The destruction of CD4 T cells in human immunodeficiency virus (HIV) infection is associated with activation of apoptotic programs, partly mediated by death receptors. The role of CD95L/CD95 in depletion of patients' CD4 T cells is well documented, but the possible contribution of the tumor necrosis factor/tumor necrosis factor receptor (TNF/TNFR) pathway has not been examined. In this study, we found that both TNFR1 and TNFR2 induced marked apoptosis in peripheral T cells from HIV-infected persons, involving both CD4 and CD8 T cells. Longitudinal follow-up of HIV+ patients suggests an association between the in vivo evolution of CD4 T-cell numbers and variations in susceptibility to TNFR-induced apoptosis. Analysis of molecular mechanisms involved showed that it was not related to altered ex vivo expression of TNFR1-associated death domain, receptor interacting protein, or TNFR-associated factor 2. Susceptibility to TNFR-mediated apoptosis was rather related to Bcl-2 expression, because patients' T cells expressing high levels of Bcl-2 were completely protected from TNFR1- and TNFR2-induced cell death, whereas T cells expressing normal levels of Bcl-2 were not protected in patients in contrast to controls. Early recruitment of caspase-8 and caspase-3 is needed to transduce the apoptotic signals, and expression of both caspases in their active form was detected in blood T cells from HIV+ patients, whereas it was hardly detected in controls. Moreover, ligation of TNFRs induced increased activation of both caspases in patients' T cells. Together these data demonstrate that exacerbated TNFR-mediated cell death of T cells from HIV-infected individuals is associated with both alteration of Bcl-2 expression and activation of caspase-8 and caspase-3 and may contribute to the pathogenesis of acquired immunodeficiency syndrome.
Introduction
Human immunodeficiency virus type-1 (HIV-1) infection is characterized by the progressive loss of CD4 T lymphocytes, which is responsible for the profound immunodeficiency observed in late stages of the disease. Accelerated and inappropriate apoptosis has been proposed as a central mechanism of HIV-dependent CD4 T-cell destruction. However, the very low proportion of productively infected cells in vivo,1 the observation in lymph nodes that apoptosis occurs in bystander cells and rarely in productively infected cells,2 and the much higher number of T cells primed for apoptosis than the number of infected cells3 in HIV-infected individuals, have challenged the concept that, in the setting of HIV disease, much of T-cell loss occurs in uninfected cells.2,4-7 Spontaneous apoptosis of both CD4 and CD8 T cells occurs following short-term culture of peripheral blood mononuclear cells (PBMCs) from HIV-infected individuals and we showed in a large cohort of patients that the degree of apoptosis in both subsets was correlated with disease evolution.8 In addition, the degree of apoptosis in vivo and ex vivo was correlated with the in vivo expression of activation markers on patients' T cells,8,9 and priming for apoptosis of T cells was found related to the down-regulation of Bcl-2.10 11 Therefore, increased priming for apoptosis of lymphocytes from HIV+individuals is the consequence of the persistent in vivo expression of HIV antigens, which trigger apoptotic programs in noninfected cells.
This leads to an aberrant up-regulation of the physiologic mechanisms controlling peripheral CD4 T-cell deletion, which depends on the expression of a family of ligands (CD95 ligand [CD95-L], tumor necrosis factor [TNF], tumor necrosis factor-related apoptosis-inducing ligand [TRAIL]) and death receptors (CD95, TNF receptor 1 [TNFR1], and TNF receptor 2 [TNFR2]) that mediate apoptosis in susceptible cells.12 T cells from HIV-infected individuals exhibit both increased CD95 expression and enhanced susceptibility to CD95-mediated death, which correlates at the CD4 T-cell level with disease evolution,13-16 and this is not observed in the nonpathogenic model of HIV-infected chimpanzees.17 CD95L is elevated in plasma and in PBMCs from HIV-infected patients,18,19 and its expression on monocytes20 or on chronically activated cytotoxic CD8 T cells21 might contribute to the in vivo depletion of activated (CD95+) but noninfected CD4 T cells. The expression of TNF-α and TNFRs is also deregulated in HIV infection. Increased serum TNF-α levels are detected in symptomatic patients and elevated levels of soluble TNFR2 are predictive of HIV disease progression.22 There is little information concerning the potential role of TNFRs in lymphocyte apoptosis in HIV-infected patients. Katsikis et al15 reported that ligation of TNFR1 or TNFR2 with specific antibodies did not result in any increase in T-cell apoptosis above that spontaneously observed in HIV+persons. Herbein et al23 proposed a mechanism of CD8 T-cell death in HIV disease dependent on TNFR2 expression induced by ligation of CXCR4 by glycoprotein (gp)120, which increased membrane-bound TNF-α on macrophages and induced apoptosis of CD8 T cells.
Regulation of TNF signaling is complex and is achieved through multiple steps. Both TNFR1 and TNFR2 can regulate TNF-mediated processes, but apoptosis is mainly induced through TNFR1.24 The binding of TNF to TNFR1 leads to the trimerization of TNFR1 and the recruitment of TNFR1-associated death domain (TRADD) protein into the receptor complex. Then TRADD serves as a platform to recruit other adapter proteins in the complex such as Fas-associated death domain (FADD), TNFR-associated factor 2 (TRAF-2), and receptor interacting protein (RIP). Then caspase-8 is recruited, and the active caspase-8 initiates a caspase cascade, which results in apoptosis.25 The mechanisms of TNFR2-mediated death remain unclear. Unlike TNFR1 and CD95, TNFR2 lacks a death domain and the precise effectors mediating activation of the apoptotic machinery by TNFR2 are not elucidated. It has been recently shown in human T cells that TNFR2-mediated death requires RIP, which induction during T-cell activation promotes a change in TNFR2 signaling from nuclear factor-κB (NF-κB) activation to apoptosis.26 Cross-linking of TNFR2 on activated T cells results in down-regulation of Bcl-x and interestingly Grell et al28 demonstrated in different cellular systems that triggering of TNFR2 induces endogenous production of TNF and autotropic or paratropic activation of TNFR1.
In the current study, we examined whether TNFR1 and TNFR2 stimulation by cross-linking with monoclonal antibodies (mAbs) induces peripheral blood T-cell apoptosis in HIV-infected individuals. We found that both receptors induced marked T-cell apoptosis in HIV-infected persons and both CD4 and CD8 T cells were involved. We then determined if this apoptotic response to TNFRs was related to virologic or immunologic parameters and to antiretroviral treatment. We also analyzed whether increased susceptibility to TNFR1- and TNFR2-mediated apoptosis was regulated by differential expression of the adapter proteins RIP, TRADD, and TRAF-2, the antiapoptotic molecule Bcl-2, and caspases-8 and caspases-3 to further assess the mechanism by which TNFR ligation induces apoptosis in peripheral T cells from HIV+ persons and not in T cells from control donors.
Materials and methods
Blood samples
Heparinized peripheral blood samples were obtained from 42 HIV-1+ persons attending the Service for Infectious Diseases (Dr R.Roué), Bégin Military Hospital, Saint Mandé, France. Eleven patients did not receive any antiretroviral treatment (nontreated), 20 received a combination of 2 HIV-reverse transcriptase inhibitors (bi-RTIs) and 11 received a highly active antiretroviral therapy (HAART) including 2 RTIs and 1 HIV protease inhibitor (PI). Study subjects gave informed consent. Clinical characteristics of the patients are shown in Table1. Control blood samples (n = 30) were drawn from HIV-seronegative healthy donors.
