Human band 3 Walton is an AE1 mutation that results in the deletion of the 11 COOH-terminal amino acids of the protein and is associated with dominant distal renal tubular acidosis. The properties of band 3 Walton expressed with normal band 3 in the heterozygous mutant erythrocytes and the kidney isoform expressed in Xenopusoocytes and in the Madin-Darby canine kidney cell line were examined. The mutant erythrocytes have normal hematology but have reduced band 3 Walton content. Transport studies showed that erythrocyte band 3 Walton has normal sulfate transport activity, and kidney band 3 Walton has normal chloride transport activity when expressed inXenopus oocytes. The mutant protein is clearly able to reach the cell surface of erythrocytes and oocytes. In contrast, while normal kidney band 3 was expressed at the cell surface in the kidney cell line, the Walton mutant protein was retained intracellularly within the kidney cells. The results demonstrate that band 3 Walton is targeted differently in erythrocytes and kidney cells and indicate that the COOH-terminal tail of band 3 is required to allow movement to the cell surface in kidney cells. It is proposed here that the mutant band 3 gives rise to dominant distal renal tubular acidosis by inhibiting the movement of normal band 3 to the cell surface. It is suggested that this results from the association of the normal and mutant proteins in band 3 hetero-oligomers, which causes the intracellular retention of normal band 3 with the mutant protein.
Introduction
Erythrocyte band 3 (AE1) comprises 2 main domains: a 40-kd NH2-terminal cytoplasmic domain, which anchors the membrane to the red cell skeleton, and a 55-kd COOH-terminal membrane–associated domain, which carries out anion exchange.1 The short cytoplasmic COOH-terminal tail binds carbonic anhydrase.2 A form of band 3 (KB3), truncated at the NH2-terminus, is expressed in the basolateral membrane of the α-intercalated cells of kidney-collecting ducts and is involved in acid secretion.3 Mutations in theAE1 gene may, by interfering with renal excretion of hydrogen ions, cause distal renal tubular acidosis (dRTA),4-10 which is often complicated by nephrocalcinosis, hypokalemia, and metabolic bone disease.11 The mutation considered here (band 3 Walton) introduces a premature stop codon at amino acid 901 of band 3 and is associated with dominant dRTA.7
We have examined the expression and functional properties of band 3 Walton in the patients' erythrocytes and also in Xenopusoocytes and a mammalian kidney cell line. Our results suggest that the COOH-terminal 11 amino acids deleted in band 3 Walton do not affect the overall structure or anion transport activity of the protein. However, deletion of this region affects the surface membrane trafficking of band 3 much more severely in kidney cells than in erythrocytes orXenopus oocytes, suggesting that this sequence contains a signal that is important for the plasma membrane targeting of band 3 in kidney cells. We propose that the mutant band 3 gives rise to dominant dRTA by inhibiting the movement of normal band 3 to the cell surface. We suggest that this results from the association of the mutant and normal proteins in band 3 hetero-oligomers, which causes the intracellular retention of normal band 3 with the mutant protein.
Patients, materials, and methods
Patients
Two brothers (B1 and B2) with thirst, polyuria, and occasional renal colic since childhood were diagnosed at ages 37 and 25 years, respectively, as having dRTA on the basis of acidosis and hypokalemia (minimum urine pH while acidotic 6.8 and 6.7, plasma potassium 3.4-3.5 mM/L), gross nephrocalcinosis, but no bone disease. Red cell morphology was normal by Wright stain, but both patients have a tendency to erythremia (hemoglobin up to 188 g/L, hematocrit up to 0.53), a recognized complication of nephrocalcinosis from various causes.12 The parents of both patients are dead, and there are no known living blood relatives. Normal control blood samples were drawn and stored under the same conditions as the brothers' blood samples.
Erythrocyte membrane protein analysis
Membranes were prepared from untreated, chymotrypsin-treated, or 2 μM–tritiated 4,4′-di-isothiocyanato-1,2-dihydrostilbene-2,2′-disulfonic acid ([3H]H2DIDS)–labeled13erythrocytes and treated with peptide N-glycosidase5 or incubated in phosphate-buffered saline (PBS) on ice.2 The membrane proteins were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and treated for fluorography13 or immunoblotted with antiband 3 antibodies.5 14
Analysis of the AE1 gene
Genomic DNA was isolated from blood samples. The coding regions of exons 2 to 20 of the human AE1 gene15 were analyzed for single-strand conformation polymorphisms and by DNA sequencing as described previously.5 The coding region of exon 20 was cloned using the TA Cloning Kit (Invitrogen, Groningen, the Netherlands) and sequenced as above.
