We adoptively transferred donor-derived cytomegalovirus (CMV)-specific T-cell lines into 8 stem cell transplant recipients lacking CMV-specific T-cell proliferation. All patients, of whom one was infected by a CMV strain that was genotypically ganciclovir resistant, had received unsuccessful antiviral chemotherapy for more than 4 weeks. CMV-specific lines had been prepared by repetitive stimulation with CMV antigen, which increased the percentage of CMV-specific T cells and ablated alloreactivity completely even against patients mismatched for 1 to 3 HLA antigens. After transfer of 107 T cells/m2 at a median of 120 days (range, 79-479 days) after transplantation, no side effects were noticed. Despite cessation of antiviral chemotherapy, the CMV load dropped significantly in all 7 evaluable patients, with a maximal reduction after a median of 20 days (range, 5-31 days). In 2 patients with high virus load, the antiviral effect was only transient. One of these patients received a second T-cell infusion, which cleared the virus completely. At a median of 11 days after transfer, CMV-specific T-cell proliferation was demonstrated in 6 patients, and an increase in CMV-specific CD4+ T cells was demonstrated in 5 patients. In 6 patients, 1.12 to 41 CMV-specific CD8+ T cells/μL blood were detected at a median of 13 days after transfer, with an increase in all patients lacking CMV-specific CD8+ T cells prior to transfer. Hence, anti-CMV cellular therapy was successful in 5 of 7 patients, whereas in 2 of 7 patients, who received an intensified immune suppression at the time of or after T-cell therapy, only transient reductions in virus load were obtained.
Introduction
Cytomegalovirus (CMV) infection after allogeneic stem cell transplantation (SCT) is frequently associated with life-threatening invasive visceral disease.1 During the last few years, the introduction of prophylactic or preemptive administration of ganciclovir has resulted in a significant reduction in the incidence of early-onset CMV disease. Unfortunately, this has been at the expense of an increase in late-onset CMV disease.2-4 Because persistent CMV infection with prolonged antiviral treatment results in a delayed CMV-specific immune reconstitution, the onset of CMV disease after day 100 has become the major CMV-related posttransplant complication.2-5
Cell-mediated immunity represents an essential host factor in the control of persistent infection and the recovery from CMV disease.6-10 An increased understanding of the mechanisms by which T cells recognize virus and tumor-specific antigens has stimulated much interest in the use of specific T cells as adoptive immunotherapy for viral and malignant diseases.11-16Peripheral blood lymphocytes of the donor usually contain CMV-specific T cells and can therefore be used to control CMV infection. However, this kind of therapy is limited by potentially fatal complications caused by the alloreactive T cells that are also present in the donor lymphocyte infusion.11-14 A further problem of using unselected populations of donor lymphocytes is the rather low frequency of CMV-specific T cells in the donor lymphocyte preparation.7 Enrichment of virus-specific T cells by in vitro culture before transfer13-18 appears to reduce the risk of graft-versus-host disease (GVHD)13-16 and can effectively restore virus-specific T-cell responses.15,16It has been shown that prophylactic treatment of patients in the early period following allogeneic SCT with CD8+ CMV-specific cytotoxic T lymphocytes (CTLs) isolated from their HLA-matched sibling donor16 results in a CD8+ T-cell response in peripheral blood equivalent to responses in healthy seropositive donors who resist CMV infection. Here, we investigated for the first time the therapeutic application of CMV-specific T-cell lines in patients lacking CMV-specific T-cell responses who suffered from persisting or recurring CMV infection in spite of prolonged antiviral chemotherapy. We show that this therapy has antiviral activity also in a patient who was infected by a CMV strain that was genotypically and phenotypically documented to be ganciclovir resistant.
Patients, materials, and methods
Transplant protocols
All patients underwent a preparative regimen with total-body irradiation (TBI, 12 Gy) or busulfan (16 mg/kg) and cyclophosphamide (2 × 60 mg/kg). Patients with advanced disease received additional etoposide (40 mg/kg) (TBI/Cy/VP-16), thiotepa (2 × 5 mg/kg), or cytosine arabinoside (2 × 1 g/m2).
All grafts from a family donor were CD34+-selected using either the Miltenyi (Miltenyi Biotech GmbH, Bergisch-Gladbach, Germany) (n = 3) or the Cell Pro device (n = 1). If less than 1 × 105 CD3+ lymphocytes/kg in the matched or 1 antigen-mismatch situation or less than 0.5 × 105CD3+ lymphocytes/kg in the 3-antigen mismatch situation were transferred, no GvHD prophylaxis was administered.
