Mildly thrombocytopenic patients with relapsed or refractory low-grade non-Hodgkin lymphoma (NHL) have an increased risk of chemotherapy-induced myelosuppression following treatment. The safety and efficacy of radioimmunotherapy with a reduced dose of90Y ibritumomab tiuxetan (0.3 mCi/kg [11 MBq/kg]; maximum 32 mCi [1.2 GBq]) was evaluated in 30 patients with mild thrombocytopenia (100-149 × 109 platelets/L) who had advanced, relapsed or refractory, low-grade, follicular, or transformed B-cell NHL. The ibritumomab tiuxetan regimen included an infusion of rituximab (250 mg/m2) and injection of 111In ibritumomab tiuxetan (5 mCi [185 MBq]) for dosimetry evaluation, followed 1 week later with rituximab (250 mg/m2) and90Y ibritumomab tiuxetan (0.3 mCi/kg [11 MBq/kg]). Patients (median age, 61 years; 90% stage III/IV at study entry; 83% follicular lymphoma; and 67% with bone marrow involvement) had a median of 2 prior therapy regimens (range, 1-9). Estimated radiation-absorbed doses were well below the study-defined maximum allowable for all 30 patients. With the use of the International Workshop criteria for NHL response assessment, the overall response rate was 83% (37% complete response, 6.7% complete response unconfirmed, and 40% partial response). Kaplan-Meier estimated median time to progression (TTP) was 9.4 months (range, 1.7-24.6). In responders, Kaplan-Meier estimated median TTP was 12.6 months (range, 4.9-24.6), with 35% of data censored. Toxicity was primarily hematologic, transient, and reversible. The incidence of grade 4 neutropenia, thrombocytopenia, and anemia was 33%, 13%, and 3%, respectively. Reduced-dose ibritumomab tiuxetan is safe and well tolerated and has significant clinical activity in this patient population.
Introduction
Myelosuppression, including its associated complications such as bleeding, infection, and fatigue, is the major toxic effect associated with systemic treatment of patients with low-grade non-Hodgkin lymphoma (NHL).1 Mild thrombocytopenia, defined as platelet counts of 100 to 150 × 109 platelets/L (plt/L), persists in a considerable number of patients who are refractory to lymphoma treatment or relapse within the first year and provides additional clinical challenges for treatment of this patient population. In an analysis of risk factors for chemotherapy-induced thrombocytopenia in 1051 patients, a platelet count of less than 150 × 109plt/L was associated with a significantly increased risk of severe thrombocytopenia requiring platelet transfusions (P < .01).2 The appearance of chemotherapy-induced thrombocytopenia may result in the interruption or delay of treatment, necessitate dose reduction, and/or require patients to receive platelet transfusions. Therefore, identification of safe and effective regimens for treatment of patients with relapsed or refractory NHL with persistent, mild thrombocytopenia is essential.
Radioimmunotherapy, the administration of radionuclides conjugated to monoclonal antibodies, is an effective approach for treating patients with relapsed or refractory NHL.3,4 Ibritumomab tiuxetan (Zevalin; IDEC Pharmaceuticals, San Diego, CA) is composed of a murine immunoglobulin G1 kappa monoclonal antibody, ibritumomab, covalently bound to the chelator, tiuxetan (isothiocyanatobenzyl MX-DTPA). Ibritumomab tiuxetan can be radiolabeled with either 111In for imaging or 90Y for therapy. Both ibritumomab tiuxetan and its chimeric counterpart, rituximab (Rituxan; IDEC Pharmaceuticals and Genentech, South San Francisco, CA) target the CD20 antigen, which is present on more than 90% of B-cell lymphomas.5 Studies conducted with radiolabeled ibritumomab tiuxetan successfully improved on the favorable clinical response rates attained by using the unconjugated antibody, rituximab, in patients with low-grade, follicular, or transformed NHL who had relapsed or who failed primary chemotherapy.6
Baseline platelet count was considered as a surrogate for prior bone marrow damage from former chemotherapy or external-beam radiation therapy/involvement of the marrow with lymphoma, or both, and was identified as an indicator of increased risk of hematologic toxicity in the phase I portion of a phase I/II trial of ibritumomab tiuxetan reported on by Witzig et al.7 Two patients with baseline platelet counts of 100 to 120 × 109 plt/L developed grade 4 thrombocytopenia (< 25 × 109 plt/L), whereas none of 8 patients with normal baseline platelet counts (≥ 150 × 109 plt/L) developed thrombocytopenia at the same dose level (0.4 mCi/kg [15 MBq/kg]). Thereafter, patients with baseline platelet counts of 100 to 149 × 109 plt/L were treated with 0.3 mCi/kg (11 MBq/kg) instead of the standard90Y ibritumomab tiuxetan dose of 0.4 mCi/kg (15 MBq/kg) for patients with normal platelet counts.8 A statistically significant correlation was noted between percentage of bone marrow involvement with NHL at baseline and hematologic toxicity following ibritumomab tiuxetan therapy.9
The current study was designed to examine whether mildly thrombocytopenic patients with relapsed or refractory, low-grade, follicular, or transformed NHL could receive safe and effective treatment with ibritumomab tiuxetan radioimmunotherapy at a reduced dose of 0.3 mCi/kg (11 MBq/kg).