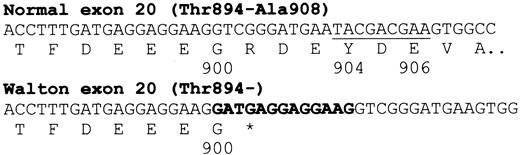
DNA sequence analysis of exon 4 showed both brothers to be heterozygous for the band 3 Memphis polymorphism Lys56Glu. Sequence analysis of cloned exon 20 showed the presence of the 13–base pair (bp) insertion after the first base of amino acid 900, as reported previously.7 In addition, a previously unreported deletion of 9 bp over the sequence that would have coded for amino acids Tyr904-Glu906 of normal band 3 was also present in band 3 Walton (Figure 1).
DNA sequence changes in band 3 Walton.
Shown is the 13-bp insertion in band 3 Walton (bold) and the 9-bp deletion (underlined) in band 3 Walton corresponding to Tyr904-Glu906 of normal band 3.
DNA sequence changes in band 3 Walton.
Shown is the 13-bp insertion in band 3 Walton (bold) and the 9-bp deletion (underlined) in band 3 Walton corresponding to Tyr904-Glu906 of normal band 3.
Preparation of mutant constructs and expression inXenopus oocytes
The cDNA clones encoding human KB3 (BSXG1.KB3) and glycophorin A (BSXG.GPA) have been described.5,16 The band 3 Walton deletion was made by polymerase chain reaction (PCR) using an internal band 3 primer (CTGGTCTTCATCCTCATAT) and a 3′ primer (GGCGGTAACCGCGGTCGACTCATCCTTCCTCCTCATCAAA) containing the deletion and a BstEII site. The cloned PCR fragment was cut and pasted into BSXG.KB3 using the BstXI-BstEII sites. The methods used for the preparation of cRNA, expression in oocytes, and assay of 36Cl− uptake have been described.5 16
Expression of normal KB3 and KB3 Walton in MDCK cells
Normal KB3 and KB3 Walton were cloned into the pcDNA3 vector to give the constructs pcDNA3-KB3 or pcDNA3-KB3 Walton, respectively, as follows: BSXGKB3 and BSXGKB3 Walton were used as templates in PCR reactions using the sense primer (CCACCATGGACGAAAAGAACCAG) that incorporated a Kozak sequence (CCACC) immediately before the initiator methionine codon in combination with an antisense primer containing the appropriate translation termination sequence using Expand Taq Polymerase (Roche, Mannheim, Germany). The PCR products were TOPO-TA–cloned into pcDNA3 as directed by the manufacturer (Invitrogen). The sequences of all constructs were confirmed by DNA sequencing.
Madin-Darby canine kidney (MDCK) cells were grown in Dulbecco modified Eagle medium containing 25 mM HEPES supplemented with 10% (vol/vol) fetal bovine serum (Gibco BRL, Paisley, United Kingdom). Cells were maintained at 37°C in 5% CO2–balanced air. MDCK cells were transfected with pcDNA3-KB3, pcDNA3-KB3 Walton, or empty pcDNA3 vector using Lipofectamine (Gibco BRL), and stable cell lines containing the constructs were cloned. MDCK cells were seeded at 5 × 104 cells per well and transfected with 1 μg of vector DNA per well. Twenty-four hours later, cells were selected with 600 μg/mL G418 (Sigma, St Louis, MO). After an additional 48 hours, the cells were washed with Hanks buffered saline and then cloned by serial dilution to allow the selection of single colonies. The MDCK clones expressing normal KB3 or the mutant KB3 were identified by immunostaining using antiband 3 antibodies. Positive clones were then expanded and continuously cultured in media containing G418.