Reagents and antibodies
The mouse anti–human surface antigen mAbs used in this study included fluorescein isothiocyanate (FITC)–, phycoerythrin (PE), or activated protein C (APC)–conjugated anti-CD4 mAb (IgG1k, clone SK3) and anti-CD8 mAb (IgG1k, clone SK1) (Becton Dickinson, Pont de Claix, France), FITC-conjugated anti-TNFR1 (IgG1, 16803.1 subclone) and anti-TNFR2 (IgG2a, clone 22235.311; R & D Systems, Abingdon, United Kingdom), and FITC-conjugated anti-CD95 mAbs (IgG2a, clone 22235.311; Becton Dickinson, San Jose, CA). Intracellular detection of Bcl-2 protein was performed using FITC-conjugated anti–Bcl-2 mAbs (IgG1k, clone 124; Dako, Glostrup, Denmark). Appropriate conjugated isotype-matched controls were obtained from Dako. Active caspase detection was performed with the CaspaTag kit for caspase-8 (FAM-LETD-FMK) and caspase-3 (FAM-DEVD-FMK; Intergen, Oxford, United Kingdom).
Induction of apoptosis was performed with anti-TNFR1 (IgG1, clone 16803.1) and anti-TNFR2 (IgG2a, 22221.311) mAbs (R & D Systems), anti-CD3 mAb (IgG2a, clone X35), anti-CD95 mAbs (IgG1, clone UB2; Immunotech, Marseille, France). Appropriate conjugated isotype-matched mAbs were used as controls (IgG1, clone 11711.11 and IgG2a, clone 20102.1, R & D Systems).
For Western blot analysis, the following antibodies were used: purified mouse anti–human RIP mAbs (IgG1, clone G322-2; Pharmingen/Becton Dickinson, Pont de Claix, France), rabbit anti–human TRAF-2 antibodies (Immunotech), mouse anti–human TRADD mAbs (IgG1, clone 37; Transduction Laboratories) and mouse anti–human actin mAbs as control (IgG1, clone C-2; Santa Cruz Biotechnology, Santa Cruz, CA).
Cell culture and apoptosis induction
Spontaneous apoptosis was induced by culturing overnight 106 freshly-isolated PBMCs in 1 mL complete medium, composed of RPMI 1640 (Sigma Chemical, St Louis, MO), supplemented with 10% (vol/vol) heat-inactivated fetal calf serum (FCS; Institut Jacques Boy, Reims, France), 2 mM l-glutamine (Life Technologies, Paisley, United Kingdom), and 10 IU/mL penicillin/streptomycin (Life Technologies), at 37°C in a 5% CO2 humidified atmosphere. Death receptor–mediated apoptosis was induced by overnight incubation of PBMCs (106 cells/mL) in 24-well plates precoated with anti-TNFR1, anti-TNFR2, anti-CD95, or anti-CD3 mAbs. Coating was performed for 1 hour at 4°C with 25 μg/mL suspension of mAbs in complete medium.
Quantification of apoptosis in CD4 and CD8 T cells
Quantification of apoptosis in cultured lymphocytes was performed following a method that we previously reported.29 Briefly, PBMCs incubated overnight in medium or in the presence of TNFR1, TNFR2, or CD95 mAbs were washed in phosphate-buffered saline (PBS)–bovine serum albumin (BSA)–NaN3 (PBS pH 7.2, supplemented with 1% [wt/vol] BSA [Sigma Chemical] and 0.1% NaN3) and double-stained for 30 minutes at 4°C with PE-conjugated anti-CD4 or anti-CD8 mAbs and 7-amino actinomycin D (7-AAD; Sigma Chemical), which detects apoptotic cells. Stained cells were then washed in PBS-BSA-NaN3 containing nonfluorescent actinomycin D (AD; Sigma Chemical), as described29 and fixed in PBS-BSA-NaN3 containing 1% paraformaldehyde (PFA) for 15 minutes at 4°C. Stained cells were immediately applied to a FACScalibur flow cytometer. For each sample 10 000-20 000 events were acquired and analyses were performed with the Cell Quest software (Becton Dickinson).
Flow cytometry detection of surface death receptors and intracellular Bcl-2 and active caspases
Detection of death receptors was performed either on freshly isolated PBMCs or on peripheral lymphocytes cultured overnight in medium or stimulated with coated anti-TNFR1, anti-TNFR2, or anti-CD3 mAbs. Cells were washed in PBS-BSA-NaN3 and double-stained for 30 minutes at 4°C with PE-conjugated anti-CD4 or anti-CD8 mAbs, and FITC-conjugated anti-TNFR1, anti-TNF-R2, or anti-CD95 mAbs. Stained cells were then washed in PBS-BSA-NaN3 and fixed in PBS-BSA-NaN3 containing 1% PFA for 15 minutes at 4°C. Fixed cells were then immediately applied to a FACScalibur (Becton Dickinson).
The intracellular expression of Bcl-2 protein was analyzed on freshly isolated PBMCs and on lymphocytes induced to die following ligation of the death receptors, using a method that we previously reported.10 30 Briefly, cells were first stained with PE-conjugated anti-CD4 or anti-CD8 mAbs, costained with 7-AAD, and fixed in PBS-BSA-NaN3 containing 1% PFA. Then, intracellular detection of Bcl-2 was performed with FITC-conjugated anti–Bcl-2 mAb in 0.05% (wt/vol) saponin buffer (Sigma Chemical) for 30 minutes at 4°C. Cells were then fixed with 1% PFA and applied to a FACScalibur (BD).
Intracellular expression of active caspase-8 and active caspase-3 was analyzed on freshly isolated PBMCs and on PBMCs stimulated overnight by anti-TNFR1 or anti-TNFR2 mAbs. Lymphocytes were first costained with 7-AAD and APC-conjugated anti-CD4 or anti-CD8 mAbs, and further incubated with LETD-FMK or DEVD-FMK (peptide inhibitors of caspase-8 and caspase-3, respectively) coupled with the FAM carboxyfluorescein dye for 1 hour at 37°C in PBS (CaspaTag kit, Intergen). Cells were then washed with washing buffer and fixed according to the manufacturer's procedure. Stained cells were immediately applied on a FACScalibur flow cytometer (Becton Dickinson) and analyses performed with the Cell Quest software (Becton Dickinson).
Western blot analyses
Western blot analyses were performed on purified CD2 T cells. These cells were positively selected from PBMCs using magnetic beads coated with anti-CD2 mAb, according to the manufacturer's instructions (Dynal, Oslo, Norway). The efficiency of positive selection was more than 90%. CD2 T cells were washed in PBS and lysed in 50 μL lysis buffer (0.5% Nonidet P-40, 0.2 mM EDTA, 150 mM NaCl, 50 mM Tris-HCl pH 7.5, 10% sodium dodecyl sulfate [SDS], 2 μg/mL leupeptin, 120 μg/mL phenylmethylsulfonyl fluoride [PMSF] and 2 μg/ml aprotinin). The lysates were denatured by boiling in SDS buffer, and protein concentrations were determined using the Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA). Proteins (1 mg/mL) were separated on SDS–polyacrylamide gel electrophoresis (SDS-PAGE) gels and transferred onto nitrocellulose membranes using standard procedures (Bio-Rad). Membranes were blocked, incubated with primary specific antibody for 1 hour at 37°C, and washed. Secondary alkaline phosphatase antibodies (goat anti–rabbit IgG or goat anti–mouse IgG + IgM; Tropix, Bedford, MA) were added subsequently for 1 hour at 37°C. After washes, Western blots were developed using the Tropix system (Tropix) according to the manufacturer's description. For each blot, β-actin was used as control for equal protein loading.
