The platelet collagen receptor, glycoprotein VI (GPVI), and GPIb-IX-V, which binds von Willebrand factor, initiate platelet aggregation at low or high shear stress, respectively. We recently reported that positively charged, membrane-proximal sequences within cytoplasmic domains of GPIbβ and GPV of GPIb-IX-V bind calmodulin. We now show that GPVI also binds calmodulin as follows—(1) calmodulin coimmunoprecipitated with GPVI from resting platelet lysates using an anti-GPVI IgG, but partially dissociated in platelets activated by collagen or collagen-related peptide; (2) calmodulin coprecipitated from platelet lysates with maltose-binding protein (MBP)–GPVI cytoplasmic domain fusion protein, but not MBP alone; (3) GPVI-related synthetic peptide based on the membrane-proximal sequence, His269-Pro287, induced a shift in calmodulin migration on nondenaturing gels, an assay that identifies calmodulin-binding peptides. His269-Pro287 is analogous to the calmodulin-binding sequence in GPIbβ. The novel interaction of GPVI and calmodulin may regulate aspects of GPVI function.
Introduction
Platelet activation and aggregation in normal hemostasis or pathologic thrombosis is initiated by the engagement of specific adhesion receptors.1-4 At low shear stress, collagen receptors such as glycoprotein VI (GPVI) initiate platelet activation. At high shear, platelet adhesion is primarily dependent on the GPIb-IX-V complex binding von Willebrand factor. Subsequent platelet aggregation involves the elevation of cytosolic Ca++ and the triggering of signaling pathway(s), leading to cytoskeletal rearrangements and activation of the integrin, αIIbβ3 (GPIIb-IIIa). Defining pathways by which ligand binding to GPVI and GPIb-IX-V activate αIIbβ3 may provide therapeutic targets for attenuating thrombosis. Recent studies have identified signaling molecules associated with the cytoplasmic domain of GPIb-IX-V, including 14-3-3ζ,5,6 the p85 subunit of phosphatidylinositol 3-kinase,7 and calmodulin.8 Consistent with their parallel physiological function, common elements involved in mechanisms of regulation and signaling are emerging for GPVI and GPIb-IX-V. For instance, a functional association with Fc receptor γ chain (FcRγ) has been implied for both receptors.1,2 9-11 In this study, we show that, like GPIbβ and GPV, the cytoplasmic domain of GPVI binds calmodulin, an interaction that is decreased after platelet activation. We further show that a synthetic peptide based on the positively charged, membrane-proximal sequence His269-Pro287 contains a conserved calmodulin recognition site and interacts with purified calmodulin.
Study design
Maltose-binding protein–GPVI fusion protein
A maltose-binding protein (MBP)–GPVI fusion protein was prepared from cDNA encoding the GPVI cytoplasmic sequence Glu266-Ser31612 subcloned into a pMAL-c2x vector (New England Biolabs, Beverly, MA) at EcoRI andXbaI sites. The construct encoded a fusion protein with the N-terminal region corresponding to Escherichia coli MBP and the GPVI sequence at the C-terminus. The correct sequence was verified by sequencing. MBP and MBP-GPVI expressed in E coli were purified on amylose–Sepharose according to the manufacturer's instructions (New England Biolabs).
