGFβR, transformed cells were incubated with the inhibitor STI571 (Figure 1, lane 10). STI571 (previously CGP57 148B) is a well-described tyrphostin small molecule that inhibits the kinase activity of the ABL and PDGFβR tyrosine kinases at micromolar concentrations.29,30Inhibition of other kinases is seen with the drug but only at does 100 to 1000 times higher. The drug completely inhibits the kinase activity of TEL/PDGFβR and causes Ba/F3-TEL/PDGFβR cells to undergo apoptosis over an 18- to 30-hour period.17 As seen in Figure 1, lane 10, STI571 inhibits the activation of PI3 kinase in TEL/PDGFβR-transformed cells growing without IL-3. To confirm the specificity of the drug, cells were treated with STI571 and IL-3 (Figure 1, lane 11). As expected, STI571 does not inhibit the IL-3–induced activation of PI3 kinase. Together, these data demonstrate that TEL/PDGFβR-transformed cells contain activated PI3 kinase and that this activity depends on the kinase activity of the fusion protein.
TEL/PDGFβR activates PI3 kinase.
Parental Ba/F3 cells (lanes 1-5) or transformed Ba/F3-TEL/PDGFβR cells (lanes 6-11) were analyzed during log phase growth (lanes 1,6) or after treatment with DMSO alone (lanes 4,7) or the PI3 kinase inhibitor, LY294002 (lanes 5,8,9) for 4 hours. LY294002 was dissolved in DMSO and used at a concentration of 25 μM to obtain complete inhibition. As a positive control, Ba/F3 cells were deprived of IL-3 for 4 hours and then restimulated with IL-3 at 50 ng/mL (lane 3). To demonstrate a necessity for the kinase activity of TEL/PDGFβR for activation of PI3 kinase, cells were treated with STI571 at 1 μM in dH20 for 4 hours (lane 10). As a control for the specificity of STI571, Ba/F3-TEL/PDGFβR cells were treated with STI571 for 4 hours and then stimulated with IL-3 50 ng/mL for 10 minutes (lane 11). All experiments were performed in the presence of 10% fetal calf serum.
TEL/PDGFβR activates PI3 kinase.
Parental Ba/F3 cells (lanes 1-5) or transformed Ba/F3-TEL/PDGFβR cells (lanes 6-11) were analyzed during log phase growth (lanes 1,6) or after treatment with DMSO alone (lanes 4,7) or the PI3 kinase inhibitor, LY294002 (lanes 5,8,9) for 4 hours. LY294002 was dissolved in DMSO and used at a concentration of 25 μM to obtain complete inhibition. As a positive control, Ba/F3 cells were deprived of IL-3 for 4 hours and then restimulated with IL-3 at 50 ng/mL (lane 3). To demonstrate a necessity for the kinase activity of TEL/PDGFβR for activation of PI3 kinase, cells were treated with STI571 at 1 μM in dH20 for 4 hours (lane 10). As a control for the specificity of STI571, Ba/F3-TEL/PDGFβR cells were treated with STI571 for 4 hours and then stimulated with IL-3 50 ng/mL for 10 minutes (lane 11). All experiments were performed in the presence of 10% fetal calf serum.
TEL/PDGFβR regulates phosphorylation of Akt and p70S6 kinase
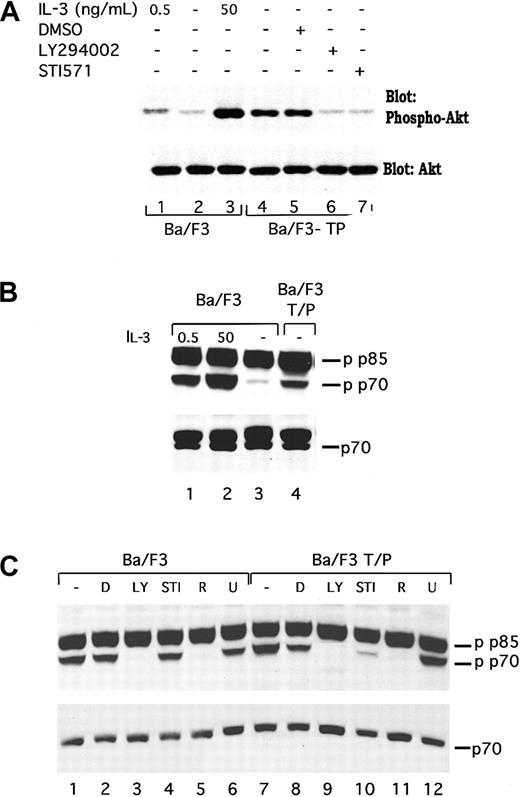
To demonstrate that induction of PI3 kinase activity in TEL/PDGFβR-transformed cells is functionally relevant, we have examined activation of 2 mediators of the effects of PI3 kinase, Akt/PKB (subsequently referred to as Akt), and p70S6 kinase. Akt is a serine threonine kinase that is regulated by PI3 kinase and has been reported to lead to phosphorylation of the Bcl2 family member BAD34-36 and other proteins. As shown in Figure2A, lane 4, TEL/PDGFβR-transformed cells contain phosphorylated Akt. This phosphorylation is increased relative to that seen in parental Ba/F3 cells either growing in IL-3 (compare lane 4 with lane 1) or deprived of IL-3 (Figure 2A, lane 2). Furthermore, the phosphorylation is inhibited by both LY294002 and STI571 (Figure 2A, lanes 6 and 7) but not by the vehicle, DMSO (Figure2A, lane 5). These results are consistent with a signaling pathway that connects TEL/PDGFβR with PI3 kinase and subsequently to Akt. Similar results were seen in 32D-TEL/PDGFβR cells (data not shown).