Statistical analyses
Univariate analyses included Spearman, Mann-Whitney, and Wilcoxon matched-pairs tests. A P < .05 was considered significant.
Results
Increased susceptibility of T lymphocytes from HIV-infected persons to TNFR1 and TNFR2-mediated apoptosis: relation to in vivo CD4 T cell evolution and HIV viral load
Figure 1A shows representative stainings of PBMCs from a healthy donor and an HIV-infected person receiving bi-RTI therapy following overnight incubation in medium or in the presence of immobilized mAbs specific for the death receptors TNFR1, TNFR2, and CD95. Apoptosis in CD4 and CD8 T cells was quantified following costaining of cultured lymphocytes with 7-AAD and subset-specific mAbs. Ligation of death receptors did not induce significant apoptosis in T lymphocytes from control donors, whereas it was very efficient in inducing apoptosis in both T-cell subsets from HIV+ donors. This analysis has been extended to 42 HIV-infected persons and 15 controls. Ligation of TNFR1 induced apoptosis in both CD4 and CD8 T cells from patients and similar observations were made following ligation of TNFR2 or CD95 (Figure1B,C). It is noteworthy that a gradient of susceptibility to apoptosis occurred in both CD4 and CD8 T-cell subsets in response to ligation of the death receptors, CD95 being more efficient that TNFR2 and TNFR1, despite a heterogeneous response (CD4 T cells, mean 26.5 ± 21.5 [CD95] versus 13.2 ± 13.3 [TNFR2] versus 10.7 ± 12.5 [TNFR1] versus 5.9 ± 5.2 [medium]; CD8 T cells, mean 31 ± 24.6 [CD95] versus 12 ± 14 [TNFR2] versus 8.6 ± 11 [TNFR1] versus 4 ± 6.1 [medium]; Figure 1B,C). Electron microscopy analysis confirmed that apoptotic cell death was induced in patients' lymphocytes by each of the death receptors (Figure1D).
T lymphocytes from HIV-infected individuals are susceptible to TNFR1-and TNFR2-mediated apoptosis.
(A) Freshly isolated PBMCs from healthy subjects (n = 15) or HIV-infected donors (n = 42) were incubated overnight with coated anti-TNFR1 or anti-TNFR2 mAbs as described in “Materials and methods.” Phenotypic identification of apoptotic cells was performed by costaining of cultured cells with CD4- or CD8-specific mAbs and 7-AAD. Representative dot plots of CD4 and CD8 T cells from a control donor and an HIV-1+ patient are shown. Numbers in each quadrant indicate the percentage of apoptotic cells (7-AAD+) within CD4 and CD8 subsets under indicated culture conditions. Median percentages (25-75th percentiles) of apoptotic cells within CD4 (B) and CD8 (C) subsets are shown in both groups of donors. Statistical significance was assessed by the Wilcoxon signed rank test for paired data. (D) Cells (1 × 106) submitted to indicated stimuli were fixed with 2.5% glutaraldehyde in Sorensen buffer (phosphate 0.1 M, pH = 7.4) and further dehydrated in a series of ethanol solutions (30%-100%). They were then embedded in epoxy. Sections were performed with a Reichter-Jung ultramicrotome before examination. Original magnification, × 5000 with a Jeol JEM 1200 EX electron microscope.
T lymphocytes from HIV-infected individuals are susceptible to TNFR1-and TNFR2-mediated apoptosis.
(A) Freshly isolated PBMCs from healthy subjects (n = 15) or HIV-infected donors (n = 42) were incubated overnight with coated anti-TNFR1 or anti-TNFR2 mAbs as described in “Materials and methods.” Phenotypic identification of apoptotic cells was performed by costaining of cultured cells with CD4- or CD8-specific mAbs and 7-AAD. Representative dot plots of CD4 and CD8 T cells from a control donor and an HIV-1+ patient are shown. Numbers in each quadrant indicate the percentage of apoptotic cells (7-AAD+) within CD4 and CD8 subsets under indicated culture conditions. Median percentages (25-75th percentiles) of apoptotic cells within CD4 (B) and CD8 (C) subsets are shown in both groups of donors. Statistical significance was assessed by the Wilcoxon signed rank test for paired data. (D) Cells (1 × 106) submitted to indicated stimuli were fixed with 2.5% glutaraldehyde in Sorensen buffer (phosphate 0.1 M, pH = 7.4) and further dehydrated in a series of ethanol solutions (30%-100%). They were then embedded in epoxy. Sections were performed with a Reichter-Jung ultramicrotome before examination. Original magnification, × 5000 with a Jeol JEM 1200 EX electron microscope.
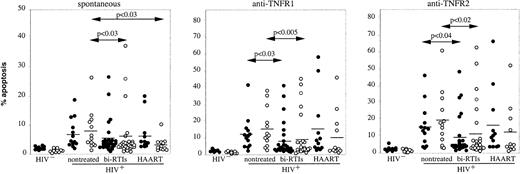
To determine whether antiretroviral treatments may influence the apoptotic response mediated by TNFRs, HIV-infected donors were subdivided into 3 groups, including 11 untreated and 20 and 11 treated with bi-RTI and HAART, respectively, as detailed in Table 1. Figure2 shows that bi-RTI treatment induced in both CD4 and CD8 subsets a significant decrease in the rate of apoptosis mediated by TNFR1 (P < .03 andP < .005, respectively, versus untreated patients) and TNFR2 (P < .04 and P < .02, respectively, versus untreated patients), whereas HAART had no significant effect compared to nontreated or bi-RTI–treated patients. In contrast, HAART and bi-RTI induced a significant decrease in spontaneous apoptosis of CD8 T cells (Figure 2). We have previously shown, in a cohort of 130 untreated HIV-infected patients, that the increased susceptibility of CD4 and CD8 T cells to spontaneous or anti-CD3–induced apoptosis was correlated with disease evolution, evaluated by the number of CD4 T cells,8 and we have confirmed this correlation in the present study (data not shown). A positive correlation has also been observed between CD95-induced apoptosis in CD4 T cells and disease evolution,16 suggesting that this cell death pathway may contribute to the depletion of CD4 T cells during HIV infection. In the present study, whether patients were untreated or received combined antiretroviral therapies, no significant correlation was found between the rate of TNFR1- or TNFR2-induced apoptosis in CD4 or CD8 T cells and plasmatic viral load or disease evolution (not shown). However, these analyses may be skewed by the low number of patients included in each group. To determine whether susceptibility to TNFR-induced apoptosis was related to levels of soluble TNF-α or TNFRs, we measured these factors in patients' serum and, although higher levels of TNF-α, TNFR1, and TNFR2 were found in HIV+ patients compared to healthy donors, they did not seem to influence the extent of apoptosis (data not shown).