The transfected cell lines were examined by immunofluorescence microscopy as follows: the cells were grown on polylysine-coated (Sigma) glass coverslips, washed with PBS (pH 7.4), and fixed in methanol:acetone 6:4 (vol/vol) at −20°C for 5 minutes. The cells were then washed twice with PBS (pH 7.4). Nonspecific binding sites were blocked with 4% bovine serum albumin for 15 minutes, and cells were then incubated with the antiband 3 monoclonal antibodies for 1 hour. The coverslips were washed with PBS 3 times and incubated with a 1:300 dilution of rabbit antimouse fluorescein isothiocyanate secondary (DAKO, Cambridgeshire, United Kingdom) for 1 hour in 4% rabbit serum. The cells were washed 3 times with PBS and mounted in Vectorshield (Vector Labs, Burlingame, CA) before visualization using a Leica TCS confocal microscope (Leica Microsystems, Milton Keynes, United Kingdom).
Results
The genetic basis of band 3 Walton has been described7: the band 3 cDNA of both brothers affected with dRTA was shown to be heterozygous for a 13-bp insertion, which results in a premature termination codon and the absence of 11 amino acids from the C-terminus of band 3 (Figure 1). In addition, band 3 Walton contains a 9-bp deletion on the 3′ side of the termination codon (Figure 1), which was not described in the original report.7
Studies on the red cells of the band 3 Walton patients
The erythrocytes of the 2 affected brothers, both heterozygous for band 3 Walton, had normal hematology.
Expression of band 3 Walton in the mutant red cell membranes.
We carried out further analysis of the AE1 gene and also found that both affected brothers are heterozygous for band 3 Memphis (Lys56Glu), a common, benign polymorphism17 that proved to be on the same allele as the Walton deletion (see below). The presence of the band 3 Memphis polymorphism is significant because the NH2-terminal chymotryptic fragments of band 3 with the Memphis polymorphism and normal band 3 have different mobilities (63 kd and 60 kd, respectively) on SDS-PAGE.13 This allowed resolution of the normal and mutant band 3 in the Walton red cells.
SDS-PAGE of membranes prepared from chymotrypsin-treated Walton erythrocytes confirmed that the 2 brothers had both the 63-kd band 3 Memphis polymorphism-containing NH2-terminal fragment and the normal 60-kd NH2-terminal fragment (Figure2A, tracks 2 and 3). Scanning densitometry showed that the abundance of the 63-kd band 3 Memphis–containing fragment was 62% ± 2% and 63% ± 3% (n = 4) of the normal band 3 fragment in the affected erythrocytes (Figure 2A, tracks 2 and 3), demonstrating that the band 3 Walton allele was present at a lower abundance than normal band 3 in the patients' red cells.
SDS-PAGE and immunoblotting of band 3 Walton.
(A-D) Membranes prepared and separated by SDS-PAGE. (A) Membranes from chymotrypsin-treated red cells stained for protein with Coomassie blue. (B) Membranes from band 6–depleted red cells2immunoblotted using sheep antihuman carbonic anhydrase II (Serotec, Oxford, England). The variation in intensity of the band reflects the different amounts of total protein loaded on each track. The total protein in each track was estimated from scans of the total spectrin bands in parallel gels stained with Coomassie blue. (C) Fluorograph of membranes from chymotrypsin-treated red cells labeled with 2 μM [3H]H2DIDS. (D) Membranes from chymotrypsin-treated red cells were digested with peptide N-glycosidase and immunoblotted using monoclonal antibodies. For panels A-D, track 1 represents control membranes; track 2, membranes from affected individual B1; track 3, membranes from affected individual B2. Arrows in panels A and C indicate the normal (60 kd) and band 3 Memphis (63 kd) NH2-terminal chymotryptic fragments, and arrows in panel D indicate the normal and band 3 Walton COOH-terminal chymotryptic fragments. (E) Location of monoclonal antibody epitopes in the C-terminal portion of normal band 3. Only the region of band 3 containing membrane spans 12 to 14 is shown. BRIC155 and BRIC130 are directed against the COOH-terminal cytoplasmic tail of band 3, while BRIC132 is directed against the final intracellular loop of band 3.
SDS-PAGE and immunoblotting of band 3 Walton.