Patients undergoing bone marrow transplantation from an unrelated donor received 3.5 mg/kg/d antithymocyte globulin (ATG; Pasteur-Merieux, MDS GmbH, Leiden, Germany) (day −4 to day −1). For additional GvHD prophylaxis, cyclosporin A (CSA) with dose modification according to blood levels, mycophenolate mofetil (MMF; 2 × 1 g/d orally), and prednisolone (1 mg · kg−1 · d−1) were administered.
Antimicrobial prophylaxis
Oral acyclovir (4 × 400 mg/d) was administered until day 30 after SCT. Additional antimicrobial prophylaxis was administered as described before.19
CMV disease
CMV disease was diagnosed according to standard criteria.20
Virus screening and antiviral therapy
All CMV-seropositive patients as well as those receiving a transplant from a seropositive donor were followed up weekly by qualitative polymerase chain reaction (PCR) from whole blood beginning on day 0 and continued until day 100 after SCT, as described before.19,21-23 After day 100, patients continued to be screened by PCR when considered at high risk for late-onset CMV disease.4 PCR-based preemptive therapy was performed as reported before.19 Patients not responding to preemptive treatment with ganciclovir for 4 weeks received foscarnet (2 × 60 mg/kg/d) or cidofovir (5 mg/kg/wk) until they tested PCR negative.
Protocol design
SCT recipients receiving a transplant from a CMV-seropositive donor and failing antiviral chemotherapy as defined by the persistence or recurrence of CMV DNA in the peripheral blood after 4 weeks of antiviral chemotherapy and/or lacking CMV-specific T-cell proliferation were eligible for study entry. T-cell transfer was performed if a sufficient number (107/m2) of CMV-specific T cells could be generated.
Patients were closely monitored for acute side effects during the first 2 to 4 hours following T-cell transfer and later on for acute and chronic GvHD. The efficacy of T-cell therapy was documented by a reduction in the virus load, increase in the absolute numbers of CMV-specific CD4+ and CD8+ T cells, and the documentation of CMV-specific T-cell proliferation. This study was performed under a protocol approved by the local human research ethics committee of the Eberhard-Karls University, Medical Faculty, Tübingen, Germany. Informed consent was obtained from all patients included in the study.
Preparation of CMV-specific T-cell lines
T-cell lines were prepared from fresh samples of peripheral blood (200 mL) collected from the stem cell donor. Donor-derived peripheral blood mononuclear cells (PBMNCs) were isolated by Ficoll-Hypaque gradient and incubated for 10 days in 10 mL RPMI (supplemented with 10% human AB serum from CMV-seronegative donors) with CMV lysate (1:200 diluted) in an upright 70-mL culture flask. Live cells (5 × 105) isolated from the culture flask were transferred into wells of a 24-well plate and restimulated with 106 autologous irradiated (3000 rad) feeder cells and CMV antigen (1:1000) in RPMI/10% human AB serum (PAN, Biotech GmbH, Aidenbach, Germany) supplemented with 25 U interleukin-2 (IL-2, Proleukin; Chiron, Ratingen, Germany) and gentamicin. Live cells isolated 7 days later were cultured at 5 × 105 per well and restimulated with irradiated autologous PBMNCs, CMV antigen, and IL-2.
CMV specificity of the T-cell lines was confirmed by CMV-specific proliferation (see below). In addition, no T-cell proliferation above background had to be documented when the T-cell lines were stimulated with allogeneic and autologous targets.
Before infusion, bacterial, fungal, or Mycoplasmacontamination was excluded (sterility testing). In addition, viral growth (CMV, herpes simplex virus) and amplification of CMV RNA had to be negative prior to administration. In addition, PCR for human immunodeficiency virus (HIV), hepatitis B virus, and hepatitis C virus was performed and was negative in all preparations analyzed. The T-cell lines were extensively washed in phosphate-buffered saline (PBS) before infusion.
Monitoring of acute and chronic toxicity of the T-cell infusions
All patients received T-cell infusions in the outpatient department, where their vital signs were monitored before; immediately after; and 1, 2, and 4 hours after each infusion. Complete blood counts and liver function were evaluated weekly until 1 month following T-cell transfer. GvHD was graded by standard criteria weekly during this time period and later on every 2 weeks until 3 months after T-cell infusion.
Monitoring of CMV-DNA load
Lymphoproliferation assay
The proliferation assay was performed as described before.2 CMV antigen, phytohemagglutinin M form (Murex, Life Technology, Karlsruhe, Germany), and IL-2 were added at final concentrations of 1:400, 10 ng/mL, and 50 U/mL, respectively. A stimulation index of 3 was considered a positive lymphoproliferative response.