Influence of antiretroviral treatment on susceptibility of T cells to TNFR1- and TNFR2-mediated apoptosis.
Freshly isolated PBMCs from HIV− donors (n = 15), HIV-infected patients either nontreated (n = 11) or treated with biRTI (n = 20) or HAART (n = 11) were incubated with coated anti-TNFR1 or anti-TNFR2 mAbs and further analyzed by flow cytometry for apoptosis quantification in CD4 (●) and CD8 T cells (○) as detailed in the legend of Figure 1. Mean values are represented as horizontal bars. Statistical significance was assessed with the Mann-Whitney test.
Influence of antiretroviral treatment on susceptibility of T cells to TNFR1- and TNFR2-mediated apoptosis.
Freshly isolated PBMCs from HIV− donors (n = 15), HIV-infected patients either nontreated (n = 11) or treated with biRTI (n = 20) or HAART (n = 11) were incubated with coated anti-TNFR1 or anti-TNFR2 mAbs and further analyzed by flow cytometry for apoptosis quantification in CD4 (●) and CD8 T cells (○) as detailed in the legend of Figure 1. Mean values are represented as horizontal bars. Statistical significance was assessed with the Mann-Whitney test.
On the other hand, we followed up 5 patients. Three cases revealed interesting links between TNFR-induced apoptosis and clinical parameters (Figure 3). Patient 1 (P1) was treated with bi-RTI (zidovudine + didanosine [ddI]) and he presented at the first time point of the study (M0) with a low viral load, a significant number of CD4 T cells (560/μL), and very low levels of TNFR-induced apoptosis. Interruption of antiviral therapy at month 8 (M8) was followed by a drop in the number of CD4 T cells (from 744 at M8 to 492 CD4/μL at M14), concomitant with a drastic increase in both TNFR1- and TNFR2-mediated apoptosis in both CD4 (from 10% to 30.2% for TNFR1 and 16% to 43% for TNFR2; Figure3A) and CD8 T cells (from 11% to 41% for TNFR1 and 18% to 59% for TNFR2). Following reintroduction of antiviral therapy (stavudine + ddI), CD4 T cells slightly increased, from 492 to 524 CD4T cells/μL and TNFR-induced apoptosis decreased to normal levels (ranging from 0.5% to 1.3%), although the viral load was elevated, confirming the lack of correlation between plasmatic viral load and apoptotic response to TNFRs. Patient 2 presented at M0 with a high viral load (214 800 copies/mL) and virtually no CD4 T cells (2/μL) although under HAART including a PI. Changing PI from indinavir (IDV) to nelfinavir (NFV) resulted in a dramatic increase in CD4 T-cell number (2 to 248 T CD4/μL) at M18, accompanied by the suppression of plasmatic viral load and normalization of TNFR1- and TNFR2-mediated apoptosis in both CD4 (Figure 3B) and CD8 T cells (not shown). Finally, patient 3 was under bi-RTI at M0 with a high number of CD4 T cells and low viral load, and very low levels of TNFR-mediated apoptosis. Six months later, a drop in CD4 T cells, from 1170 to 913 CD4/μL, occurred, concomitant with a slight increase in the low viral load and an increased susceptibility to TNFR1-, TNFR2- and CD95- (not shown) induced apoptosis detected in T cells (Figure3C). Therefore, in these cases, the in vivo evolution of CD4 T-cell numbers in the course of HIV infection was associated with variations in susceptibility to death receptor–mediated apoptosis.
Relation between TNFR-induced apoptosis in CD4 T cells and virologic and immunologic parameters.
Patients were studied in a longitudinal follow-up for CD4 T-cell apoptotic response to TNFR1 (▨) and TNFR2 (░). Determination of the percent of apoptosis was performed as detailed in the legend of Figure1 and Δ of apoptosis corresponds to the percent apoptosis in TNFR-stimulated cultures minus (nCD4/μL) percent apoptosis in medium. The parallel evolution of in vivo CD4 T-cell number, plasmatic HIV RNA viral load, and therapy (d4T, stavudine; 3TC, zidovudine) is indicated from the first time point of the study (M0) to the last one, varying from 6 to 18 months. Data from 3 representative patients are shown.
Relation between TNFR-induced apoptosis in CD4 T cells and virologic and immunologic parameters.
Patients were studied in a longitudinal follow-up for CD4 T-cell apoptotic response to TNFR1 (▨) and TNFR2 (░). Determination of the percent of apoptosis was performed as detailed in the legend of Figure1 and Δ of apoptosis corresponds to the percent apoptosis in TNFR-stimulated cultures minus (nCD4/μL) percent apoptosis in medium. The parallel evolution of in vivo CD4 T-cell number, plasmatic HIV RNA viral load, and therapy (d4T, stavudine; 3TC, zidovudine) is indicated from the first time point of the study (M0) to the last one, varying from 6 to 18 months. Data from 3 representative patients are shown.
Susceptibility to apoptosis mediated by TNFR1 and TNFR2 is not associated with increased expression of these receptors on peripheral CD4 and CD8 T cells from HIV+ donors
The expression of TNFRs on peripheral T cells was assessed by flow cytometry performed on freshly isolated or anti-CD3–stimulated PBMCs from HIV+ and control donors. Table2 shows that, in contrast to CD95,13,15,17 both TNFR1 and TNFR2 were hardly detected on peripheral CD4 and CD8 T cells from control donors and HIV-infected patients. Ligation of either receptor had no effect on their respective expression (data not shown). In contrast, TCR/CD3 ligation induced a dramatic increase in TNFR2 expression on both CD4 and CD8 T cells from control donors and HIV+ patients, in agreement with previous observations in normal human T cells.31 In contrast, only a slight increase in TNFR1 expression was detected under these conditions of stimulation (Table 2). No significant differences ex vivo following incubation in medium or stimulation with anti-CD3 mAbs in TNFR1 and TNFR2 expression were observed between HIV+ donors and controls (Table 2), suggesting that HIV infection did not alter expression of these death receptors. To confirm that expression of these receptors is not sufficient to transduce the apoptotic signals, TNFR1 and TNFR2 expression was induced in PBMCs from control donors following CD3 ligation, and activated lymphocytes were incubated short-term in the presence of coated anti-TNFR antibodies, under conditions that induce apoptosis in patients' lymphocytes. No significant apoptosis could be detected in CD4 or CD8 T cells (not shown), indicating that susceptibility of peripheral T cells to TNFR-mediated apoptosis is rather related to differential expression of regulatory proteins than to membrane expression of the death receptors.