(A-D) Membranes prepared and separated by SDS-PAGE. (A) Membranes from chymotrypsin-treated red cells stained for protein with Coomassie blue. (B) Membranes from band 6–depleted red cells2immunoblotted using sheep antihuman carbonic anhydrase II (Serotec, Oxford, England). The variation in intensity of the band reflects the different amounts of total protein loaded on each track. The total protein in each track was estimated from scans of the total spectrin bands in parallel gels stained with Coomassie blue. (C) Fluorograph of membranes from chymotrypsin-treated red cells labeled with 2 μM [3H]H2DIDS. (D) Membranes from chymotrypsin-treated red cells were digested with peptide N-glycosidase and immunoblotted using monoclonal antibodies. For panels A-D, track 1 represents control membranes; track 2, membranes from affected individual B1; track 3, membranes from affected individual B2. Arrows in panels A and C indicate the normal (60 kd) and band 3 Memphis (63 kd) NH2-terminal chymotryptic fragments, and arrows in panel D indicate the normal and band 3 Walton COOH-terminal chymotryptic fragments. (E) Location of monoclonal antibody epitopes in the C-terminal portion of normal band 3. Only the region of band 3 containing membrane spans 12 to 14 is shown. BRIC155 and BRIC130 are directed against the COOH-terminal cytoplasmic tail of band 3, while BRIC132 is directed against the final intracellular loop of band 3.
Immunoblotting was used to detect the COOH-terminal chymotryptic fragment of band 3 in peptide N-glycosidase–treated erythrocytes (Figure 2D). Monoclonal antibodies BRIC130 and BRIC155 both bind close to the region deleted in the COOH-terminal tail of band 3 Walton.14 The COOH-terminal chymotryptic band 3 fragments from each affected sibling gave a single band that showed reduced binding of BRIC155 (49% ± 7% and 54% ± 6%, n = 4, respectively) and of BRIC130 (50%, 45% and 40%, 47%, respectively, in 2 determinations; Figure 2D, tracks 2 and 3) compared with a normal control. This result indicates that the mutant protein does not react with BRIC155 and BRIC130. Monoclonal antibody BRIC132 binds the final intracellular loop of band 3,14 and should react with both the mutant and normal band 3. Immunoblotting of the affected samples with BRIC132 gave 2 closely spaced bands (Figure 2D, tracks 2 and 3). One band, with the same mobility as the band detected by BRIC155 and BRIC130, is derived from normal band 3. The other, faster-migrating, COOH-terminal chymotryptic fragment is derived from band 3 Walton. This band had Mr 1000 to 2000 less than the normal fragment, consistent with the presence of the 11-residue COOH-terminal truncation. The band 3 Walton fragments were present at 62% ± 6% and 60% ± 3% (n = 3) of the amounts of the normal fragment (Figure 2D, tracks 2 and 3), confirming that band 3 Walton is expressed at a lower level than the normal protein in the patients' red cells. This reduced expression relative to the normal band 3 is quantitatively similar to the reduction in expression of the band 3 Memphis polymorphism–containing isoform in the mutant erythrocytes (Figure 2A, tracks 2 and 3) and also demonstrates that the Walton deletion and Memphis mutation are on the same allele.
Anion transport properties of band 3 Walton.
DIDS titration of sulfate uptake into the erythrocytes of the 2 affected brothers (Figure 3A) showed that the sulfate uptake was 82% and 87%, respectively, of the control red cells, and the number of DIDS binding sites (which measures the number of band 3 molecules present18) was also reduced to 79% and 81%, respectively, of control red cells. This indicates that the specific sulfate transport activity per band 3 molecule in the mutant red cells is the same as normal red cells and shows that the specific sulfate transport activity of band 3 Walton is unchanged from normal. [3H]H2DIDS-labeling studies5 13were done on the affected erythrocytes (Figure 2C). Quantitation by scanning showed that the amount of [3H]H2DIDS covalently bound to band 3 Walton in the 2 samples was 64% and 60% and was 64% and 63%, respectively (2 determinations), of that bound to the normal band 3 in the mutant cells. This reduction in covalent [3H]H2DIDS binding to the mutant protein corresponds with the reduction in the amount of band 3 Walton protein compared with normal band 3 in the Walton red cells, as estimated by protein staining and from the anion transport data and shows that the specific H2DIDS binding per molecule to band 3 Walton is unchanged from normal.
Anion transport studies of band 3 Walton.