Synthesis of peptides and tetramers
CMV pp65 peptides for intracellular cytokine staining and generation of major histocompatibility complex (MHC)–peptide tetrameric complexes were synthesized as described15 using standard Fmoc chemistry on an automated peptide synthesizer (432A; Applied Biosystems, Weiterstadt, Germany) and purified by reverse-phase high-performance liquid chromatography (HPLC, Varian star; Zinsser, München, Germany) and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (G2025A; Hewlett-Packard, Palo Alto, CA). Fast-protein liquid chromatography (FPLC)–purified, biotinylated MHC-peptide tetrameric complexes were prepared as described previously.24 25 The 3 CMV pp65 epitopes used in this study (amino acid [AA] 363-373: YSEHPTFTSQY restricted by A*0101, AA 495-503: NLVPMVATV restricted by A*0201, and AA16-24: GPISHGHVLK restricted by A*1101) represent dominant epitopes in the anti-CMV response, so that in individuals expressing the respective HLA antigens, the bulk of the responding CTLs can be detected by staining with only a single tetramer or by detection of interferon-γ (IFN-γ) production following stimulation with the immunodominant peptide antigen.
Staining with MHC-peptide tetrameric complexes
Generation of and staining with MHC-peptide tetrameric complexes (HLA A2/CMV pp65 peptide NLVPMVATV) were performed as described before.24,25 The biotinylated MHC complexes were recovered by FPLC purification, and tetramers were generated as previously described.25 A total of 2 to 5 × 105 PBMNCs were stained in a 96-well plate with 20 μg/mL phycoerythrin-labeled HLA A2 tetramer. After tetramer staining, cells were incubated with anti-CD8 and -CD3 antibodies and analyzed with a FACS Calibur (Becton Dickinson, Heidelberg, Germany) as previously described.25
Quantification of circulating CMV-specific CD4+ and CD8+ T cells
The absolute numbers of CMV-specific T cells were determined by measuring IFN-γ production and/or MHC-peptide tetrameric complex binding in 2 to 5 × 105 PBMNCs stained with anti-CD8 and anti-CD3 antibodies. For the detection of IFN-γ production, 106 PBMNCs had been stimulated overnight with either peptides (10 μg/mL) or CMV antigen (10 μg/mL) in 200 μL RPMI 1640 supplemented with Glutamax (Gibco), 10% fetal calf serum (CC-pro; Neustadt, Germany), and 10 μg/mL Brefeldin A (Sigma, Deisenhofen, Germany). Before analysis, the cells were stained with monoclonal antibodies directed against CD4 or CD8 and fixed in Dulbecco PBS (dPBS; Gibco BRL) containing 2% formaldehyde (Sigma) for 15 minutes at room temperature, washed, and resuspended in dPBS containing 0.5% saponin (Roth, Karlsruhe, Germany), 1% bovine serum albumin (Roth), and 0.2 μg/well anti–IFN-γ antibody (Becton Dickinson, Heidelberg, Germany). All samples were analyzed on a FACS Calibur flow cytometer using the CellQuest software package. Absolute numbers of peptide- and protein-specific T cells were calculated on the basis of the absolute numbers of CD3+CD4+ and CD3+CD8+ lymphocytes per milliliter blood and the relative numbers of these lymphocyte fractions staining positive for IFN-γ after specific stimulation.
Results
Preparation of CMV-specific T-cell lines
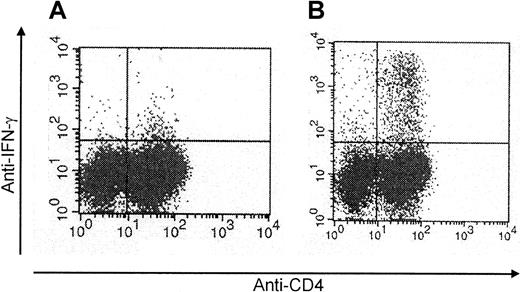
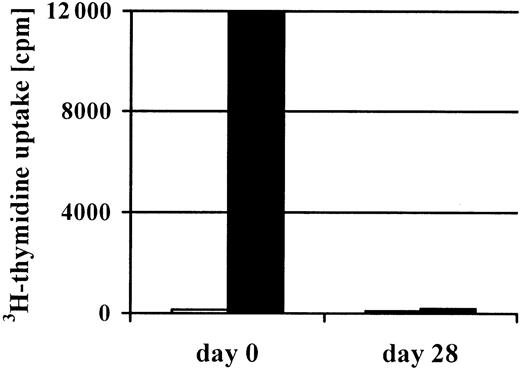
To obtain CMV-specific T-cell lines, we stimulated the mononuclear cells isolated from 200 mL peripheral blood of the stem cell donor 4 times with CMV antigen. In 14 of 21 cultures, this procedure efficiently increased the percentage of CMV-specific T cells. Figure1 shows that already after 2 stimulations, the percentage of CMV-specific cells could be as high as 7.4%. After 4 stimulations, the T-cell lines (n = 14) were 77% ± 10% CD4+ and 6% ± 3% CD8+. CMV-specific cells were predominantly found in the CD4+population (Figure 1). Furthermore, after the 4 stimulations, the T-cell lines had lost their initial alloreactivity toward the patient. Even in the donor-recipient combination that was mismatched for 3 HLA antigens, the mixed lymphocyte culture had become entirely negative (Figure 2).