Patients, materials, and methods
Eligibility
Eligible patients were required to be at least 18 years of age and to have histologically confirmed, relapsed, or refractory low-grade, follicular, or transformed CD20+ NHL. Patients were to have mild thrombocytopenia (platelet counts of 100-149 × 109 plt/L), an absolute neutrophil count (ANC) of 1.5 × 109 cells/L or more, and a total lymphocyte count of more than 5 × 109 cells/L. In addition, patients were to have bidimensionally measurable disease with at least one lesion measuring 2.0 cm in a single dimension; less than 25% bone marrow involvement with lymphoma (as measured by biopsy); a prestudy World Health Organization performance status of 0, 1, or 2; no prior myeloablative therapy with autologous bone marrow transplantation or peripheral blood stem cell support; normal hepatic and renal function; no concurrent systemic corticosteroid therapy; and no prior radioimmunotherapy or anti-CD20 therapy. All patients were required to provide written informed consent in accordance with the Declaration of Helsinki, and each participating clinical site had to obtain approval from the institutional review board to conduct this study.
Study design
This phase II, open-label, single-arm, multicenter study was designed to assess the safety and efficacy of ibritumomab tiuxetan radioimmunotherapy in mildly thrombocytopenic patients with advanced, relapsed, or refractory low-grade, follicular, or transformed NHL. Additionally, the study examined whether baseline platelet counts were predictive of a safe and effective dose of ibritumomab tiuxetan in this defined patient population, thus obviating the need for dosimetry.
The components of the ibritumomab tiuxetan treatment regimen were described in detail, elsewhere.7 10 Briefly, ibritumomab tiuxetan is composed of ibritumomab, a murine anti-CD20 monoclonal antibody covalently bound to tiuxetan, an isothiocyanatobenzyl derivative of MX-DTPA (a chelating agent), which allows stable binding of the 90Y radionuclide for therapy. The radionuclide,111In, can be substituted for 90Y for imaging and dosimetry, if desired. Clinical sites were provided with the unlabeled antibody-chelate conjugate, ibritumomab tiuxetan, and radiolabeling was performed onsite with either 111In for gamma camera imaging or 90Y for therapy. Radiometal incorporation of the radiolabeled antibody was determined by using instant thin-layer chromatography and was required to be 95% or more prior to injection. The formulated conjugate and other components of the radiolabeling kit were provided to the clinical sites by IDEC Pharmaceuticals.