Increased TNFR1- and TNFR2-induced apoptosis in HIV infection is not associated with altered expression of the adapter proteins TRADD, TRAF-2, and RIP
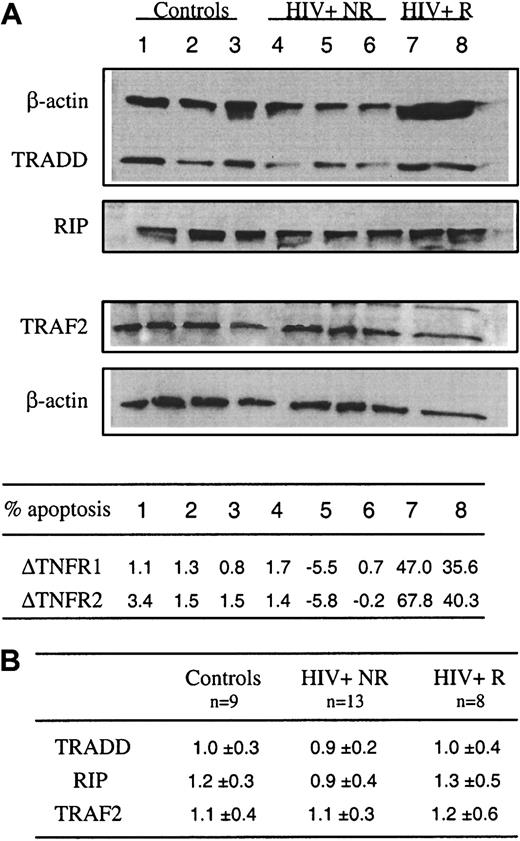
The binding of TNF to TNFR1 leads to the recruitment of TRADD that serves as a platform to recruit FADD, TRAF-2, and RIP, which interact with TRADD directly.24 Recent studies showed that, in human T cells, RIP is needed for TNFR1- and TNFR2-mediated cell death, RIP playing an indispensable role in converting cells from life to death in response to TNF treatment.26 32 To further explore the factors responsible for the increased susceptibility of patients' T cells to TNFR-induced apoptosis, messenger RNA (mRNA) and protein expression of TRADD, RIP, and TRAF-2 were analyzed in freshly purified CD2 T cells from control donors and HIV+ subjects. Because we observed that in some patients, T cells did not undergo apoptosis in response to cross-linking of TNFR1 and TNFR2, HIV+ donors were subdivided into 2 groups, susceptible/responders (R group) and nonsusceptible/nonresponders (NR group). Figure 4A shows that comparable expression of RIP was detected ex vivo in lymphocytes from the R group (lanes 7 and 8) versus NR group (lanes 4-6) versus controls (lanes 1-3), and similarly no alteration of TRADD and TRAF-2 expression was found in patients' lymphocytes. This was confirmed when the expression of these proteins was normalized to that of actin (Figure 4B). These molecules were also analyzed at the level of mRNA in purified freshly isolated CD4 and CD8 T cell subsets and no difference was detected between the different groups of donors (not shown).
Constitutive expression of RIP, TRADD, and TRAF-2 in blood T lymphocytes from control donors and HIV-infected patients.
(A) Ex vivo expression of TRADD, RIP, and TRAF-2 was assessed by Western blot analysis from whole cell extracts prepared from sorted CD2+ T cells. As a control, β-actin expression is reported for each donor for TRADD and RIP (upper part) and TRAF2 (lower part) expression after restripping of each membrane. Data are shown for 3 controls (lanes 1-3), 5 HIV-1+ patients including 3 nonresponders (NR) (lanes 4-6) and 2 responders (R) (lanes 7 and 8) to TNFR1- and TNFR2-mediated apoptosis. In the same experiment, the percent of T-cell apoptosis was determined in response to TNFR1 or TNFR2 ligation, as described in the legend of Figure 1. Data are given as Δ of apoptosis corresponding to percent apoptosis in TNFR-stimulated cultures minus percent apoptosis in medium. (B) Scanning of autoradiograms has been performed using Color It !TM 3.0. The values were obtained using the NIH Image 1.62b7. Data represent the ratio of the peak area for each protein divided by the peak area for actin.
Constitutive expression of RIP, TRADD, and TRAF-2 in blood T lymphocytes from control donors and HIV-infected patients.
(A) Ex vivo expression of TRADD, RIP, and TRAF-2 was assessed by Western blot analysis from whole cell extracts prepared from sorted CD2+ T cells. As a control, β-actin expression is reported for each donor for TRADD and RIP (upper part) and TRAF2 (lower part) expression after restripping of each membrane. Data are shown for 3 controls (lanes 1-3), 5 HIV-1+ patients including 3 nonresponders (NR) (lanes 4-6) and 2 responders (R) (lanes 7 and 8) to TNFR1- and TNFR2-mediated apoptosis. In the same experiment, the percent of T-cell apoptosis was determined in response to TNFR1 or TNFR2 ligation, as described in the legend of Figure 1. Data are given as Δ of apoptosis corresponding to percent apoptosis in TNFR-stimulated cultures minus percent apoptosis in medium. (B) Scanning of autoradiograms has been performed using Color It !TM 3.0. The values were obtained using the NIH Image 1.62b7. Data represent the ratio of the peak area for each protein divided by the peak area for actin.
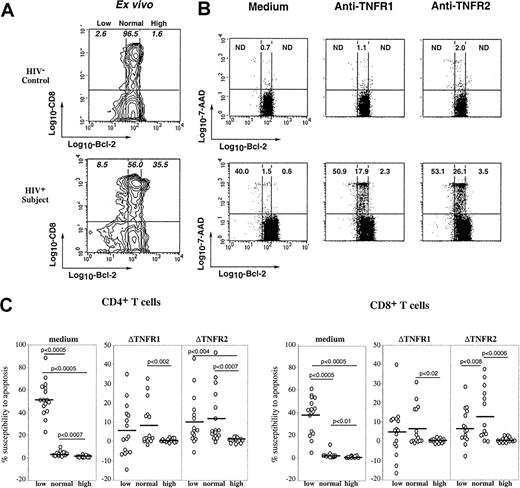
Susceptibility of T cells from HIV+ persons to TNFR1- and TNFR2-mediated apoptosis is related to Bcl-2 expression
Because HIV infection is associated with in vivo down-regulation of Bcl-2 expression in CD8 T cells, which primes lymphocytes for spontaneous apoptosis,10,11 we asked whether susceptibility to TNFR-mediated cell death was associated with differential expression of Bcl-2. Figure5A shows ex vivo expression of intracellular Bcl-2 in gated CD8 T cells from a control donor and an HIV-infected person. As we previously reported,10 the majority of CD8 T lymphocytes from healthy donors constitutively expressed homogeneous (normal) levels of Bcl-2, whereas patients' CD8 T lymphocytes often included cells expressing low levels and very high levels of Bcl-2. To analyze the relationship between expression of Bcl-2 and susceptibility to TNFR-mediated apoptosis, freshly isolated PBMCs underwent either short-term stimulation with anti-TNFR1 or anti-TNFR2 mAbs or were cultured in medium, and the combined detection at the single-cell level of apoptosis (7-AAD staining), membrane CD4 or CD8 molecule, and intracellular Bcl-2 was performed, as we previously described.30 Figure 5B shows that down-regulation of Bcl-2 expression is associated with increased susceptibility of T cells to spontaneous apoptosis because 40% of low Bcl-2 cells died when cultured in medium, as opposed to 1.5% of normal Bcl-2 and 0.6% of high Bcl-2 cells, confirming our previous data.10Interestingly, Bcl-2 expression influenced the apoptotic response to TNFR of T cells from HIV+ patients, but not that from control donors. Indeed, patients' T cells expressing normal levels of Bcl-2 and resistant to spontaneous apoptosis were susceptible to TNFR1- and TNFR2-mediated apoptosis (18% and 26% of them died, respectively), whereas normal Bcl-2 T cells from the control donor were resistant (1.1% and 2% of them died, respectively). In contrast, high expression of Bcl-2, frequently observed in patients' T cells,10 efficiently protected these cells from TNFR-induced apoptosis (Figure 5B). We have performed this analysis on lymphocytes from 16 patients and studied both CD4 and CD8 T-cell subsets. Data in Figure 5C confirm that the apoptotic response to TNFR ligation on CD4 or CD8 T cells from patients was detected in low and normal Bcl-2, not in high Bcl-2 cells. Because ligation of TNFR1 or TNFR2 did not modify by itself Bcl-2 expression, whether T cells came from patients or control donors (data not shown), these observations suggest that in vivo HIV-induced alteration of Bcl-2 expression predisposes T cells to TNFR-mediated apoptosis.