Enrichment for CMV-specific CD4+ T cells following repetitive stimulation with CMV antigen.
CMV protein–specific CD4+ T cells generated by repetitive stimulation with CMV antigen were analyzed by intracellular IFN-γ staining by flow cytometry after stimulation with CMV antigen (representative example of a T-cell line analyzed after 2 stimulations) (A, day 0; B, day 14).
Enrichment for CMV-specific CD4+ T cells following repetitive stimulation with CMV antigen.
CMV protein–specific CD4+ T cells generated by repetitive stimulation with CMV antigen were analyzed by intracellular IFN-γ staining by flow cytometry after stimulation with CMV antigen (representative example of a T-cell line analyzed after 2 stimulations) (A, day 0; B, day 14).
Loss of alloreactivity after 4 repetitive in vitro stimulations with CMV antigen.
After 4 repetitive stimulations with CMV antigen, a complete loss of alloreactivity (black bars) was documented in the CMV-specific T-cell line generated from the patient's haploidentical (3 Ag-mismatched) father (patient no. 2). In addition, no autoreactivity (gray bars) was detected prior to and after stimulation with CMV antigen.
Loss of alloreactivity after 4 repetitive in vitro stimulations with CMV antigen.
After 4 repetitive stimulations with CMV antigen, a complete loss of alloreactivity (black bars) was documented in the CMV-specific T-cell line generated from the patient's haploidentical (3 Ag-mismatched) father (patient no. 2). In addition, no autoreactivity (gray bars) was detected prior to and after stimulation with CMV antigen.
Characteristics of patients and their response to cellular therapy
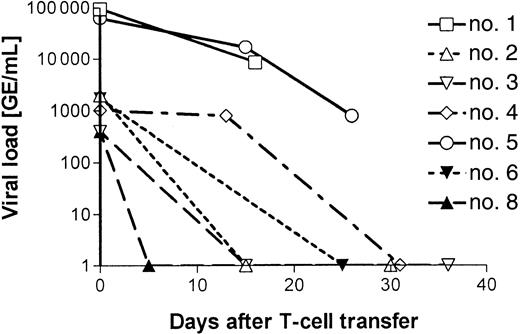
On the basis of our study design, 21 patients were eligible for cellular anti-CMV therapy. The criteria to be enrolled in the study were as follows: (1) a CMV-seropositive donor, (2) the presence of CMV DNA in the peripheral blood after a minimum of 4 weeks of antiviral chemotherapy, and (3) absence of CMV-specific in vitro proliferative responses. For 6 patients, we did not succeed in preparing the CMV-specific T-cell lines, either because we were not able to expand the cells to the numbers required by our study design (107/m2) or because, after 4 rounds of stimulation in vitro, little or no anti-CMV specificity could be detected. One patient died before the start of the cellular therapy, one patient was excluded because he developed an Epstein-Barr virus lymphoma that was treated by infusion of unmodified donor lymphocytes, and 5 patients were excluded because they responded to the continuing antiviral therapy. The characteristics of the remaining 8 patients are shown in Table 1. Two patients (nos. 6 and 7) underwent transplantation with stem cells from their HLA-identical sibling donor, 3 patients (nos. 1, 5, and 8) underwent transplantation with stem cells from HLA-matched unrelated donors, and the other 3 patients (nos. 2-4) received grafts that were mismatched for 1 to 3 antigens. With the exception of the patients undergoing transplantation with the marrow from an HLA-identical sibling donor, all patients received ATG as GvHD prophylaxis. Preemptive anti-CMV chemotherapy was started on the day of the second positive PCR result (median, 30 days; range, 16-65 days). After a median of 8 weeks (range, 4-10 weeks) of unsuccessful antiviral chemotherapy, patients received a single dose of 107 CMV-specific T cells/m2 at the time indicated in Table2, which was at a median of 120 days (range, 79-479 days) after SCT. At that moment, with 7 of 8 patients still receiving some form of immunosuppression, antiviral chemotherapy was stopped. After infusion of the T-cell lines, no pulmonary toxicity or other acute side effects occurred. In addition, in none of the patients was induction or aggravation of acute or chronic GvHD observed. Within 5 to 31 days, viral DNA could no longer be detected in 5 of 7 evaluable patients (Table 2 and Figure3). Patient no. 7 was antigenemia-positive at the time of T-cell transfer, but was not evaluated for CMV-DNA load. Following T-cell therapy, this patient became and remained negative by antigenemia assay. Patient no. 5 cleared a residual viral load (1000 virus copies/mL blood) only after a second infusion of CMV-specific T-cell lines given 40 days after the first infusion. Only in patient no. 1 did CMV DNA remain detectable throughout the entire period, which, after she had refused further treatment, resulted in a CMV encephalitis at 6 weeks after cellular therapy. Patient no. 8 again became PCR-positive after high-dose corticosteroid treatment for relapse of her original disease. However, all other patients remained CMV-negative during the entire period of the study.