(A) DIDS titration of sulfate influx into erythrocytes. The number of DIDS binding sites was determined by titration of the influx of [35S]sulfate into erythrocytes, as described.5 18 Control erythrocytes (▾); B1 erythrocytes (○); B2 erythrocytes (●). Three replicate measurements were taken for each point. The results show the mean and the error bars the SD of the 3 replicate measurements. (B) Chloride influx intoXenopus oocytes. Oocytes were injected with 1.5 ng kidney band 3 cRNA (K) or kidney band 3 Walton cRNA (KW) with or without 0.15 ng glycophorin A cRNA (A). After 24 hours, Cl− influx over 1 hour was measured individually on 13 to 15 oocytes, in the presence or absence of 2 mM 4,4′-dinitrostilbene-2,2′-disulfonate. Control oocytes were injected with water. The results show the mean stilbene disulfonate–sensitive Cl− influx for each cRNA, and the error bars indicate the SEM for each sample.
Anion transport studies of band 3 Walton.
(A) DIDS titration of sulfate influx into erythrocytes. The number of DIDS binding sites was determined by titration of the influx of [35S]sulfate into erythrocytes, as described.5 18 Control erythrocytes (▾); B1 erythrocytes (○); B2 erythrocytes (●). Three replicate measurements were taken for each point. The results show the mean and the error bars the SD of the 3 replicate measurements. (B) Chloride influx intoXenopus oocytes. Oocytes were injected with 1.5 ng kidney band 3 cRNA (K) or kidney band 3 Walton cRNA (KW) with or without 0.15 ng glycophorin A cRNA (A). After 24 hours, Cl− influx over 1 hour was measured individually on 13 to 15 oocytes, in the presence or absence of 2 mM 4,4′-dinitrostilbene-2,2′-disulfonate. Control oocytes were injected with water. The results show the mean stilbene disulfonate–sensitive Cl− influx for each cRNA, and the error bars indicate the SEM for each sample.
Carbonic anhydrase binding to Walton erythrocyte membranes.
The amino acid residues D887ADD in the COOH-terminal tail of band 3 are critical for binding erythrocyte carbonic anhydrase II.19 Although these residues are retained in band 3 Walton, because of the proximity of the deletion to this region we examined whether carbonic anhydrase II binding was altered in band 3 Walton erythrocyte membranes. Immunoblotting studies of band 6–depleted erythrocyte membranes2 with anticarbonic anhydrase II antibodies were carried out (Figure 2B). Scanning of the immunoblots showed that, after correction for the different amounts of total membrane protein in each gel track, the 2 band 3 Walton samples contained 89% and 98%, respectively, of the carbonic anhydrase II present in control membranes. It is not clear why these values are slightly higher than the reduction in band 3 content in the Walton membranes. However, the data suggest that band 3 Walton binds carbonic anhydrase II like the normal protein.
Expression of the kidney isoform of band 3 Walton
KB3, the kidney isoform of band 3, lacks the N-terminal 65 residues of erythroid band 3. Because KB3 Walton is involved in kidney disease and because of the possibility that the combination of NH2- and COOH-terminal truncations in KB3 Walton may affect its functional properties, we investigated the anion transport activity and surface membrane expression of the mutant KB3 using theXenopus oocyte system and transfected kidney cell lines.
Expression of KB3 Walton in Xenopus oocytes.
A KB3 construct containing the band 3 Walton deletion was expressed inXenopus oocytes, allowing measurement of its chloride transport properties in the absence of normal band 3. The band 3–specific chloride transport induced by the mutant protein was similar to that induced by normal kidney band 3, in the presence or absence of glycophorin A (Figure 3B), confirming that the Walton deletion does not affect the chloride transport activity of the mutant KB3. The results also suggest that the kidney isoform of the mutant protein retains the ability to be expressed at the cell surface inXenopus oocytes as efficiently as normal KB3.
Expression of normal KB3 and KB3 Walton in kidney cells.