Influence of Bcl-2 expression on TNFR-induced apoptotic response of CD4 and CD8 T cells.
(A) Ex vivo expression of Bcl-2 on CD8 T lymphocytes from a representative control and an HIV-infected patient. Populations expressing low, normal and high levels of Bcl-2 were determined as we previously reported.10 (B) PBMCs were stimulated overnight by coated anti-TNFR1 or anti-TNFR2 mAbs or incubated in medium, and further triple stained with anti-CD8 and anti–Bcl-2 mAbs and 7-AAD. Each dot plot shows the relationship between the level of Bcl-2 expression and the rate of apoptosis in gated CD8 T cells. Numbers in quadrants indicate the percentage of apoptotic cells within the corresponding Bcl-2 subset. (C) This experiment has been performed on PBMCs from 16 HIV+ patients. Data represent the susceptibility to apoptosis induced by medium, TNFR1, or TNFR2 of CD4 or CD8 T cells expressing low, normal, or high levels of Bcl-2. The ΔTNFR1 and ΔTNFR2 represent the difference between the percent apoptosis in TNFR-stimulated cultures and the percent apoptosis in medium. Statistical significance was assessed by the Wilcoxon signed rank test for paired data.
Influence of Bcl-2 expression on TNFR-induced apoptotic response of CD4 and CD8 T cells.
(A) Ex vivo expression of Bcl-2 on CD8 T lymphocytes from a representative control and an HIV-infected patient. Populations expressing low, normal and high levels of Bcl-2 were determined as we previously reported.10 (B) PBMCs were stimulated overnight by coated anti-TNFR1 or anti-TNFR2 mAbs or incubated in medium, and further triple stained with anti-CD8 and anti–Bcl-2 mAbs and 7-AAD. Each dot plot shows the relationship between the level of Bcl-2 expression and the rate of apoptosis in gated CD8 T cells. Numbers in quadrants indicate the percentage of apoptotic cells within the corresponding Bcl-2 subset. (C) This experiment has been performed on PBMCs from 16 HIV+ patients. Data represent the susceptibility to apoptosis induced by medium, TNFR1, or TNFR2 of CD4 or CD8 T cells expressing low, normal, or high levels of Bcl-2. The ΔTNFR1 and ΔTNFR2 represent the difference between the percent apoptosis in TNFR-stimulated cultures and the percent apoptosis in medium. Statistical significance was assessed by the Wilcoxon signed rank test for paired data.
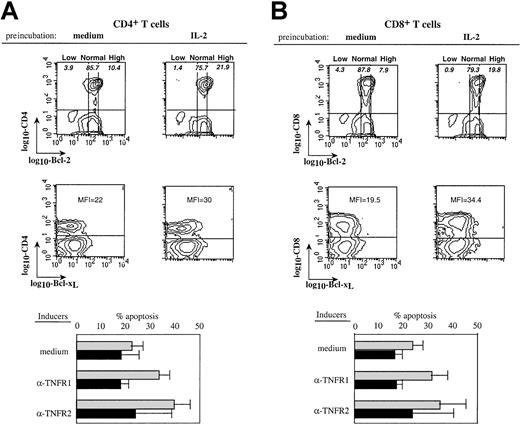
To confirm that high expression of Bcl-2 could protect patients' T cells from TNFR-induced apoptosis, PBMCs from HIV-infected donors were pretreated overnight with interleukin-2 (IL-2) to increase Bcl-2 expression, and then stimulated by anti-TNFR mAbs. Figure6A shows that IL-2 pretreatment induced an increased expression of Bcl-2 and Bcl-xL in both CD4 and CD8 T cell subsets. This was associated in both subsets with a protection from the proapoptotic effects of the cross-linking of TNFR1 and TNFR2 (Figure 6A,B). Therefore, high expression of Bcl-2 and Bcl-xL could inhibit the TNFR-dependent apoptotic pathway in T cells from HIV+ persons, whereas normal levels could not, although normal levels were sufficient to protect T cells from control donors.
IL-2–induced up-regulation of Bcl-2 expression is associated with protection from TNFRs-induced apoptosis.
PBMCs from an HIV-infected donor were pretreated overnight with IL-2 (50 ng/mL) or medium. Dot plots (upper part) show the influence of IL-2 on Bcl-2 and Bcl-xL expression in CD4 (A) or CD8 (B) T cells. Low, normal, and high Bcl-2 populations are shown and numbers in quadrants indicate the percentage of apoptotic cells within the corresponding Bcl-2 subset. Mean fluorescence intensity (MFI) of Bcl-xL expression on gated CD4 or CD8 T cells is indicated. These cells were further stimulated with anti-TNFR1 or anti-TNFR2 mAbs and apoptosis in each T- cell subset was quantified. The lower part of the figure shows data from experiments performed with PBMCs from 5 patients (mean ± SD). Preincubation in ░, medium; ▪, IL-2.
IL-2–induced up-regulation of Bcl-2 expression is associated with protection from TNFRs-induced apoptosis.
PBMCs from an HIV-infected donor were pretreated overnight with IL-2 (50 ng/mL) or medium. Dot plots (upper part) show the influence of IL-2 on Bcl-2 and Bcl-xL expression in CD4 (A) or CD8 (B) T cells. Low, normal, and high Bcl-2 populations are shown and numbers in quadrants indicate the percentage of apoptotic cells within the corresponding Bcl-2 subset. Mean fluorescence intensity (MFI) of Bcl-xL expression on gated CD4 or CD8 T cells is indicated. These cells were further stimulated with anti-TNFR1 or anti-TNFR2 mAbs and apoptosis in each T- cell subset was quantified. The lower part of the figure shows data from experiments performed with PBMCs from 5 patients (mean ± SD). Preincubation in ░, medium; ▪, IL-2.