TEL/PDGFβR stimulates phosphorylation of Akt and p70S6 kinase through a LY294002-responsive pathway.
(A) Ba/F3 (lanes 1-3) were harvested in log phase growth (lane 1) or deprived of IL-3 for 4 hours and then restimulated with media containing no IL-3 (lane 2) or 50 ng/mL IL-3 for 15 minutes. Ba/F3/TEL/PDGFβR cells were either harvested in log phase growth (lane 4) or treated with the indicated compound for 16 hours. Cells were lysed in 1% Triton lysis buffer, proteins were separated by SDS-PAGE and blotted for phospho-Akt. Blot was stripped and reprobed for expression of total Akt. Similar inhibition of Akt was seen in Ba/F3 TEL/PDGFβR cells treated with LY294002 (25 μM; lane 6) or STI571 (1 μM; lane 7). (B) In separate experiments, phosphorylation of p70S6 kinase was examined. Ba/F3 cells were either analyzed during log phase growth with 0.5 ng/mL IL-3 (lane 1) or deprived of IL-3 for 4 hours and then restimulated with media containing 50 ng/mL IL-3 for 15 minutes (lane 2) or no IL-3 (lane 3). Ba/F3-TEL/PDGFβR cells were harvested in log phase growth (lane 4) in the absence of IL-3. (C) Ba/F3 and Ba/F3-TEL/PDGFβR cells were harvested in log phase growth or treated with indicated inhibitors for 4 hours. D indicates DMSO; LY, LY294002 25 μM; STI, STI571 1 μM; R, rapamycin; and U, U0126 20 μM. Cells were lysed in 1% Triton, proteins were separated by SDS-PAGE and blotted by using the indicated antibodies. Phospho-p70S6 kinase antibody cross-reacts with a splice variant of p70, p85 S6 kinase, which is not known to be regulated by cytokines.
TEL/PDGFβR stimulates phosphorylation of Akt and p70S6 kinase through a LY294002-responsive pathway.
(A) Ba/F3 (lanes 1-3) were harvested in log phase growth (lane 1) or deprived of IL-3 for 4 hours and then restimulated with media containing no IL-3 (lane 2) or 50 ng/mL IL-3 for 15 minutes. Ba/F3/TEL/PDGFβR cells were either harvested in log phase growth (lane 4) or treated with the indicated compound for 16 hours. Cells were lysed in 1% Triton lysis buffer, proteins were separated by SDS-PAGE and blotted for phospho-Akt. Blot was stripped and reprobed for expression of total Akt. Similar inhibition of Akt was seen in Ba/F3 TEL/PDGFβR cells treated with LY294002 (25 μM; lane 6) or STI571 (1 μM; lane 7). (B) In separate experiments, phosphorylation of p70S6 kinase was examined. Ba/F3 cells were either analyzed during log phase growth with 0.5 ng/mL IL-3 (lane 1) or deprived of IL-3 for 4 hours and then restimulated with media containing 50 ng/mL IL-3 for 15 minutes (lane 2) or no IL-3 (lane 3). Ba/F3-TEL/PDGFβR cells were harvested in log phase growth (lane 4) in the absence of IL-3. (C) Ba/F3 and Ba/F3-TEL/PDGFβR cells were harvested in log phase growth or treated with indicated inhibitors for 4 hours. D indicates DMSO; LY, LY294002 25 μM; STI, STI571 1 μM; R, rapamycin; and U, U0126 20 μM. Cells were lysed in 1% Triton, proteins were separated by SDS-PAGE and blotted by using the indicated antibodies. Phospho-p70S6 kinase antibody cross-reacts with a splice variant of p70, p85 S6 kinase, which is not known to be regulated by cytokines.