GPVI-calmodulin association
Washed platelets (2 × 108/mL-5 × 108/mL)13-15were resuspended in buffer containing 1 mM RGDS and 10 μM indomethacin and were treated with 10 μg/mL collagen or collagen-related peptide2 or 200 nM A23187 for 5 to 60 seconds at 37°C. Some samples were preincubated with 20 μM PP1 (Src-family kinase inhibitor) for 20 minutes at 37°C. Platelets were lysed by the addition of an equivalent volume of ice-cold lysis buffer (2% vol/vol Triton X-100, 40 mM Tris-HCl, 10 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 1 μg/mL pepstatin-A, pH 7.3), centrifuged at 15 000g for 10 minutes, and precleared with 30 μL of a 50% suspension of protein A–Sepharose in TBS-T buffer (20 mM Tris-HCl, pH 7.3, 136 mM NaCl, 0.1% vol/vol Tween 20). Lysates were immunoprecipitated using 3 μg/mL anti-GPVI antibody MM20411 and 30 μL protein A–Sepharose for 2 hours at 4°C as previously described.12-14 MM20411 was characterized and provided by Dr Masaaki Moroi (Kurume University, Japan; unpublished results, 2000). Lysates were also precipitated with amylose–Sepharose beads and either MBP or MBP-GPVI fusion protein as described elsewhere.13-15
Blotting
Gel shift assay
The interaction of synthetic peptides corresponding to His269-Pro287 of GPVI12 or Lys529-Gly544 of GPV16 (Chiron Mimotopes, Clayton, Australia) with bovine calmodulin (Sigma, St Louis, MO) was analyzed on nondenaturing gels.8 17-20 Calmodulin (0.3 nmol) in 0.1 M Tris-HCl, pH 7.5, containing 4 M urea was incubated with GPVI or GPV peptides for 30 minutes at 22°C in the presence of 1 mM Ca++ or 10 mM EGTA.
Results and discussion
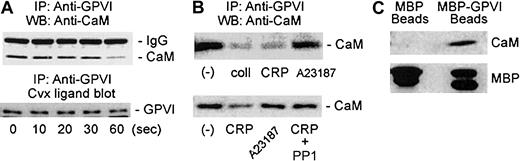
In this study, we found that the cytoplasmic domain of GPVI binds calmodulin. First, immunoblotting with anticalmodulin antibody showed that calmodulin coimmunoprecipitated with GPVI from resting platelet lysates (Figure 1). We previously showed that up to 25% of total platelet calmodulin was redistributed from the cytosol to the cytoskeleton following platelet stimulation.8 After platelet stimulation with collagen or collagen-related peptide, there was decreased association of calmodulin with GPVI (Figure 1A-B). Ligand blotting with convulxin showed levels of immunoprecipitated GPVI were essentially unaffected on this time scale (Figure 1A). In different experiments with these agonists, dissociation occurred after a lag of 30 to 60 seconds (typically approximately 60% loss after 60 seconds), and this loss was maintained up to 90 seconds (Figure 1A). Dissociation of calmodulin from GPVI induced by collagen-related peptide was delayed relative to stimulation of tyrosine phosphorylation (not shown) and was blocked by the Src-family kinase inhibitor PP1 (Figure 1B), suggesting an activation-dependent mechanism for the observed dissociation. In contrast to the GPVI agonists, ionophore A23187 that induces GPVI-independent platelet activation did not affect calmodulin-GPVI association (Figure 1B). Second, we used an MBP fusion protein containing the entire cytoplasmic sequence of GPVI, Glu266-Ser316 (MBP-GPVI), to specifically coprecipitate calmodulin from platelet lysates (Figure 1C). In contrast, calmodulin was not precipitated by MBP alone.
Coprecipitation of GPVI and calmodulin from platelet lysates.
(A) Immunoprecipitation (IP) by anti-GPVI antibody (MM20411) of untreated platelet lysates or lysates of platelets (5 × 108/mL) treated with 10 μg/mL collagen-related peptide. Samples were electrophoresed on 10% or 15% polyacrylamide gels under reducing conditions and were immunoblotted with anticalmodulin (Anti-CaM) IgG. The position of the antibody light chain is shown. Blots were visualized using horseradish peroxidase–coupled second antibody (Silenus, Hawthorn, Australia) and enhanced chemiluminescence detection (ECL; Amersham, Buckinghamshire, United Kingdom). Samples were also ligand blotted with convulxin (Cvx; lower panel) to determine GPVI levels.12 WB indicates Western blot. (B) Immunoprecipitation by anti-GPVI IgG of untreated platelet lysates or lysates of platelets treated with 10 μg/mL collagen (coll) or collagen-related peptide (CRP), or 200 nM A23187 for 90 seconds (upper panel) or 30 seconds (lower panel) at 37°C. Where indicated, platelets were preincubated with 20 μM PP1 before stimulation with collagen-related peptide. Samples were immunoblotted with anticalmodulin IgG as described above. Experiments with collagen-related peptide were performed 6 times, and those with collagen were performed 3 times. (C) Coprecipitation of calmodulin with amylose–Sepharose beads from platelet lysates in the presence of MBP or MBP-GPVI (Glu266-Ser316) fusion protein. Precipitates were electrophoresed on 5% to 20% sodium dodecyl sulfate–polyacrylamide gels under reducing conditions, electrotransferred to nitrocellulose, probed with anticalmodulin IgG or anti-MBP IgG, and visualized using the ECL reagent. Results are typical of 3 separate experiments.