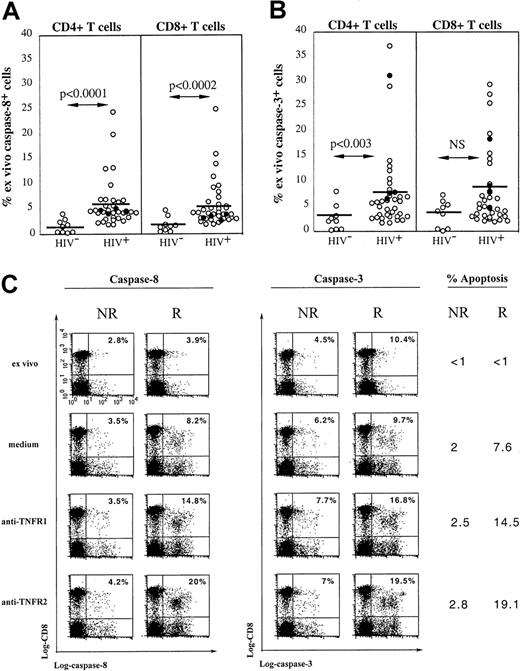
HIV infection is associated with increased in vivo expression of active caspase-8 and caspase-3: relation to TNFR-dependent cell death
Caspase-8 recruitment by the adapter protein FADD is an essential event in TNF-induced apoptosis because the blockade of TNF-induced NF-κB activation and conversion of life to death has been shown to depend on the cleavage of RIP by active caspase-8.26,32The active caspase-8 then initiates a caspase cascade, recruiting effector caspases such as caspase-3, resulting in apoptosis.33 Freshly isolated peripheral human T cells from healthy donors do not spontaneously express active caspase-8 and caspase-3 detected by Western blot analysis,34 and we used a flow cytometric approach using FAM-labeled peptide inhibitors of active caspases to analyze expression of these molecules. Figure7A shows that very low expression of active caspase-8 is detected in CD4 and CD8 T cells from control donors (n = 10; mean, 1.5% ± 1.4%; range, 0.2%-4% and mean, 2% ± 1.5%; range, 0.5%-4.9% in CD4 and CD8 T-cell subsets, respectively). In contrast, when the same analysis was performed on 35 HIV-infected persons, we found that a significant proportion of both CD4 and CD8 T cells expressed active caspase-8 (mean, 5.8% ± 4.7%; range, 1.8%-24.5% and mean, 5.6% ± 4.5%; range, 1.9%-25%, respectively), the percentage of positive cells reaching in some patients very high values. Similarly, both T-cell subsets in HIV-infected patients expressed in vivo active caspase-3 (mean, 7.7% ± 8%; range, 1.7-37.1%, and mean, 8% ± 7.3%; range, 2-29.5% of CD4 and CD8 T cells, respectively), whereas T cells from control donors expressed very low levels of this caspase (mean, 2.6% ± 2.6%; range, 0.2%-8%, and mean, 3.6 ± 2.6%, range, 0.2%-7.3% of CD4 and CD8 T cells, respectively; Figure 7B). No correlation was found between the in vivo expression of either caspase and the R (black dots) or NR (white dots) status of patients (Figure 7B).
Active caspase-8 and caspase-3 are expressed in freshly isolated blood T cells from HIV+ patients and are recruited following TNFR ligation.
Expression of intracellular active caspase-8 (A) and caspase-3 (B) was detected by flow cytometry in both CD4 or CD8 T lymphocytes from 10 healthy controls and 35 HIV+ patients including 4 patients susceptible to TNFR-mediated apoptosis (●). (C) PBMCs from a responder (R) or a nonresponder (NR) HIV-1–infected patient were incubated overnight with anti-TNFR1 or anti-TNFR2 mAbs. Detection of intracellular caspase-8 and caspase-3 on CD8 and CD4 (not shown) T cells was performed by flow cytometry. Data from a representative experiment are shown. The percentage of caspase-positive cells within CD8 T cells is indicated in each quadrant. The percentage of CD8 T cells dying of apoptosis under indicated conditions of stimulation is given for each donor.
Active caspase-8 and caspase-3 are expressed in freshly isolated blood T cells from HIV+ patients and are recruited following TNFR ligation.
Expression of intracellular active caspase-8 (A) and caspase-3 (B) was detected by flow cytometry in both CD4 or CD8 T lymphocytes from 10 healthy controls and 35 HIV+ patients including 4 patients susceptible to TNFR-mediated apoptosis (●). (C) PBMCs from a responder (R) or a nonresponder (NR) HIV-1–infected patient were incubated overnight with anti-TNFR1 or anti-TNFR2 mAbs. Detection of intracellular caspase-8 and caspase-3 on CD8 and CD4 (not shown) T cells was performed by flow cytometry. Data from a representative experiment are shown. The percentage of caspase-positive cells within CD8 T cells is indicated in each quadrant. The percentage of CD8 T cells dying of apoptosis under indicated conditions of stimulation is given for each donor.
The involvement of caspase-8 and caspase-3 in TNFR-mediated apoptosis of T cells from HIV+ donors has been studied. Cross-linking of TNFR1 or TNFR2 by specific mAbs induced in a fraction of CD4 (not shown) and CD8 T cells (Figure 7C) from R patients an increased expression of active caspase-8 and caspase-3 compared to that induced by incubation in medium. Of note, the percentage of caspase-8+ and caspase-3+ cells matched the percentage of apoptotic cells in each subset and accordingly, caspase-expressing cells were all stained by 7-AAD. In contrast, cross-linking of TNFR1 or TNFR2 on T cells from NR patients did not trigger the expression of either caspase (Figure 7C).
Discussion
Inappropriate priming for apoptosis of lymphocytes from HIV+ individuals may account for the destruction of CD4 and CD8 T cells in acquired immunodeficiency syndrome (AIDS). It is thought to be the consequence of the state of chronic immune activation characteristic of HIV disease, leading to an aberrant up-regulation of the physiologic mechanisms controlling peripheral T-cell deletion, which depends on the expression of death receptors, including CD95 and TNFRs, that mediate apoptosis in susceptible cells.12Peripheral lymphocytes from HIV+ patients exhibit both increased CD95 expression and enhanced susceptibility to CD95-mediated death, which correlates at the CD4 T-cell level with disease evolution.13-18 This report shows for the first time that ligation of TNFR1 or TNFR2 on peripheral T cells from chronically infected patients induces apoptosis in both CD4 and CD8 T-cell subsets, whereas T cells from control donors are resistant. The in vivo relevance of priming for TNFR-mediated apoptosis of patients' lymphocytes was suggested in longitudinal studies showing an association between the in vivo evolution of CD4 T-cell numbers and variations in susceptibility to death receptor-induced apoptosis (Figure 3). Analysis of molecular factors involved in susceptibility of patients' T lymphocytes to TNFR-mediated apoptosis showed that it was not related to altered in vivo expression of TRADD, RIP, or TRAF-2 (Figure 4), but it was rather related to the in vitro expression of Bcl-2 (Figure 5). Moreover, we reported the in vivo increased expression of active caspase-8 and caspase-3 in freshly isolated CD4 and CD8 T cells from patients, and cross-linking of TNFR1 or TNFR2 triggered the activation of both caspases in patients' T cells, whereas it had no effect in T cells from healthy donors (Figure7).