Patients were to receive one course of ibritumomab tiuxetan as outpatient treatment. In this treatment regimen, rituximab is infused prior to ibritumomab tiuxetan to deplete B cells from the peripheral circulation, bone marrow, and lymph nodes and to optimize ibritumomab tiuxetan biodistribution. Patients were to receive an initial infusion of rituximab (250 mg/m2) and immediately thereafter an intravenous injection of an imaging dose of 111In ibritumomab tiuxetan (5 mCi [185 MBq]; 1.6 mg). One week later, patients meeting dosimetry requirements (ie, a predicted radiation exposure of < 2000 cGy to normal organs and < 300 cGy to red marrow) were to receive a second infusion of rituximab (250 mg/m2) and a therapeutic intravenous injection of90Y ibritumomab tiuxetan based on body weight (0.3 mCi/kg [11 MBq/kg]; 1.6 mg) and not to exceed 32 mCi (1.2 GBq). This dose represents a reduction of the standard dose of 0.4 mCi/kg (15 MBq/kg) that was defined previously as the maximum tolerated dose in patients with platelet counts of 150 × 109 plt/L or more. Given that this refractory patient population was at risk of serious hematologic toxicity, a dose-reduction rule was in place if patients exhibited an unacceptable rate of myelotoxicity.
A treatment period was defined as the interval from the first rituximab infusion to 12 weeks following 90Y ibritumomab tiuxetan injection. A follow-up period was defined as the interval from 12 weeks following 90Y ibritumomab tiuxetan injection to 4 years following the first rituximab infusion.
Toxicity was evaluated according to the National Cancer Institute Adult Toxicity Criteria (version 2.0). Safety evaluations included clinical adverse events, hematology and blood chemistry laboratory results, analysis of peripheral blood lymphocyte subpopulations, quantitation of immunoglobulins, and measurement of serum antichimeric and antimurine antibodies (HACA and HAMA, respectively). Duration of hematologic toxicity for each hematologic variable (ANC, platelet count, and hemoglobin concentration) was calculated by using 2 methods. In method A, duration was measured from the date of the last laboratory value prior to development of grade 3 toxicity to the date of the next value in grade 2 (or grade 1) following nadir. In method B, duration was measured from the date of the first laboratory value in grade 3 toxicity to the date of the last value in grade 3 following nadir. No attempt was made to separately ascribe adverse events to rituximab,111In ibritumomab tiuxetan, or 90Y ibritumomab tiuxetan. Serum for HACA and HAMA testing was obtained at 4 and 12 weeks following 90Y ibritumomab tiuxetan treatment. Peripheral blood B cells were defined as those expressing the CD19 (pan B) cell-surface antigen.
Statistical methods
Efficacy was assessed as response to treatment and was calculated by using 2 sets of response criteria. At the inception of this study, no standardized guidelines existed for the evaluation of response to therapy in patients with NHL and, thus, the criteria defined originally in the study protocol were used. In 1999, a report published by the International Workshop conducted at the National Cancer Institute defined specific criteria for the uniform evaluation of response in patients with NHL.11 These response criteria gained broad acceptance and are used currently in clinical trials. Therefore, the International Workshop criteria were also applied in this study to assess patients' responses.
The primary endpoint, the overall response rate (ORR) in intent-to-treat patients, was defined in the study protocol as the percentage of patients with responses classified as complete, complete clinical, or partial (CR, CCR, and PR, respectively). According to protocol-defined criteria, CR patients had no evidence of disease, ie, all lesions had to regress to a “normal” size of less than 1.5 cm × 1.5 cm for at least 28 days. CCR patients met CR criteria but had a single residual mass that decreased by 75% and remained stable or improved for at least 3 months. PR patients had a 50% or more decrease from baseline in the sum of the products of the greatest perpendicular diameters (SPD) of measured lesions and no progressive disease observed for at least 28 days. Patients with stable disease (SD) exhibited neither a 50% decrease nor a 50% increase in the SPD of measured lesions compared with baseline. Those with progressive disease (PD) had a 50% or more increase over nadir in the SPD of measured lesions or the appearance of a new lesion, in any single observation.
As noted, International Workshop NHL response criteria described by Cheson et al11 were used to evaluate efficacy. These criteria include no CCR category but define an additional ORR category designated as complete response/unconfirmed (CRu). CRu describes patients who meet the criteria of CR classification, but who either display a 1.5-cm or more residual mass reduced more than 75% by therapy or who have an indeterminate bone marrow assessment of involvement with lymphoma.