Coprecipitation of GPVI and calmodulin from platelet lysates.
(A) Immunoprecipitation (IP) by anti-GPVI antibody (MM20411) of untreated platelet lysates or lysates of platelets (5 × 108/mL) treated with 10 μg/mL collagen-related peptide. Samples were electrophoresed on 10% or 15% polyacrylamide gels under reducing conditions and were immunoblotted with anticalmodulin (Anti-CaM) IgG. The position of the antibody light chain is shown. Blots were visualized using horseradish peroxidase–coupled second antibody (Silenus, Hawthorn, Australia) and enhanced chemiluminescence detection (ECL; Amersham, Buckinghamshire, United Kingdom). Samples were also ligand blotted with convulxin (Cvx; lower panel) to determine GPVI levels.12 WB indicates Western blot. (B) Immunoprecipitation by anti-GPVI IgG of untreated platelet lysates or lysates of platelets treated with 10 μg/mL collagen (coll) or collagen-related peptide (CRP), or 200 nM A23187 for 90 seconds (upper panel) or 30 seconds (lower panel) at 37°C. Where indicated, platelets were preincubated with 20 μM PP1 before stimulation with collagen-related peptide. Samples were immunoblotted with anticalmodulin IgG as described above. Experiments with collagen-related peptide were performed 6 times, and those with collagen were performed 3 times. (C) Coprecipitation of calmodulin with amylose–Sepharose beads from platelet lysates in the presence of MBP or MBP-GPVI (Glu266-Ser316) fusion protein. Precipitates were electrophoresed on 5% to 20% sodium dodecyl sulfate–polyacrylamide gels under reducing conditions, electrotransferred to nitrocellulose, probed with anticalmodulin IgG or anti-MBP IgG, and visualized using the ECL reagent. Results are typical of 3 separate experiments.
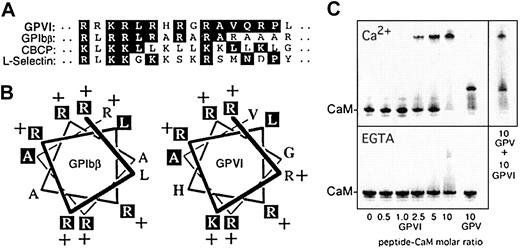
A membrane-proximal 16-residue sequence, His269-Pro287, within the cytoplasmic domain of GPVI was analogous to calmodulin-binding motifs found in GPIbβ,8 L-selectin,20 and CBCP19 (Figure 2A). CBCP is a nonphysiological, calmodulin-binding control peptide representative of positively charged, amphipathic α-helices typical of calmodulin recognition sites in other proteins.19,21-23 A helical-wheel representation of the calmodulin-binding sequences of GPVI and GPIbβ8 revealed an almost identical charge distribution between the 2 peptides (Figure 2B). To examine whether this sequence in GPVI bound calmodulin, we used a nondenaturing gel shift assay previously used to identify calmodulin-binding peptides.8,17-20 The peptide His269-Pro287 induced a concentration-dependent shift in calmodulin migration in the presence of Ca++, but not in the presence of EGTA (Figure 2C). Maximal shift was observed at approximately 10-fold molar excess of peptide to calmodulin, suggesting the affinity of the interaction was comparable to that of the GPV-related peptide8 and within the range reported for other calmodulin-binding peptides, 1:1 (Kd approximately 30 nM)17 or 50:1 (Kd approximately 2 μM).18 The location of the calmodulin shift for the GPVI-related peptide was different from that induced by the GPV peptide (Figure 2C). When both peptides were included in the assay at equimolar concentrations, approximately 50% of each GPVI-calmodulin and GPV-calmodulin complex resulted, suggesting the peptides interacted with calmodulin at the same site and with comparable affinity. Like GPIbβ, GPV, and other proteins,8,22,23 the Ca++ dependence for the interaction of GPVI-related peptide with calmodulin was in contrast with the coassociation of calmodulin with immunoprecipitated GPVI or MBP-GPVI fusion protein containing the full-length cytoplasmic domain that occurred in the presence of EGTA. However, decreased calmodulin association with GPVI in activated platelets (this study) and corresponding redistribution of calmodulin to the activated cytoskeleton8 suggest that the elevation of cytosolic Ca++ in activated platelets2-4 may potentially regulate calmodulin interactions with GPVI.