Decrease in CMV load following T-cell therapy.
The viral load of all 7 patients with detectable CMV DNA at the time of T-cell transfer is presented (virus copies/mL blood). In 3 patients (nos. 1, 5, and 8), virus load increased again, but responded to a second T-cell infusion in patient no. 5 (Figure 5). The increase in virus load in the later posttransplant period in these 3 patients (nos. 1, 5, and 8) is described in detail in the Results and in Figure5 (patient no. 5) and Figure 6 (patient no. 8).
Decrease in CMV load following T-cell therapy.
The viral load of all 7 patients with detectable CMV DNA at the time of T-cell transfer is presented (virus copies/mL blood). In 3 patients (nos. 1, 5, and 8), virus load increased again, but responded to a second T-cell infusion in patient no. 5 (Figure 5). The increase in virus load in the later posttransplant period in these 3 patients (nos. 1, 5, and 8) is described in detail in the Results and in Figure5 (patient no. 5) and Figure 6 (patient no. 8).
Reconstitution of CMV-specific T cells after adoptive transfer of polyclonal CMV-specific T-cell lines
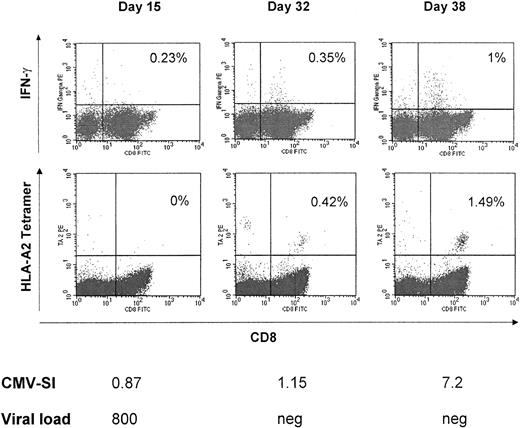
To assess the recovery of CMV-specific T-cell reactivity, we scheduled blood samples to be drawn every 14 days after adoptive immunotherapy. The following assays were performed: (1) lymphoproliferative responses after stimulation with CMV protein, (2) staining for intracellular IFN-γ after stimulation with CMV peptides in vitro (n = 7), (3) analysis of ex vivo binding HLA/peptide tetrameric complexes (n = 3), and (4) staining for intracellular IFN-γ after stimulation with CMV protein in vitro (n = 6). These data were correlated with the clinical course and the virus load in each patient. Table 3 shows that following T-cell therapy, 6 of 8 patients who all lacked anti-CMV reactivity before adoptive transfer responded in vitro to the CMV protein (stimulation index [SI] >3). Only a borderline response was observed in patient no. 7 (SI = 2.7), while patient no. 1 remained negative. Both patients had received CSA/MMF/prednisolone or FK506/prednisolone as intensified immunosuppression at the time of T-cell therapy. Interestingly, the viral load already decreased considerably before the CMV-specific T cells appeared in the blood. In patient no. 1, no CMV-specific proliferative T-cell response was detected in the blood at 20 days after cellular therapy, although the virus load had diminished to less than 10% of the level before infusion. This is also seen in Figure 4, which shows the anti-CMV proliferative response, the intracellular IFN-γ staining, as well as the tetramer analysis in patient no. 4 over time. At day 15 after infusion, the viral load had decreased from an initial 11 800 virus copies/mL blood to 800. However, at that time, very few CMV-specific T cells were detected in the blood. At day 32, when the virus had been cleared entirely, some IFN-γ–producing CD8+ as well as some tetramer-binding CD8+ T cells became apparent. However, even higher numbers of CMV-specific cells were present 6 days later. At day 38, more than 1% of the peripheral blood T cells were CMV specific, which was reflected by the significant increase of the in vitro proliferative response to the CMV protein. In this patient (no. 4), a T-cell clone generated from the peripheral blood after adoptive immunotherapy revealed specific killing of T2 cells pulsed with the CMV peptide NLVPMVATV in a 51Cr-release assay.