Restaging was performed by using computed tomography or magnetic resonance imaging at 1 month following treatment with ibritumomab tiuxetan. For responders, restaging was performed at least 28 days following response onset and again at 6 months. For nonresponders, restaging was repeated 3 months following ibritumomab tiuxetan treatment. For all patients who did not demonstrate PD, restaging was performed every 3 months for the first year and at 6-month intervals for years 2, 3, and 4.
Secondary efficacy endpoints included CR rate, PR rate, time to progression (TTP), and duration of response (DR; PD-free interval). TTP was measured from the date of the first rituximab infusion to the earliest of the following 2 dates: date of PD or date of last contact. DR was measured from the date of the first observation of 50% or more shrinkage of tumor response to the earliest of the following 2 dates: date of PD or date of last contact.
Response rates were analyzed using the 2-sided confidence limits method with normal approximation. The time-to-event efficacy variables, TTP and DR, were summarized by presenting the sample size, median, and maximum/minimum values. By definition, the analysis of DR data included only patients achieving clinical response (CR or PR). Median TTP was estimated by using the Kaplan-Meier product-limits method. To describe the relationship between ORR and prognostic factors, the Fisher exact 2-tailed test was performed for each prognostic factor. For the analyses of the relationship between TTP and DR versus prognostic factors, the log-rank test was performed for each factor.
Safety variables were tabulated and descriptive statistics (mean, median, standard deviation, etc.) were calculated. Clinical adverse event data were assigned preferred terms by using COSTART (Food and Drug Administration, Rockville, MD) and analyzed by calculating the number and percentage of patients and events for each dosing group. If the same adverse event was reported on consecutive days, then the specific number of times the event occurred was recorded as a single event. The most severe grading among the individual events was used to characterize this unified event.
Results
Patient characteristics
Thirty eligible patients were treated at 12 study sites between May 27, 1998, and August 24, 1999. All patients received both rituximab infusions and injections of 111In ibritumomab tiuxetan and90Y ibritumomab tiuxetan. Patient characteristics included a median age of 61 years (range, 29-85); 60% men; 90% stage III/IV at study entry; 83% follicular histology; 67% bone marrow involvement with lymphoma; and involvement of 2 or more extranodal sites in 20%. Most patients (97%) had a prestudy World Health Organization performance status of 0 or 1; 23% had splenomegaly (Table1).
Patients entering the study were treated previously with a median of 2 therapy regimens (range, 1-9); 5 received radiotherapy, and 2 received bioimmunotherapy. Nineteen patients (63%) were chemoresistant, ie, either had no response (CR or PR) or progressed within 6 months of therapy to one or more prior chemotherapy regimens. Fourteen patients (47%) were resistant to their last chemotherapy regimen administered prior to enrollment.
Response to ibritumomab tiuxetan
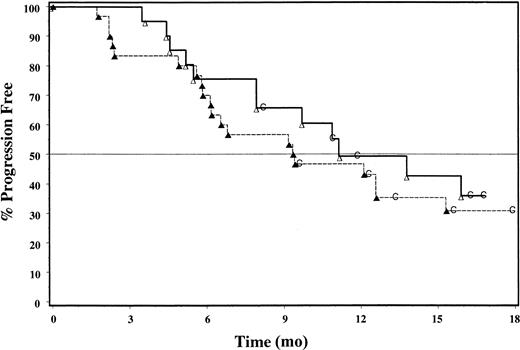
An ORR of 83% (25 of 30 intent-to-treat patients) was achieved based on International Workshop criteria (Table2). A CR was achieved in 11 patients (37%), CRu in 2 (6.7%), and PR in 12 (40%). Patients with follicular histology had an ORR of 92%. Response assessment according to criteria defined by the study protocol included a 67% ORR, with 33% CR (10 patients) and 33% PR (10 patients); patients with follicular histology achieved an ORR of 76%. No patient had a response classified as CCR. Median time to onset of response was 35 days. TTP was estimated by Kaplan-Meier analysis (Figure 1); the median TTP was 9.4 months (range, 1.7-24.6) in intent-to-treat patients. Responders had an estimated median TTP of 12.6 months (range, 4.9-24.6), with 35% of data censored. In patients with follicular histology, estimated median TTP was 10.8 months and was 12.6 months for responders. The Kaplan-Meier estimated median DR was 11.7 months (range, 3.6-23.4+) for intent-to-treat patients.