We have also examined phosphorylation of the PI3 kinase mediator, p70S6 kinase. p70S6 kinase regulates a variety of functions including ribosomal activity. In IL-3–stimulated Ba/F3 cells, p70S6 kinase has been reported to be necessary for the full mitogenic effect of IL-3.37 Phosphorylation of p70S6 kinase in TEL/PDGFβR-transformed cells was examined by using Western blotting for phosphorylated p70S6 kinase as shown in Figure 2B,C. As previously reported, IL-3 stimulates the phosphorylation of p70S6 kinase (Figure2B, compare lane 2 with lane 3; note that antibody recognizes both p70S6 kinase and the related protein, p85 S6 kinase, that is constitutively phosphorylated). Ba/F3-TEL/PDGFβR cells, growing in the absence of IL-3, contain phosphorylated p70S6 kinase (Figure 2B, lane 4). As above, phosphorylation of p70S6 kinase in TEL/PDGFβR-expressing cells is inhibited by both LY294002 and STI571 (Figure 2C, lanes 9 and 10) as well as the p70 inhibitor, rapamycin (lane 11) but not by the unrelated inhibitor of MEK, U0126 (lane 12). Similar results are seen in the parental cells with the exception that STI571 has no effect of p70 phosphorylation in these cells (Figure 2C, lanes 1-6).
Inhibition of PI3 kinase in transformed cells decreases cell growth
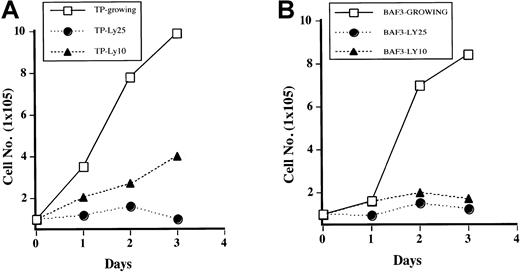
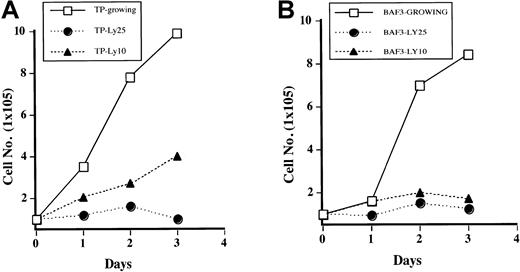
It has been previously reported that PI3 kinase is necessary for growth of Ba/F3 cells stimulated with IL-3.24 To determine if this is true for TEL/PDGFβR-transformed cells, cells were treated with LY294002, and growth curves were performed by counting the cells daily by using trypan blue exclusion. As shown in Figure3A, Ba/F3-TEL/PDGFβR cells treated with LY294002 at 10 μM have decreased cell growth (compare open boxes with closed triangles). At the completely inhibitory concentration of 25 μM, cells almost completely stop growing, although they do not undergo apoptosis for several days. In fact, examination of cultures 24 to 48 hours after addition of LY294002 shows that there is little cell death present in treated cultures on the basis of cell morphology and trypan blue exclusion (data not shown). Sensitivity of the transformed cells to LY294002 is similar to parental cells growing in IL-3 (Figure3B). Inhibition of cell growth in transformed cells is probably not due to decreased activity of p70S6 kinase, as treatment of cells with rapamycin led to only a slight and delayed decrease in cell growth in our hands (data not shown).
Inhibition of PI3 kinase blocks growth of transformed cells.
Ba/F3 TEL/PDGFβR cells (A) or Ba/F3 cells (B) were either treated with DMSO (“Growing”) or treated with LY294002 at indicated concentrations. Viable cells were enumerated daily by using trypan blue exclusion.
Inhibition of PI3 kinase blocks growth of transformed cells.
Ba/F3 TEL/PDGFβR cells (A) or Ba/F3 cells (B) were either treated with DMSO (“Growing”) or treated with LY294002 at indicated concentrations. Viable cells were enumerated daily by using trypan blue exclusion.