The apoptotic response to TNFR1 and TNFR2 was not related to an increased expression of either receptor on patients' CD4 or CD8 T cells compared to healthy donors, either in vivo or following anti-CD3 stimulation (Table 2). Regulation of TNFR signaling is complex and is achieved through multiple steps. A consensus signaling pathway for TNFR1-mediated apoptosis proposes that on TNF engagement, a multiprotein complex including the effectors TRADD, TRAF-2, and RIP is formed at the receptor, recruiting and activating caspase-8, which initiates a caspase cascade resulting in apoptosis.25 The mechanisms by which death domain (DD)–lacking TNFR2 mediates cell death remain unclear. It has been shown that triggering of TNFR2 leads to an up-regulation of TNF production and subsequent stimulation of TNFR1-mediated cytotoxicity.28 Another study reported that RIP, a DD-containing protein Ser/Thr kinase required for NF-κB activation by TNFR1, is required for TNFR2-mediated death and the induction of RIP during human T-cell activation switches TNFR2 signaling from activation to death.26 Furthermore, RIP has been shown to be a critical substrate of caspase-8 and its cleavage increases cell sensitivity to TNF-induced apoptosis.32 It was therefore of interest to determine whether increased susceptibility to TNFR-mediated apoptosis of T cells from HIV+ donors was related to altered expression of RIP. No modification in in vivo RIP expression, tested either at the mRNA level or at the protein level (Figure 4), was detected in purified CD2+ T cells from HIV+ patients compared to controls and in R versus NR patients. However, we cannot exclude that the R versus NR status of patients is associated to differential expression of RIP following TNFR cross-linking. For technical problems (the requirement of high amounts of peripheral T cells from patients), this hypothesis has not been tested. Similar to RIP, TRADD and TRAF-2 expressions were unchanged in T cells from HIV-infected patients compared to controls.
Priming for spontaneous apoptosis of T cells from HIV+ patients is related to in vivo down-regulation of the antiapoptotic protein Bcl-2,10,11 which is particularly observed in CD8 T cells expressing the phenotype of activated effectors (CD28−, CD45R0+, CD38+),10 and can be restored by cytokines such as IL-2 and IL-15.5,35 To determine whether susceptibility to TNFR-mediated apoptosis was related to Bcl-2 levels, the combined detection at the single-cell level of apoptosis and intracellular Bcl-2 on CD4 or CD8 T cells submitted to TNFR ligation was performed. Interestingly, we found that in patients T cells expressing normal levels of Bcl-2 were not protected from TNFR1- or TNFR2-mediated apoptosis, whereas in control donors, they were completely protected (Figure 5). It is noteworthy that in patients but not in controls, these normal Bcl-2 T cells included a significant proportion of cells with the activated/memory phenotype (CD45R0+, HLA-DR+; data not shown), suggesting a relationship between susceptibility to TNFR-mediated apoptosis of T cells and their activation state, as we previously showed for CD3-mediated apoptosis.8 Thus, physiologic levels of Bcl-2 are not sufficient to prevent TNFR-induced apoptosis in T cells from HIV+ patients. In contrast, T cells expressing high levels of Bcl-2, detected in the blood of HIV+ patients but not in control donors, and exhibiting the phenotype of naı̈ve quiescent cells,10 were completely resistant to TNFR-induced cell death. The relationship between the level of Bcl-2 and susceptibility of T cells to TNFR-mediated apoptosis was confirmed in experiments in which Bcl-2 was up-regulated by IL-2, leading to an almost complete resistance to TNFR signaling (Figure 6). In addition to Bcl-2, Bcl-xL was also up-regulated, suggesting that other members of Bcl-2 family may be involved in inhibiting apoptotic signals mediated by TNFRs, as suggested by Lin et al27 who reported that susceptibility to TNFR2-induced apoptosis of activated T cells was associated with down-regulation of Bcl-xL.
Cross-linking of death receptors leads to activation of an array of cysteine proteases or caspases that require proteolytic processing for activation and cleave selected substrates including proteins that protect living cells from apoptosis.33 Caspases have been implicated in apoptosis of T cells from HIV-infected individuals36-38 and a number of HIV-1 proteins such as Tat, Env, Nef, or Vpr have been shown to induce in vitro activation of caspases.39-42 In the present study we show that the active forms of both the initiator caspase-8 and the effector caspase-3 are expressed in vivo in both CD4 and CD8 T cells from HIV-infected patients, and at very low levels in T cells from control donors (Figure7). Our findings are consistent with recent reports showing that another caspase, ICE, is expressed in patients' T cells, although predominantly found in CD4 T cells,37 and patients with progressive HIV disease were reported to express increased caspase-3 activity.43 The expression of active caspase-8 that we observed in lymphocytes from HIV+ patients may account for their greater sensitivity to CD95- and TNFR-mediated apoptosis, but more studies are needed to elucidate this point because no correlation was found in the small cohort that we tested. Several mechanisms may contribute to in vivo caspase activation, including the direct effect of HIV proteins,39-42 HIV-driven immune activation because cleavage of procaspases occurs early during T-cell activation and is required for T cell expansion . Interestingly, we found that ligation in vitro of TNFR1, TNFR2, or CD95 specifically increased the expression of both caspases in T lymphocytes susceptible to these death receptors (Figure 7C and data not shown). Activation of caspases might have beneficial effects by decreasing viral replication, either by acting directly on viral machinery or by inducing cell death.
What are the consequences of the priming of patients' T cells for TNFR-mediated cell death during HIV infection? It may contribute to the depletion of CD8 T cells that occurs in the chronic phase of HIV infection,46 through a mechanism proposed by Herbein et al23 involving TNFR2 expression on CD8 T cells and membrane TNF-α on macrophages consequently to the binding of gp120 on CXCR4. It may also contribute to depletion of CD4 T cells because recent evidence indicates that interaction of TNF-α and TNFR2 plays an important role in down-regulating antigen-activated T-cell responses, chronically induced in HIV disease. Indeed, in this study, we have found an association during the follow-up of patients between the in vivo evolution of CD4 T-cell numbers and variations in susceptibility to death receptor–induced apoptosis. Several studies suggest a central role for TNF/TNFR in the immunopathogenesis of HIV-1 infection. For example, a direct correlation was found between serum levels of TNF-α and soluble TNFRs and disease progression during primary and chronic HIV infection, and we reported that potent antiviral therapy may be associated with alterations in TNF-α T-cell homeostasis, which contributes to lipodystrophy-associated dyslipidemia associated with the therapy.49
In conclusion, in addition to be responsive to the CD95 death receptor, peripheral CD4 and CD8 T cells from HIV-infected patients are susceptible to proapoptotic signaling through both TNFR1 and TNFR2, and this is associated with the expression of caspase-8 and caspase-3 and the lack of physiologic protection by Bcl-2. This apoptotic pathway may contribute in vivo to the depletion of antiviral T-cell effectors during the progression of HIV disease.
We thank Nicola Wahsen and Deborah Hirt for their participation in RNA transducer analysis and enzyme-linked immunosorbent assay detection of TNF and TNFRs, respectively, and Marie-Christine Prevost for electron microscopy experiments.
Supported by grants from the Agence Nationnale de Recherche sur le SIDA (ANRS), the Fondation pour la Recherche Médicale (Sidaction), Pasteur Institute, the Centre National de la Recherche Scientifique (CNRS), and the European Union (contracts BMH4-CT 97-2055 and ERB-IC15-CT97-O901). L.M.O.P. was supported by a grant from Conselho Nacional de Pesquisa e Desenvolvimento (CNPq) of the Brazilian government.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Marie-Lise Gougeon, Institut Pasteur, Département SIDA et Rétrovirus, 28 Rue du Dr Roux, 75724 Paris, Cedex 15, France; e-mail: mlgougeo@pasteur.fr.