Kaplan-Meier analysis of TTP for all patients (▴—▴; n = 30) and for responders (▵ — ▵; n = 20); 35% of responders' data remain censored (C).
Kaplan-Meier analysis of TTP for all patients (▴—▴; n = 30) and for responders (▵ — ▵; n = 20); 35% of responders' data remain censored (C).
Analysis of patient characteristics at baseline and response to ibritumomab tiuxetan therapy revealed significantly higher response rates in patients with follicular versus small lymphocytic lymphoma or transformed histology (P = .014), and in those with a low baseline peripheral blood B-cell count (< 32 × 106cells/L; P = .041). Higher response rates were associated with fewer prior chemotherapy regimens, an absence of splenomegaly, and with 0 or 1 extranodal disease sites. Responders included 7 (70%) of 10 patients classified as International Prognostic Index (IPI) low risk, 7 (70%) of 10 low/intermediate risk, 4 (67%) of 6 intermediate/high risk, 1 (50%) of 2 high risk, and 1 (50%) of 2 whose IPI risk group was unknown. Length of TTP and DR correlated significantly with the following baseline characteristics: absence of bone marrow involvement, 0 to 1 extranodal sites, low to normal lactic dehydrogenase concentration, and lower IPI risk group.
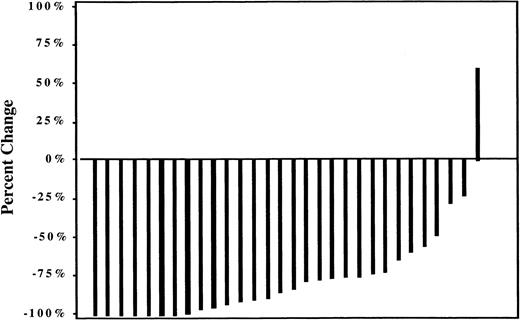
All but one patient experienced a reduction of 20% or more in overall tumor size according to maximum percentage lesion change (Figure2). Mean tumor size declined by the first evaluation (1 month following ibritumomab tiuxetan treatment) and continued to decline in responders through 15 months.
Dosimetry
Estimated radiation absorbed doses were below the maximum allowable of 2000 cGy for uninvolved major organs and 300 cGy for red marrow in all 30 patients. Median radiation absorbed doses were 48 cGy to red marrow, 522 cGy to spleen, 393 cGy to liver, 162 cGy to lungs, and 14 cGy to kidneys. Although a statistically significant correlation was noted between the severity or duration of thrombocytopenia and blood effective half-life, blood residence time, and red marrow radiation absorbed dose in some analyses, a wide range of hematologic nadirs for neutrophils and platelets was observed in patients with similar red marrow dose estimates. Therefore, none of these factors were reliably predictive of hematologic toxicity. No correlation was noted between response to treatment and blood half-life, residence time, or blood-derived red marrow radiation absorbed dose. Dosimetry and pharmacokinetic data collected in this study were analyzed and will be discussed in detail elsewhere (G.A.W. et al, manuscript submitted).
Safety
Toxicity was primarily hematologic, transient, and reversible. No patient received a reduced dose of ibritumomab tiuxetan because of an unacceptable rate of myelotoxicity, ie, no implementation of the dose-reduction rule was required. Median hematologic nadir values were as follows: ANC, 0.6 × 109 cells/L; platelets, 26.5 × 109 plt/L; and hemoglobin, 10.1 g/dL (Table3). Ten patients (33%) experienced grade 4 neutropenia; 4 (13%) experienced grade 4 thrombocytopenia; and 1 (3%) experienced grade 4 anemia. The median duration of neutropenia, thrombocytopenia, and anemia was calculated by using 2 methods as presented in Table 3. Nine patients with thrombocytopenia (30%) received platelets, and 6 patients with anemia (20%) received red blood cell transfusions. Growth factor use was limited; one patient each received erythropoietin and oprelvekin, and 2 patients received both filgrastim and epoetin alfa.