Treatment with LY294002 arrests cells in the G1 phase of the cell cycle
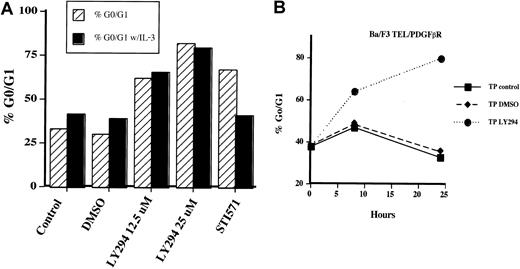
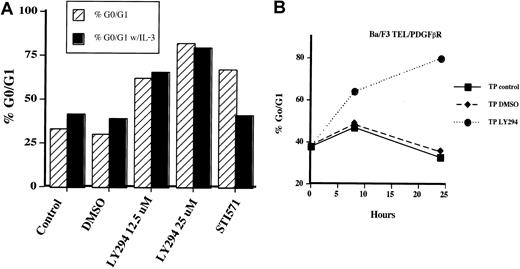
The presence of decreased cell growth with no apoptosis suggested to us that Ba/F3-TEL/PDGFβR cells treated with LY294002 may be undergoing a cell cycle arrest. To test this hypothesis, cells were treated with LY294002 and examined for DNA content by using propidium iodide staining at various time points. Initial analysis demonstrated that cells began to arrest in the G1 phase of the cell cycle within 8 to 16 hours of addition of LY294002. As shown in Figure4A, cells treated with LY294002 at 12.5 μM for 24 hours showed an accumulation of cells in the G1 phase of the cell cycle (66% versus approximately 40% or less for control cells). When LY294002 was used at 25 μM, 80% of cells arrested in G1. This finding was true whether IL-3 was present or not, confirming that TEL/PDGFβR and IL-3 may both regulate cell cycle through a PI3 kinase–dependent pathway. Interestingly, when cells are treated with STI571, which inactivates the kinase activity of TEL/PDGFβR, a cell cycle arrest was also seen. However, 50% of these cells have undergone apoptosis after 24 hours (sub 2N DNA content by propidium iodide staining), in contrast to the LY294002-treated cells (data not shown). This finding demonstrates that there are alternative pathways to cell survival that are activated by TEL/PDGFβR and not blocked by LY294002. Consistent with previous results of TEL/PDGFβR-transformed cells treated with IL-3 and STI571,17 IL-3 completely rescues cells from the effects of STI571. Unlike cells transformed with BCR/ABL, we have never seen an inability of IL-3 to rescue TEL/PDGFβR-transformed cells treated with STI571 in short- or long-term assays. The time course of cell cycle arrest is shown in Figure 4B. Again cells were treated with LY294002 at 25 μM. Cells were harvested at the indicated time and analyzed for DNA content again. Accumulation of cells in the G1 phase of the cell cycle is seen as early as 8 hours after addition of LY294002, and cells continue to accumulate for 24 hours.
Inhibition of PI3 kinase leads to a G1 cell cycle arrest in Ba/F3-TEL/PDGFβR cells.
Ba/F3-TEL/PDGFβR cells were grown to subconfluence and replated at 2 × 105 cells/mL in the absence (striped bars) or presence of 0.5 ng/mL IL-3 (black bars). (A) Cells were left untreated (control), treated with DMSO, or indicated inhibitor for 24 hours. Cells were harvested by centrifugation, stained with propidium iodide, and analyzed for DNA content. Percentage of cells with 2N DNA content was estimated by using the Modfit software. Data are the average of 2 experiments with Ba/F3-TEL/PDGFβR cells; similar results were obtained with 32D-TEL/PDGFβR cells (n = 2). (B) To determine the time course of cell cycle arrest, Ba/F3-TEL/PDGFβR cells were left untreated (closed squares), treated with DMSO alone (closed diamonds), or treated with LY294002 at 25 μM (closed circles) for the indicated time periods. Data are representative of 4 experiments with both Ba/F3-TEL/PDGFβR and 32D-TEL/PDGFβR cells.
Inhibition of PI3 kinase leads to a G1 cell cycle arrest in Ba/F3-TEL/PDGFβR cells.
Ba/F3-TEL/PDGFβR cells were grown to subconfluence and replated at 2 × 105 cells/mL in the absence (striped bars) or presence of 0.5 ng/mL IL-3 (black bars). (A) Cells were left untreated (control), treated with DMSO, or indicated inhibitor for 24 hours. Cells were harvested by centrifugation, stained with propidium iodide, and analyzed for DNA content. Percentage of cells with 2N DNA content was estimated by using the Modfit software. Data are the average of 2 experiments with Ba/F3-TEL/PDGFβR cells; similar results were obtained with 32D-TEL/PDGFβR cells (n = 2). (B) To determine the time course of cell cycle arrest, Ba/F3-TEL/PDGFβR cells were left untreated (closed squares), treated with DMSO alone (closed diamonds), or treated with LY294002 at 25 μM (closed circles) for the indicated time periods. Data are representative of 4 experiments with both Ba/F3-TEL/PDGFβR and 32D-TEL/PDGFβR cells.