GPVI cytoplasmic sequence and other calmodulin-binding sequences.
(A) GPVI cytoplasmic sequence, His269-Pro287, compared with calmodulin-binding sequences in GPIbβ (Arg149-Leu167),8a model calmodulin-binding control peptide CBCP,19 and L-selectin.20 Identical amino acids or conserved substitutions are boxed. (B) Helical wheel representation of the GPIbβ and GPVI cytoplasmic sequences. (C) Nondenaturing gel shift of calmodulin (0.3 nmol) in the presence of increasing concentrations of GPVI peptide, His269-Pro287. The shift is shown relative to that induced by a peptide based on the GPV calmodulin-binding sequence Lys529-Gly544.8 Upper and lower panels were electrophoresed in the presence of 1 mM Ca++ or 10 mM EGTA, respectively. Gels were stained with Coomassie blue.
GPVI cytoplasmic sequence and other calmodulin-binding sequences.
(A) GPVI cytoplasmic sequence, His269-Pro287, compared with calmodulin-binding sequences in GPIbβ (Arg149-Leu167),8a model calmodulin-binding control peptide CBCP,19 and L-selectin.20 Identical amino acids or conserved substitutions are boxed. (B) Helical wheel representation of the GPIbβ and GPVI cytoplasmic sequences. (C) Nondenaturing gel shift of calmodulin (0.3 nmol) in the presence of increasing concentrations of GPVI peptide, His269-Pro287. The shift is shown relative to that induced by a peptide based on the GPV calmodulin-binding sequence Lys529-Gly544.8 Upper and lower panels were electrophoresed in the presence of 1 mM Ca++ or 10 mM EGTA, respectively. Gels were stained with Coomassie blue.
The cytoplasmic domain of GPVI consists of 51 residues.12Arg249 within the transmembrane domain, together with elements within the cytoplasmic domain, mediates the association of GPVI with FcRγ.10 FcRγ, containing an immunoreceptor tyrosine-based activation motif, is critical for GPVI-dependent platelet activation.2 10 Binding of calmodulin to a membrane-proximal sequence of GPVI raises the possibility that this interaction may regulate some aspect of the association of GPVI with FcRγ or FcRγ-independent events. It is unclear whether decreased calmodulin association following stimulation would be consistent with the down-regulation of functional GPVI-FcRγ association. In conclusion, this study has identified calmodulin as a GPVI-associated protein in platelets. Current studies aim to define functional consequences of the GPVI-calmodulin interaction.
We thank Carmen Llerena and Andrea Aprico for outstanding technical assistance. We also thank Drs Moroi and Jung for the antibody to GPVI.
Prepublished online as Blood First Edition Paper, April 17, 2002; DOI 10.1182/blood-2001-11-0008.
Supported by the National Health and Medical Research Council of Australia and by the British Heart Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Robert K. Andrews, Baker Medical Research Institute, PO Box 6492, St Kilda Rd Central, Melbourne, Australia, 8008; e-mail: rkandrews@hotmail.com.