The incidence of grade 4 platelet nadir correlated significantly with increased bone marrow involvement (P = .049) as shown in Table 4. The incidence of grade 4 platelet nadir was 10%, 0%, and 37% in patients with bone marrow involvement of 0% to less than 5%, 5% to less than 20%, and more than 20% to less than 25%, respectively. Although not significantly different, the incidence of grade 4 ANC nadir (10%, 33%, and 63%) increased with increasing bone marrow involvement of 0% to less than 5%, 5% to less than 20%, and more than 20% to less than 25%, respectively (P = .074). No correlation was noted between the incidence of grade 4 hemoglobin nadir and bone marrow involvement (P = .600).
Median peripheral blood B cells declined following treatment onset and returned to the normal range by 9 months; median serum immunoglobulin concentrations remained generally within the normal range. Serious infections were rare; no grade 4 cases and only 2 grade 3 cases (urinary tract infection, febrile neutropenia) were reported during the treatment period. Three of 10 patients with neutropenia received oral antibiotics. All patients with infections recovered.
The most common nonhematologic adverse events reported were infusion-related and included asthenia (50% of patients), nausea (40%), chills (37%), vomiting (30%), fever (30%), and headache (27%). Of 264 adverse events, most (96%) were grade 1 or 2. Most treatment-related events occurred on an infusion day (58%; 110 of 189) and included asthenia, chills, and nausea; the frequency of events decreased between the first rituximab infusion and treatment 1 week later with 90Y ibritumomab tiuxetan. Nausea was infusion-related in 4 of 5 patients with grade 2 nausea; no patient experienced grade 3 or 4 nausea. Overall, grade 3 events were rare, with abdominal pain, malaise, febrile neutropenia, and urinary tract infection occurring in one patient each. Table5 lists the incidence of adverse events that occurred during the treatment period.
No clinically significant chemistry abnormalities were associated with treatment; no grade 3 or 4 shift in any chemistry variable was reported. No patient discontinued treatment because of an adverse event. Although all patients were tested, no HAMA or HACA responses to treatment were detected. One patient (3%) had successful engraftment of peripheral blood stem cells collected on day 377 following ibritumomab tiuxetan treatment.
One patient (0.5%) with a history of chronic alkylator exposure was diagnosed with acute myelogenous leukemia (AML) at 20.3 months after study entry. Two patients died on study from disease progression; neither death was considered related to study treatment.
Discussion
Myelosuppression, including thrombocytopenia, is the major toxicity associated with most cytotoxic systemic therapies for patients with NHL. The incidence of grade 3 and 4 thrombocytopenia ranged from 21% to 55% in several studies using a variety of treatment regimens, including oral alkylating agents,12 purine nucleoside analogs,13-18 and single19-21 and multiagent chemotherapy.22-28 Thrombocytopenic patients have a compromised bone marrow reserve, either because of prior chemotherapy and radiotherapy exposure, bone marrow involvement with lymphoma, an inherent stem cell defect, or, more likely, a combination of these factors. Therefore, treatment of this patient population provides additional clinical challenges.
The study population was extensively pretreated, with a median of 2 prior therapies and a range extending to 9. Most patients were chemotherapy-resistant; 40% received previous cyclophosphamide, hydroxydaunomycin, vincristine, and prednisone (CHOP) chemotherapy and 43% received other aggressive chemotherapy regimens such as etoposide, Solu-Medrol, high-dose ara-c, and Platinol (ESHAP) and prednisone methotrexate, Adriamycin, cyclophosphamide, etoposide plus mechlorethamine, vincristine, procarbazine, and prednisone (ProMACE-MOPP). Nearly half of the patients were resistant to their last chemotherapy, 20% were resistant to all prior therapies, and 63% were resistant to at least one prior chemotherapy. Finally, this group had many adverse prognostic factors, including bone marrow involvement in two thirds of patients and a high prevalence of extranodal disease. Nearly half of the patients had lesions more than 5 cm, 10% had lesions 7 cm to 10 cm, and 7% had lesions 10 cm or more.