PI3 kinase regulates cdk4 kinase in transformed cells
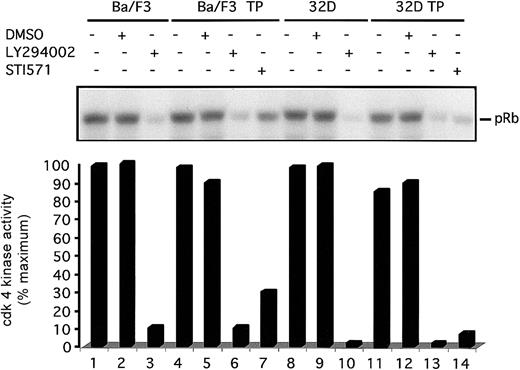
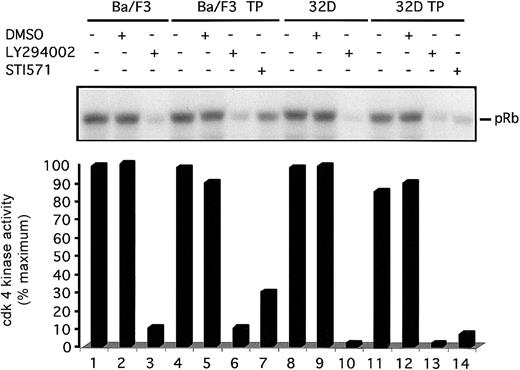
To understand the mechanism of G1 arrest in TEL/PDGFβR-transformed cells treated with LY294002, we have analyzed activation of the cdk4 complex. In these murine hematopoietic cells, cdk4 is a major regulator of the G1 to S phase transition.38 Furthermore, cdk4 appears to couple with cyclin D2 or D3 in these cells. Cyclin D1 is not expressed in these cells at an appreciable level. To analyze cdk4 kinase activity, cells were treated with DMSO or inhibitor for the indicated times, cells were lysed, and immunoprecipitations were performed with cdk4 antibodies. Immunoprecipitated complexes were incubated with purified GST-Rb fusion protein. Rb is a known substrate of cdk4 kinase.39Phosphorylated proteins were separated by SDS-PAGE and analyzed for phosphorylation of GST-Rb by using a phosphoimager. Visualized autoradiogram is shown at the top of the figure, and quantitation of bands is graphed below. Both Ba/F3 and Ba/F3-TEL/PDGFβR cells treated with LY294002 for 24 hours show a 80% decrease in activation of the cdk4 complex (Figure 5, lanes 2 versus 3 and 5 versus 6). No difference is seen between untreated cells and DMSO-treated cells at this time point. Similar results are seen in 32D and 32D-TEL/PDGFβR cells (Figure 5, lanes 8-14). These results demonstrate that inhibition of PI3 kinase in these cells leads to an inhibition of cdk4 kinase activity.
Inhibition of PI3 kinase decreases cdk4 kinase activity.
Indicated cells were grown to subconfluence, harvested by centrifugation, and replated for 24 hours with either media alone, DMSO (0.1%), LY294002 (25 μM), or STI571 (1 μM). After incubation, cells were harvested and lysed. Lysates were immunoprecipitated with cdk4 antibody and cdk4-associated Rb kinase activity was assayed. Kinase reaction products were separated by SDS-PAGE. Gel was dried and visualized by autoradiography with the use of a Molecular Dynamics PhosphoImager. Visual display of results is shown in top of figure and quantitation of results is shown below.
Inhibition of PI3 kinase decreases cdk4 kinase activity.
Indicated cells were grown to subconfluence, harvested by centrifugation, and replated for 24 hours with either media alone, DMSO (0.1%), LY294002 (25 μM), or STI571 (1 μM). After incubation, cells were harvested and lysed. Lysates were immunoprecipitated with cdk4 antibody and cdk4-associated Rb kinase activity was assayed. Kinase reaction products were separated by SDS-PAGE. Gel was dried and visualized by autoradiography with the use of a Molecular Dynamics PhosphoImager. Visual display of results is shown in top of figure and quantitation of results is shown below.