Reconstitution of CMV-specific T-cell responses and viral load after T-cell transfer.
In patient no. 4, after a high viral load at the time of T-cell transfer, already on day +15 viral load decreased to 800 viral copies/mL blood. At day 38, when the virus had been cleared entirely, reconstitution of CMV-specific lymphoproliferation and CMV peptide-specific A*0201-restricted CD8+ T cells was also shown by intracellular IFN-γ and tetramer staining.
Reconstitution of CMV-specific T-cell responses and viral load after T-cell transfer.
In patient no. 4, after a high viral load at the time of T-cell transfer, already on day +15 viral load decreased to 800 viral copies/mL blood. At day 38, when the virus had been cleared entirely, reconstitution of CMV-specific lymphoproliferation and CMV peptide-specific A*0201-restricted CD8+ T cells was also shown by intracellular IFN-γ and tetramer staining.
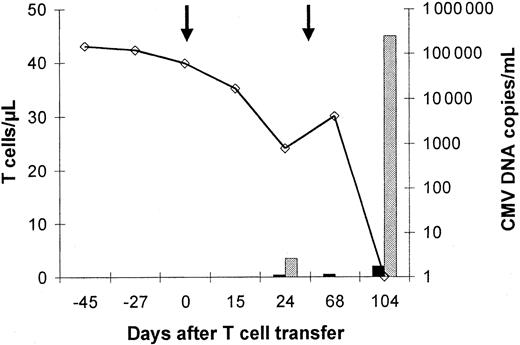
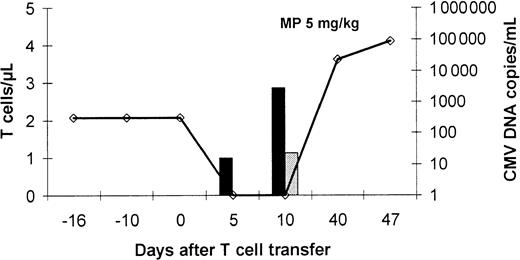
The relation between the increase of CMV-specific IFN-γ–producing CD4+ T cells, tetramer-binding CD8+ T cells, and anti-CMV immunity is further corroborated in Figure5. In patient no. 5, who needed a second infusion of CMV-specific T cells to clear the virus, very few specific T cells were detected at day 24 and day 68, when the virus was still present. However, at day 104, when the second infusion had cleared the virus entirely, the peripheral blood contained as many as 40 to 50 specific CD8+ T cells per microliter. Likewise, in patient no. 8, who cleared the virus already 5 days after infusion, very few specific cells were present in the blood (Figure6). Unfortunately, further analysis was impossible after the patient had received high-dose corticosteroid treatment for relapse of her original disease. This treatment decreased the number of lymphocytes in the peripheral blood, rapidly followed by the reappearance of the virus at very high copy numbers.
Virus load and reconstitution of CMV peptide-specific CD8+ and protein-specific CD4+ T cells.
This patient (no. 5) showed only a transient reduction in virus load and a transient occurrence of low numbers of CMV-specific CD4+ and CD8+ T cells 24 days after the first infusion of a CMV-specific T-cell line (1 × 107/m2). After a second T-cell infusion (1 × 107/m2), a rapid reduction of viral load was followed by an increase in the number of circulating CMV-specific CD4+ and CD8+ T cells and a permanent control of CMV infection. Peptide- and protein-specific T cells were analyzed by intracellular IFN-γ staining by flow cytometry after specific stimulation. Virus load was assessed by quantitative PCR (determined as virus copies/mL blood). The line represents the CMV-DNA load in the blood (virus copies/mL blood), the dark bars represent the number of circulating CMV protein-specific CD4+ T cells, and the gray bars represent the number/μL blood of circulating CD8+ T cells specific for the NLVPMVATV CMV peptide, which represents the immunodominant epitope in HLA A*0201-positive individuals. Arrows indicate T-cell infusions.
Virus load and reconstitution of CMV peptide-specific CD8+ and protein-specific CD4+ T cells.
This patient (no. 5) showed only a transient reduction in virus load and a transient occurrence of low numbers of CMV-specific CD4+ and CD8+ T cells 24 days after the first infusion of a CMV-specific T-cell line (1 × 107/m2). After a second T-cell infusion (1 × 107/m2), a rapid reduction of viral load was followed by an increase in the number of circulating CMV-specific CD4+ and CD8+ T cells and a permanent control of CMV infection. Peptide- and protein-specific T cells were analyzed by intracellular IFN-γ staining by flow cytometry after specific stimulation. Virus load was assessed by quantitative PCR (determined as virus copies/mL blood). The line represents the CMV-DNA load in the blood (virus copies/mL blood), the dark bars represent the number of circulating CMV protein-specific CD4+ T cells, and the gray bars represent the number/μL blood of circulating CD8+ T cells specific for the NLVPMVATV CMV peptide, which represents the immunodominant epitope in HLA A*0201-positive individuals. Arrows indicate T-cell infusions.