Even though this refractory population was at risk for toxicity, ibritumomab tiuxetan treatment was very well tolerated. Most nonhematologic adverse events (such as nausea) were grade 1 or 2 and were consistent with those associated with rituximab infusion.29 The frequency of adverse events decreased from the time of the first rituximab infusion to the administration of rituximab and 90Y ibritumomab tiuxetan 1 week later. Most treatment-related cardiovascular and respiratory system adverse events occurred on infusion days and were mild and transient.
A dose-reduction rule was in place in case patients experienced an unacceptable rate of myelotoxicity, as defined by hematologic nadir values. However, no implementation of this rule was necessary because toxicity was below the study protocol–defined limit. Most treatment-related grade 3 and 4 toxicities consisted of reversible depletion of one or more hematologic cell lines. Overall, there was a 33% incidence of grade 4 neutropenia, a 13% incidence of grade 4 thrombocytopenia, and a 3% incidence of grade 4 anemia. Because of the mechanism of action of ibritumomab tiuxetan treatment, extended but reversible depletion of peripheral lymphocytes (lymphopenia) was expected in most patients. Cell counts recovered in all patients except in those who went on to receive other therapy or who had preexisting cytopenias. Recovery was not due to heavy use of hematopoietic growth factors, as only 4 patients were treated with one or more growth factors during the study.
The observed correlation between percentage of bone marrow involvement with lymphoma and hematologic toxicity confirms the relevance of clinical parameters in predicting toxicity, as defined by nadir grade. Patients with greater lymphoma infiltration of bone marrow were more likely to experience grade 4 neutropenia and thrombocytopenia than those with no bone marrow disease. The fact that this correlation reached statistical significance in this small patient population confirms the relative homogeneity of this population based on eligibility criteria and establishes that clinical characteristics, rather than dosimetry, can be used to safely administer ibritumomab tiuxetan.
Serious infections were rare; only one patient had a grade 3 urinary tract infection and one had a grade 3 episode of culture-negative febrile neutropenia. The low incidence of infections was not due to heavy use of prophylactic oral antibiotics, as only 3 patients (10%) received those medications while neutropenic. In part, the low rate of infection was likely due to nondisruption of gastrointestinal mucosal barriers and maintained serum immunoglobulin concentrations, despite B-cell depletion. All bleeding-related adverse events were grade 1 or 2.
To our knowledge, toxicity results of other radioimmunotherapy trials have not been reported specifically for NHL patients with mild thrombocytopenia, although a reduced dose of iodine 131 I tositumomab (Bexxar; Corixa, Seattle, WA, and GlaxoSmithKline, Philadelphia, PA) is specified for such patients.30 Thrombocytopenia is dose-limiting for iodine 131 I tositumomab and for other radioimmunotherapies in development.30-32 The incidence of severe (grade 3 or 4) thrombocytopenia for patients in the current study was significantly higher than the incidence for 130 patients with normal baseline platelet counts (> 150 × 109 plt/L) treated with the standard ibritumomab tiuxetan dose in 2 phase III randomized trials (P = .043; unpublished data). These results are not unexpected given that an increased risk of severe thrombocytopenia for this population has been documented.2 It is noteworthy that the incidence of infection and hospitalization was similar (P > .999 andP = .418, respectively) among patients in all 3 trials (unpublished data), suggesting that the higher incidence of toxicity did not translate into a greater number of clinically significant events.