We examined the expression of normal KB3 and KB3 Walton in mammalian kidney cells using the MDCK cell line. Constructs expressing normal KB3 or KB3 Walton were transfected into the MDCK cells, and stable cell lines were cloned. The expression of band 3 in the transfected cell lines was examined by immunofluorescence and confocal microscopy using the monoclonal antiband 3 BRIC170, which reacts with both KB3 and KB3 Walton. Figure 4A shows that in cells transfected with the normal protein, KB3 was clearly located at the cell surface, although, as expected, some intracellular staining was also present (Figure 4A). In contrast, cells transfected with KB3 Walton did not show detectable expression of the mutant protein at the cell surface but was retained within the cell (Figure4C). The intracellular distribution of the KB3 Walton appeared subtly different from the intracellular fraction of normal band 3 in the 2 types of transfected cells (Figure 4A,C). The normal protein appeared to be more concentrated in a region close to the nucleus (possibly the Golgi system), while the mutant protein was distributed more evenly around the nucleus and throughout the cell interior, suggestive of endoplasmic reticulum. Similar experiments were done using the antiband 3 BRIC155, which reacts with normal KB3 but not the Walton protein. Cells expressing normal KB3 showed a pattern of cell surface and internal staining similar to that observed with BRIC170 (Figure 4B). As expected, no fluorescent staining was observed when BRIC155 was used on cells expressing KB3 Walton (Figure 4D). Neither BRIC170 nor BRIC155 antibodies gave any fluorescent staining on MDCK cells transfected with the empty vector alone (results not shown).
Expression of normal KB3 and KB3 Walton in transfected MDCK cells.
MDCK cells stably transfected with KB3 (A,B) or KB3 Walton (C,D) were treated for immunofluorescence using the antiband 3 Bric170 (A,C) or the antiband 3 Bric155 (B,D) and fluorescein isothiocyanate–conjugated rabbit antimouse secondary antibody as described in “Patients, materials, and methods.” The cells were imaged using confocal microscopy. Representative fields of view are shown. Bright field microscopy showed that all the cells in fields A through C were reactive with the antibody and fields C and D contained a similar number of cells. Bar = 10 μM.
Expression of normal KB3 and KB3 Walton in transfected MDCK cells.
MDCK cells stably transfected with KB3 (A,B) or KB3 Walton (C,D) were treated for immunofluorescence using the antiband 3 Bric170 (A,C) or the antiband 3 Bric155 (B,D) and fluorescein isothiocyanate–conjugated rabbit antimouse secondary antibody as described in “Patients, materials, and methods.” The cells were imaged using confocal microscopy. Representative fields of view are shown. Bright field microscopy showed that all the cells in fields A through C were reactive with the antibody and fields C and D contained a similar number of cells. Bar = 10 μM.
Discussion
Characterization of the erythroid isoform of band 3 Walton expressed in red cells
The 2 brothers affected with dRTA were both heterozygous for band 3 Walton, the deletion of the COOH-terminal 11 amino acids of band 3, but had red cells with normal hematology. The band 3 Walton allele was also found to carry the Lys56Glu Memphis polymorphism. This decreases the SDS-PAGE mobility of NH2-terminal fragments of band 3 and allowed us to distinguish the normal and mutant band 3 in the patients' red cells. In addition, the COOH-terminal truncation in band 3 Walton resulted in the loss of reactivity with 2 antiband 3 monoclonal antibodies, BRIC155 and BRIC130, which have epitopes in this region. The truncation also increased the SDS-PAGE mobility of the deglycosylated COOH-terminal chymotrypsin fragment of band 3 Walton and allowed it to be resolved from the corresponding fragment of normal band 3. Quantitation of the relative amounts of normal and mutant band 3 by Coomassie blue staining, immunoblotting, and the covalent binding of [3H]H2DIDS gave similar results, indicating that the band 3 Walton was present at a level of approximately 60% that of the normal band 3. The total amount of normal and mutant band 3 in the patients' red cells was approximately 80% of that found in normal red cells, a value confirmed by DIDS titration of sulfate transport into the mutant red cells.
Structure and function of red cell band 3 Walton and role of the C-terminal residues of band 3
Band 3 Walton has normal sulfate and chloride transport activity, showing that the COOH-terminal 11 amino acids are not involved in the process of monovalent or divalent anion transport. This result, together with the observation that the mutant protein shows normal [H2]DIDS binding, indicates that the COOH-terminal truncation does not significantly alter the structure of the remainder of the membrane domain of the protein. In addition, the normal shape and hematology of the mutant red cells confirms that the residues truncated in the Walton protein are not involved in interactions with the red cell skeleton, consistent with the lack of any other data indicating the involvement of this region of the protein in cytoskeletal interactions.