Limited antiviral effect of T-cell therapy in a patient receiving high-dose corticosteroids.
Patient no. 8 demonstrated an impressive expansion of CMV protein-specific CD4+ T cells after T-cell transfer associated with recovery of CMV peptide-specific CD8+ T cells already on day +10 after T-cell therapy and clearance of the viral DNA from the blood. After initiation of high-dose corticosteroid treatment for relapse of the underlying disease, a rapid increase of the virus load was observed. Peptide- and protein-specific T cells were analyzed by intracellular IFN-γ staining by flow cytometry after specific stimulation. Virus load was assessed by quantitative PCR (determined as virus copies/mL blood). The line represents the CMV-DNA load in the blood (virus copies/mL blood), the dark bars represent the number of circulating CMV protein-specific CD4+ T cells, and the gray bars represent the number/μL blood of circulating CD8+ T cells specific for the GPISHGHVLK CMV peptide, which represents the immunodominant epitope in HLA A*1101-positive individuals.
Limited antiviral effect of T-cell therapy in a patient receiving high-dose corticosteroids.
Patient no. 8 demonstrated an impressive expansion of CMV protein-specific CD4+ T cells after T-cell transfer associated with recovery of CMV peptide-specific CD8+ T cells already on day +10 after T-cell therapy and clearance of the viral DNA from the blood. After initiation of high-dose corticosteroid treatment for relapse of the underlying disease, a rapid increase of the virus load was observed. Peptide- and protein-specific T cells were analyzed by intracellular IFN-γ staining by flow cytometry after specific stimulation. Virus load was assessed by quantitative PCR (determined as virus copies/mL blood). The line represents the CMV-DNA load in the blood (virus copies/mL blood), the dark bars represent the number of circulating CMV protein-specific CD4+ T cells, and the gray bars represent the number/μL blood of circulating CD8+ T cells specific for the GPISHGHVLK CMV peptide, which represents the immunodominant epitope in HLA A*1101-positive individuals.
Discussion
T-cell immunity is crucial for protection against CMV disease. After the initial response, the virus becomes latent and may therefore reactivate upon immunodeficiency. Consequently, infection of either the patient or the donor with CMV represents a serious risk factor after SCT. It has been shown that more than half of the patients lacking detectable anti-CMV T-cell responses develop CMV disease.8Prophylaxis with ganciclovir, foscarnet, cidofovir, or a combination thereof may decrease the risk only temporarily. If during treatment, no CMV-specific T-cell immunity develops, the risk of CMV disease remains considerable.2,3 8
Pioneering work by Riddell et al and Walter et al has shown that adoptive transfer of CMV-specific CD8+ T-cell clones into patients at risk of CMV disease protected the patients from CMV-related complications.15 16 They demonstrated that when 1 to 2 × 109 CMV-specific CD8+ T cells were infused, these cells remained in the circulation for at least 8 weeks. Although the cells declined progressively in patients who did not develop a concomitant CMV-specific CD4+ T-helper response, prophylaxis against CMV infection was effective, as none of the patients developed a CMV viremia. Our approach differed in several aspects. We decided, instead of giving prophylactic anti-CMV cellular therapy, to treat patients with antiviral chemotherapy–resistant CMV viremia. To simplify our analysis, only patients who lacked a CMV-specific CD4+ T-helper response were enrolled in the study. In these patients, we have attempted to reconstitute the lacking immunity by the adoptive transfer of CMV-specific T-cell lines that had been established from the donor blood after stimulation with CMV protein. Although it is too early to draw a final conclusion based on the results in 8 patients, some of the findings on virus load, immunosuppression, and presence of CMV-specific T cells in the blood are noticeable.
First, although we transferred only 1 to 5 × 106CMV-specific CD4+ T cells, a rapid antiviral effect was seen in all patients. We believe that in most patients, this was the result of the immunotherapy rather than of a late effect of the antiviral chemotherapy that had been stopped prior to T-cell infusion. It is true that 5 of 21 patients who had been initially eligible for the study on the basis of their refractoriness to ganciclovir treatment did not enter the study because they responded just before the scheduled start of the immunotherapy. In addition, patient no. 7, whose virus load was already low during the period before immunotherapy, may essentially have responded to the ganciclovir treatment. However, in most patients, the CMV load did not decrease before 1 to 2 weeks after the CMV-specific cells had been infused; that is, with a considerable lag time after antiviral chemotherapy, or even later only after a second load of CMV-specific cells had been transferred.