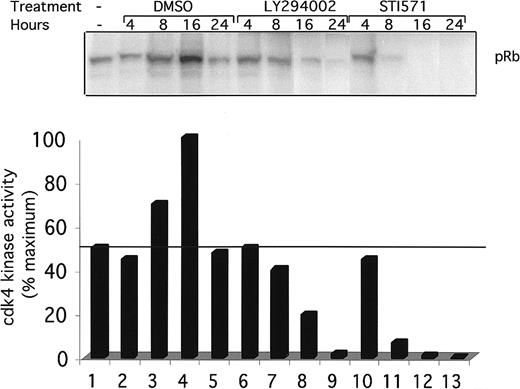
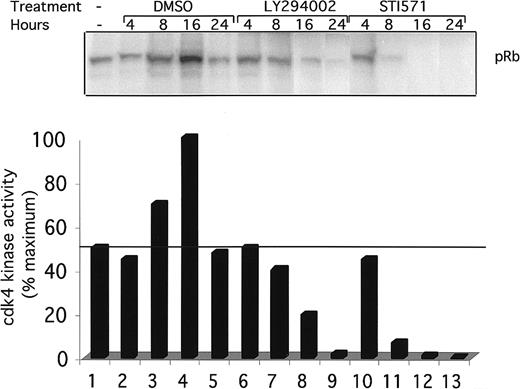
To see if the inhibition of cdk4 kinase activity is temporally associated with the block in the G1 phase of the cell cycle, a kinetic analysis of cdk4 kinase activity was performed (Figure6). Ba/F3 and Ba/F3-TEL/PDGFβR cells were again treated with DMSO (lanes 2-5), LY294002 (lanes 6-9), or STI571 (lanes 10-13) for the indicated time. Cells were harvested, and cdk4 kinase activity was assayed as above. DMSO had a delayed and reproducible stimulatory effect on cdk4 kinase activity after 8 to 16 hours (Figure 6, lanes 3 and 4). In contrast, LY294002-treated cells show a decrease in activity at 8 hours and an almost complete loss of activity by 24 hours of treatment. Interestingly, STI571-treated cells show a more rapid decrease in cdk4 kinase activity, suggesting that regulation of cdk4 in these cells may go through 2 or more signaling pathways. The partial decrease in cdk4 kinase activity seen after 8 hours of treatment correlates with the partial G1 arrest seen at this time point (Figure 4B).
Time course of cdk4 kinase activity inhibition.
Indicated cells were grown to subconfluence, harvested by centrifugation, and replated for indicated times in the presence of DMSO (0.1%), alone, LY294002 (25 μM), or STI571 (1 μM). Cells were harvested and cdk4 kinase assays performed as in Figure 5.
Time course of cdk4 kinase activity inhibition.
Indicated cells were grown to subconfluence, harvested by centrifugation, and replated for indicated times in the presence of DMSO (0.1%), alone, LY294002 (25 μM), or STI571 (1 μM). Cells were harvested and cdk4 kinase assays performed as in Figure 5.
Inhibition of cdk4 kinase activity is associated with decreased Cyclin D2 and increased p27Kip1
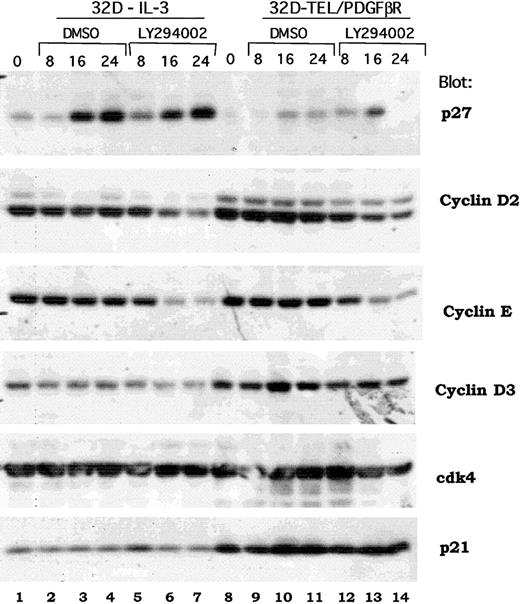
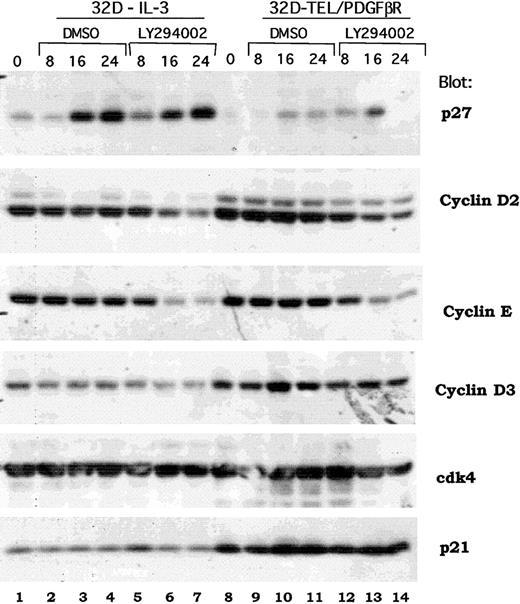
Previous reports have demonstrated a signaling cascade though which Akt activates the forkhead transcription factor, FKHR-L1, that in turn inhibits the transcription of the p27Kip1 protein and regulates progression through the G1/S cell cycle checkpoint.40 An analogous signaling cascade has also been proposed downstream of BCR/ABL in transformed Ba/F3 cells.41 To determine if the same or distinct mechanisms were regulated by TEL/PDGFβR, we examined the expression of multiple cell cycle regulatory proteins in both wild-type 32D cells and in 32D-TEL/PDGFβR cells treated with either DMSO alone or LY294002. As shown in Figure 7, Western blotting does demonstrate an increase in p27Kip1 in IL-3–stimulated cells (Figure 7, compare lane 1 with lanes 6 and 7). However, in several experiments, this induction was also seen with DMSO alone (Figure 7, lane 1 versus 3 and 4). DMSO was present at a final concentration of 0.1%. Furthermore, in 32D-TEL/PDGFβR–transformed cells, the increase was less marked and not sustained (Figure 7, compare lane 8 versus lanes 13 and 14). However, cyclin D2 was consistently down-regulated by LY294002 and not by DMSO in both IL-3–stimulated parental cells and 32D-TEL/PDGFβR cells (Figure 7, lane 1 versus 7 and lane 8 versus 14). There was also consistent down-regulation of cyclin E (Figure 7, lanes 7 and 14). Although some decrease in cyclin D3 is present in this experiment, this was not reproducible (Figure 7, lane 7). Cdk4 protein level and p21 levels were not altered in our experiments by LY294002 (Figure 7). Basal levels of both cyclin D3 and p21 were increased in 32D-TEL/PDGFβR compared with nontransformed parental cells (Figure 7, compare lane 8 with lane 1), but this increase was not seen in Ba/F3-TEL/PDGFβR cells and is of unclear significance. Although these data do not show that cyclin D2 is the critical regulator of cdk4 kinase activity in these cells, they do raise the question of whether p27Kip1 is the sole cell cycle protein regulated by the PI3 kinase/Akt pathway.