Carbonic anhydrase II binds band 3 at residues 887 to 890, which are adjacent to the truncation in band 3 Walton. It was of interest to examine whether the mutant protein bound carbonic anhydrase II because defective binding of carbonic anhydrase to band 3 in the basolateral membrane of the kidney intercalated cell could be a potential cause of the dRTA in these patients. However, the Walton red cell membranes contained amounts of carbonic anhydrase II similar to normal membranes, suggesting that this is not the case and that carbonic anhydrase II binding to band 3 Walton is normal.
The abundance of band 3 Walton in the mutant red cell membranes is only 60% that of the normal band 3 in the cells. The deletion of the COOH-terminal 11 amino acids clearly affects some stage in the biosynthetic pathway of band 3 in red cells. Either the deleted region contains trafficking signals that act to enhance red cell membrane expression or the deletion results in the exposure of other regions in the band 3 molecule, which has a deleterious effect on membrane expression. As discussed above, the normal transport activity of band 3 Walton suggests that it is not misfolded, so that is unlikely that the impaired red cell expression results from misfolding of the mutant protein.
Earlier expression studies of band 3 in Xenopus oocytes showed that COOH-terminal truncations of band 3 do not usually cause instability of the protein in oocytes, because large truncations from the COOH-terminus, including some of the membrane spans, had no effect on the stability or cell surface movement of the expressed truncated proteins in oocytes, as was the case with band 3 Walton.20,21 Interestingly, however, a 30-residue truncation at the COOH-terminus did result in the intracellular retention and enhanced turnover of this truncated protein.20
Expression of KB3 and KB3 Walton in kidney cells
Because the Walton mutation results in the defective acid secretion in the kidney associated with dRTA, it was of interest to examine the expression of normal KB3 and KB3 Walton in a kidney cell line. We prepared stable cloned cell lines of MDCK cells transfected with normal KB3. Immunofluorescence studies showed that the KB3 was clearly expressed predominantly at the cell surface. The intracellular KB3 observed, possibly in the Golgi or endosomes, most likely represents KB3 in transit from the endoplasmic reticulum to the plasma membrane or undergoing endocytosis from the plasma membrane. Successful cell surface expression of KB3 in a stable kidney cell line has not previously been reported.
In contrast, stable cloned cell lines transfected with KB3 Walton showed no evidence of cell surface expression of the protein. Instead, the mutant protein was retained within the cell, possibly in the endoplasmic reticulum, and showed an intracellular distribution different from that observed for intracellular normal KB3.
Glycophorin A is known to enhance the cell surface expression of band 3.16 Although erythrocytes contain glycophorin A, kidney cells do not.8 In one form of recessive dRTA due to the band 3 mutation Gly701Asp, the mutant protein has an absolute requirement for glycophorin A for movement to the cell surface. This can be demonstrated by expression of the mutant protein inXenopus oocytes.8,22 It is suggested that this mutant protein is retained internally and turned over in kidney cells but expressed normally in red cells.8 Band 3 Walton clearly shows a different behavior because it is expressed as efficiently as normal band 3 at the oocyte cell surface regardless of whether glycophorin A is absent or present.
Although the membrane expression of the erythroid isoform of B3 Walton was reduced compared with the normal protein in the mutant red cell membranes, this difference was slight compared with the difference in cell surface expression of the KB3 isoforms in kidney cells. The additional NH2-terminal 65 amino acids in erythroid B3 Walton may modulate the deleterious effects of the COOH-terminal truncation on cell surface expression. Interestingly, the difference in trafficking properties observed in the kidney cell line was not apparent when the normal and mutant KB3 proteins were expressed inXenopus oocytes. It is likely that each of the cell types has innate differences in trafficking mechanisms and these respond differently to the deletion present in the mutant protein. Other dominant dRTA band 3 mutations also show normal surface membrane incorporation in erythrocytes and Xenopusoocytes,5 and expression of these mutant proteins in kidney cells may be required to expose their defective function.