Within 5 to 31 days after transfer, 5 of 7 patients evaluable by PCR assay had cleared the virus from their blood. Thereafter, the CMV-specific cells persisted at numbers comparable to those in healthy individuals, showing that a normal immune response with expansion of antigen-specific cells had taken place. This significant increase of CMV-specific T cells stands in contrast to the decline that is observed after the infusion of CD8+ cells only.15 16Therefore, we feel that although transfer of large amounts of CD8+ CMV-specific clones may protect the patient from CMV disease, adoptive-transfer protocols will be more effective when CD4+ antigen-specific T cells are given. It is interesting to note that although during the in vitro culture with CMV antigen, no detectable numbers of tetramer-binding CMV-specific CD8+ T cells had been generated, in half of the patients, the number of CMV pp65 peptide-specific CD8+ cells had increased significantly within 2 months after therapy. Furthermore, we were able to clone CMV-specific CD8+ T cells that specifically lysed NLVPMVATV peptide-pulsed T2 cells in vitro from the peripheral blood of patient no. 4. This strongly suggests that the transfer of CMV-specific CD4+ T cells may induce expansion of CMV-specific CD8+ CTLs from precursors that without T-cell help would not have been activated. Currently, we are investigating whether infusion of CD8+ CMV-specific T-cell lines together with the CD4+ cells will be able to increase the efficacy in patients with high virus loads or shorten the time of virus clearance in others.
We noticed that during the viremia, very few CMV-specific T cells were found in the blood. Given the low number of cells infused, this finding is not unanticipated. In kidney transplant recipients, the CMV-specific cells that arise at the onset of viremia disappear shortly thereafter, 1 month before the virus is cleared.26Probably, this peak of CMV-specific cells represents the freshly activated T cells that home to the infected tissues. After adoptive transfer of in vitro stimulated cells, homing to the tissues or possibly to the lymph nodes where the antigen is presented is most likely to be instantaneous. Thereafter, most CD4+CMV-specific T cells will migrate to the blood only when the viral replication has been stopped, a phenomenon previously observed during HIV infection.27
The efficacy of the therapy in the 2 patients with the highest virus load (>105 CMV-DNA copies/mL) was less. One patient needed a second infusion to clear the virus completely. In the other patient, who was infected with a ganciclovir-resistant CMV strain, the virus load decreased initially from more than 105 CMV-DNA copies/mL blood to less than 10 CMV-DNA copies/mL blood.4Thereafter, the patient, who refused further treatment, proceeded to a fatal CMV encephalitis. We think that also in this patient, a second infusion of CMV-specific T cells would have been capable of clearing the virus because there is no reason to believe that CTLs would be less efficient against a ganciclovir-resistant strain than against the unmutated virus. In fact, we believe that an infection with a drug-resistant strain would be one of the first indications for antiviral cellular therapy. However, it is conceivable that the limited efficacy of the therapy in this particular patient is due to the combination of the high virus load and the intensified immunosuppression that the patient received. It is obvious that any form of immunosuppression would have a negative effect on the further expansion of the CMV-specific T cells transferred. This was also evident in the patient who received high-dose corticosteroid treatment for relapse of her original disease. This patient cleared the virus rapidly but remained PCR-negative only until the immunosuppression was started.
In conclusion, our results demonstrate that anti-CMV cellular therapy represents a therapeutic option in viremic patients after SCT. We show that this can be performed by infusion of low numbers of CD4+ CMV-specific T-cell lines obtained after an in vitro culture of 3 to 4 weeks. These lines can be infused without side effects, even in patients undergoing transplantation with stem cells of a donor mismatched for 1 to 3 HLA antigens. The substantial gain in time as compared with the generation of CD8+ CMV-specific clones15 16 may allow a more flexible clinical response to CMV viremia in selected patients at high risk for CMV disease.
Supported by grants from the Deutsche Forschungsgemeinschaft (SFB 510, projekt B3); from the Federal Ministry of Education and Research (Fö. 01KS9602) and the Interdisciplinary Center of Clinical Research Tübingen (IZKF), project C2; from the Swiss National Science foundation (no. 31-53774.98); and from the foundation of Dr Henri Dubois-Ferrière-Dinu Lipatti.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Hermann Einsele, Medizinische Klinik, Abteilung II, Otfried-Müller Str 10, D-72076 Tübingen, Germany; e-mail: hneinsel@med.uni-tuebingen.de.