Effect of PI3 kinase inhibition on expression of cell cycle regulatory proteins.
Parental 32D or 32D-TEL/PDGFβR cells were collected in log phase growth (lanes 1, 8) or replated in the presence of DMSO or LY294002 at 25 μM for the indicated period of time. Cells were lysed in 1% Triton lysis buffer; protein quantitated, separated by SDS-PAGE, and blotted. Lysates were probed with antibodies for the indicated proteins.
Effect of PI3 kinase inhibition on expression of cell cycle regulatory proteins.
Parental 32D or 32D-TEL/PDGFβR cells were collected in log phase growth (lanes 1, 8) or replated in the presence of DMSO or LY294002 at 25 μM for the indicated period of time. Cells were lysed in 1% Triton lysis buffer; protein quantitated, separated by SDS-PAGE, and blotted. Lysates were probed with antibodies for the indicated proteins.
Discussion
We have demonstrated that TEL/PDGFβR activates PI3 kinase in transformed cells and that such activation requires the kinase activity of TEL/PDGFβR and is inhibited by the pharmacologic inhibitor of PDGFβR kinase activity, STI571 (formerly CGP57148B). Furthermore, TEL/PDGFβR activates Akt and p70S6 kinase through a PI3 kinase–dependent pathway. Inhibition of PI3 kinase leads to decreased growth of transformed cells and a growth arrest in the G1 phase of the cell cycle. Interestingly, this growth arrest is associated with a decrease in the activity of cdk4 kinase. Cdk4 kinase activity is inhibited within 8 hours of addition of the PI3 kinase inhibitor, LY294002, and continues to decrease up to 24 hours after addition of the agent. These studies demonstrate the presence of a signaling pathway from TEL/PDGFβR to PI3 kinase and subsequently to regulation of the cdk4 kinase complex. Activation of this pathway is necessary for transformation of cytokine-dependent cells to IL-3 independence by TEL/PDGFβR.
Several comments are warranted about the methods we have used. First, we have used STI571 as a specific inhibitor of TEL/PDGFβR, and our conclusions are based on the assumption that this agent does not inhibit other kinases. This compound has been extensively studied and reported to inhibit ABL family kinases, wild-type PDGFβR kinases, and c-kit receptor kinase. It has not been reported to inhibit PI3 kinases themselves, small GTP proteins, MAP family kinases, or any other known signaling molecules29 30 (E. Buchdunger, Novartis Pharmaceuticals, personal communication, 2000; M. Carroll, unpublished data, 2000). Ba/F3 cells do not express wild-type kit receptor or PDGFβR, and c-abl activity is not necessary for growth of the cells. Thus, we feel justified in using STI571 as a specific inhibitor of TEL/PDGFβR. In the case of LY294002, however, nonspecific effects must be considered. LY294002 may inhibit other phosphoinositide kinases, and further experiments with dominant-negative PI3 kinase constructs will be necessary to demonstrate that the effects described depend solely on PI3 kinase activity and not on other lipid kinases.