One case of AML was reported at 20.3 months after study entry in a patient with a history of chronic exposure to alkylator therapy. Such events are not unanticipated in this patient population. In a study of 262 patients with low-grade NHL, Stone et al33 identified thrombocytopenia and extensive use of alkylator therapy among the primary factors indicating risk of myelodysplasia and AML. A cumulative incidence of myelodysplasia in NHL patients who had no prior dose-intensification therapy has been reported as approximately 4% to 8%,34 or about a general risk of 1% to 1.5% per year from 2 years to at least 9 years after the start of therapy.35
Clinical efficacy was impressive at this reduced dose. According to International Workshop criteria, an ORR of 83% was achieved, with rates of 37% CR, 6.7% CRu, and 40% PR. The 83% ORR is comparable with the 81% ORR achieved by 13 of 16 patients in the 0.3 mCi/kg (11MBq/kg) dose group in the phase I/II trial of ibritumomab tiuxetan.7 Those patients had a higher median baseline platelet count (143 × 109 plt/L versus 127.5 × 109 plt/L) and platelet nadir (46 × 109 plt/L versus 26.5 × 109 plt/L) compared with patients in the current study. Similarly, 73 patients with normal baseline platelet counts who received a standard dose of ibritumomab tiuxetan (0.4 mCi/kg [15 MBq/kg]) on the ibritumomab tiuxetan arm of a phase III randomized trial36,38 achieved an 80% ORR, 30% CR, and 4% CRu (International Workshop criteria). However, response assessments for patients in the phase III trial were performed by an independent panel of radiologists and oncologists expert in lymphoma (LEXCOR; Lymphoma Experts Confirmation of Response); therefore, results are not strictly comparable. Also, similar rates were reported for patients with normal platelet counts treated with iodine I 131 (131I) tositumomab,37 but no published data are available for patients with mild thrombocytopenia. Although no patient received maximal radiation-absorbed doses to red marrow or major organs, to potentially improve response rates by increasing the dose of 90Y ibritumomab tiuxetan was not practicable given the rate of severe neutropenia.
Clinical activity was particularly notable in patients with follicular histology (92% ORR using International Workshop criteria; 76% using study protocol–defined criteria). Because 25 of the 30 patients had follicular lymphoma, these patients dominated the ORR assessment. Unfortunately, the small number of patients with small lymphocytic lymphoma and transformed lymphoma prevents a meaningful estimate of ORR in these subsets. All but one patient experienced at least a 20% reduction in tumor burden. Therefore, even patients with stable disease benefited from receiving ibritumomab tiuxetan therapy, as bulky masses often cause discomfort and pain.
The estimated median TTP for responders, 12.6 months, is consistent with the updated estimated median TTP of 13.3 months for responders who received 0.3 mCi/kg (11 MBq/kg) in the phase I/II trial of ibritumomab tiuxetan (unpublished data) and with the 12.3 months progression-free survival achieved by the 42 patients in the iodine131I tositumomab trial.37 Therefore, responders in this study have an excellent chance of achieving a comparable ORR and TTP, despite their increased risk at baseline for hematologic toxicity.
Clinical activity was attained in patients with a diverse range of adverse prognostic factors such as tumor lesions more than 5 cm, more than one site of extranodal disease, bone marrow involvement, splenomegaly, and elevated IPI risk group. As expected, these prognostic variables influenced clinical efficacy as measured by TTP and DR. Length of TTP and DR correlated significantly with absence of bone marrow involvement, 0 to 1 extranodal sites, low to normal lactic dehydrogenase, and lower IPI risk group. No statistically significant correlation between ORR and TTP versus the number of prior chemotherapy regimens was observed, although the small number of patients may have limited the ability to detect this effect. Of note, prior radiotherapy was not associated with a lower ORR or shorter TTP.
In conclusion, patients with mild thrombocytopenia can safely and effectively receive dose-reduced ibritumomab tiuxetan radioimmunotherapy. Clinical activity is significant, and toxicity is primarily hematologic, transient, and reversible. There appears to be no excessive risk of bleeding events or serious infections. Although a single case of acute leukemia was observed in this study, such events are to be expected, given the characteristics of this extensively pretreated, refractory study population. Finally, results demonstrate that patients can be effectively screened and safely treated on the basis of clinical selection criteria, including baseline platelet count, bone marrow involvement with lymphoma, and individualized dosing based on patient weight.
Supported by IDEC Pharmaceuticals Corporation, San Diego, CA.
Several of the authors (P.S.M., A.J.G.-L., C.A.W.) have declared a financial interest in a company whose product was studied in the present work. Several of the authors (P.S.M., R.S.A., C.A.W.) are employed by a company (IDEC Pharmaceuticals) whose product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Gregory A. Wiseman, Mayo Clinic and Mayo Foundation, Division of Nuclear Medicine, Charlton Bldg 1-223, 200 First St SW, Rochester, MN 55905; e-mail: gwiseman@mayo.edu.