We have shown that IL-3–stimulated Ba/F3 and 32D cells arrest in G1 and down-regulate cdk4 kinase activity after treatment with LY294002 (Figures 3 and 5 and data not shown). This result is consistent with previous results that expression of a dominant-negative p85 PI3 kinase inhibits the growth of Ba/F3 cells,24 although this study did not demonstrate an effect of the dominant-negative construct on cdk4 kinase activity. The similarity in the results between IL-3–stimulated cells and TEL/PDGFβR-transformed cells raises the question of whether this result is determined by the cell system used rather than a unique property of TEL/PDGFβR. Analysis of other cells, particularly primary cells from patients and animals with TEL/PDGFβR-induced disease, will be important for determining if these results can be extended to primary cells; however, a comparison to BCR/ABL-transformed cells is informative. It has previously been noted that BCR/ABL activates PI3 kinase in transformed cells.5,6 This result has recently been confirmed in primary cells from a murine model of BCR/ABL-induced myeloproliferative disease.3 The consistency between the cell line and the primary results for BCR/ABL is encouraging that there will be a similar consistency for TEL/PDGFβR-transformed cells.
The exact signaling pathway between PI3 kinase and cdk4 kinase activity in these cells is unclear. It has previously been reported in fibroblasts that there is a signaling pathway from PI3 kinase through glycogen synthase kinase 3 (GSK3) to Cyclin D1.23 GSK3 activation leads to phosphorylation of Cyclin D1 and promotion of the G1 to S phase transition.23,42 However, Cyclin D1 is not detectable in Ba/F3 cells. Furthermore, an experiment using LY294002 and lithium (an inhibitor of GSK3 activity) showed no change in the cell cycle arrest seen with LY294002 alone, suggesting that this effect may not be mediated through GSK3 (data not shown). Alternatively, it has recently been reported that IL-3 regulates the forkhead transcription factor through PI3 kinase and that this pathway regulates expression of the cyclin inhibitor, p27Kip1.40 In addition, Gesbert et al41 recently reported that BCR/ABL regulates p27Kip1 through a PI3 kinase/Akt-dependent pathway.41 Although we see reproducible up-regulation of p27Kip1 in our experiments, we also see consistent down-regulation of Cyclin D2. Additional experiments will be necessary in TEL/PDGFβR-transformed cells to determine how cdk4 kinase activity is regulated. The differences between the role of PI3 kinase in regulating Cyclin D1 in fibroblasts, p27Kip1 in some hematopoietic cells, and Cyclin D2 (our results) in others suggests that PI3 kinase may regulate the G1/S phase checkpoint in multiple cells but through different mechanisms.
Tyrosine kinase fusion proteins such as BCR/ABL, TEL/PDGFβR, TEL-JAK2, and NPM/ALK are implicated in a large variety of hematopoietic neoplasms. It is unclear to what extent these proteins may activate the same or distinct signaling pathways. Recent data suggests that there are critical differences between BCR/ABL and TEL/JAK2. Although all of these proteins have been reported to activate STAT5, transplantation of STAT5-deficient cells infected with BCR/ABL into recipient, irradiated animals still causes a myeloproliferative syndrome, suggesting that BCR/ABL does not require STAT5 for activation.43 However, transplantation of STAT5-deficient cells infected with TEL/JAK2 does not cause a disease in the bone marrow transplantation model.44 Thus, in vivo, BCR/ABL and TEL/JAK2 differ in a requirement for STAT5. In contrast, BCR/ABL,5,6 NPM/ALK,45 46 and TEL/PDGFβR (this report) apparently share a requirement for PI3 kinase in transformation. It will be interesting to see if TEL/JAK2 and other fusion proteins require PI3 kinase and to determine if the requirement for PI3 kinase activation extends to primary cells. If it does, inhibition of PI3 kinase activity would present a possible therapeutic modality for treatment of a variety of neoplasms.
We thank Charles Abrams and Terri Laufer for helpful discussions and Jidong Zhang for technical assistance.
Supported by grants 73747 (M.C.) from the National Cancer Institute, National Institutes of Health. M.C. is a recipient of a G&P Charitable Foundation Award.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Martin Carroll, Division of Hematology/Oncology, BRB2/3, Rm 708, 421 Curie Blvd, Philadelphia, PA 19104; e-mail:carroll2@mail.med.upenn.edu.