Our results suggest that the COOH-terminal 11 amino acids deleted in band 3 Walton do not affect the overall structure or anion transport activity of the protein. However, deletion of this region has a very much greater effect on the trafficking of band 3 in kidney cells than in erythrocytes or Xenopus oocytes, suggesting that this sequence contains a signal that is important for the plasma membrane targeting of band 3 in kidney cells. Cytoplasmic COOH-terminal tail regions like that deleted in band 3 Walton are involved in the trafficking of other transmembrane proteins.23-26 Tyrosine residues in these tail regions are known to be important for intracellular protein sorting and basolateral targeting,23 24 and Tyr904, which is deleted in band 3 Walton, may have this role in the kidney.
The present expression studies were carried out in unpolarized kidney cells. Polarization of kidney cells is obviously associated with changes in the endogenous trafficking pathways that result in the generation of discrete apical and basolateral membrane domains. Expression studies on the mutant protein will clearly be required in polarized kidney cells to confirm whether KB3 Walton is also retained within the cell or is mistargeted in polarized cells. Nevertheless, the present results demonstrate a clear difference in the localization of normal KB3 and KB3 Walton expressed in kidney cells. It provides the first direct evidence that a band 3 mutation may cause dominant dRTA by abnormal trafficking of the KB3 protein in kidney cells.
The acid secretion process in the distal nephron depends on the proper location of KB3 in the basolateral membrane of the α-intercalated cell. We suggested that dominant dRTA could result from several possible mechanisms that could disrupt the ionic gradients necessary for acid secretion by these cells. These mechanisms include the mistargeting of the mutant KB3 to the apical membrane in the intercalated cells or, also, the mutant protein altering the usual basolateral targeting of the normal protein as a result of the association of the 2 proteins in band 3 hetero-oligomers.5Our observation that the Walton KB3 remains intracellular and does not reach the plasma membrane of transfected kidney cells suggests that the latter mechanism may be responsible for the disease in this case and perhaps also some of the other mutations associated with dominant dRTA. The tight association of normal band 3 with Walton band 3 in band 3 hetero-oligomers may cause the normal kidney KB3 in the affected individuals to be retained with the Walton band 3 in the cell interior and not to reach the surface of the α-intercalated cells. This would result in the absence or gross deficiency of chloride-bicarbonate exchange activity in the basolateral membrane of these kidney cells, disrupting acid secretion in the distal nephron, and giving rise to dRTA.
We thank the Medical Research Council for providing an Infrastructure Award to establish the School of Medical Sciences Cell Imaging Facility, Dr Mark Jepson and Alan Leard for their assistance with the imaging studies, Prof D. Anstee for monoclonal antibodies, Prof N. L. Simmons for the MDCK cells, and Dr Mark Parker for help with Figure 2.
Supported in part by grants from the Wellcome Trust and the National Kidney Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Michael J. A. Tanner, Dept of Biochemistry, School of Medical Sciences, University of Bristol, University Walk, Bristol, BS8 1TD, United Kingdom; e-mail: m.tanner@bris.ac.uk.

![Fig. 2. SDS-PAGE and immunoblotting of band 3 Walton. / (A-D) Membranes prepared and separated by SDS-PAGE. (A) Membranes from chymotrypsin-treated red cells stained for protein with Coomassie blue. (B) Membranes from band 6–depleted red cells2immunoblotted using sheep antihuman carbonic anhydrase II (Serotec, Oxford, England). The variation in intensity of the band reflects the different amounts of total protein loaded on each track. The total protein in each track was estimated from scans of the total spectrin bands in parallel gels stained with Coomassie blue. (C) Fluorograph of membranes from chymotrypsin-treated red cells labeled with 2 μM [3H]H2DIDS. (D) Membranes from chymotrypsin-treated red cells were digested with peptide N-glycosidase and immunoblotted using monoclonal antibodies. For panels A-D, track 1 represents control membranes; track 2, membranes from affected individual B1; track 3, membranes from affected individual B2. Arrows in panels A and C indicate the normal (60 kd) and band 3 Memphis (63 kd) NH2-terminal chymotryptic fragments, and arrows in panel D indicate the normal and band 3 Walton COOH-terminal chymotryptic fragments. (E) Location of monoclonal antibody epitopes in the C-terminal portion of normal band 3. Only the region of band 3 containing membrane spans 12 to 14 is shown. BRIC155 and BRIC130 are directed against the COOH-terminal cytoplasmic tail of band 3, while BRIC132 is directed against the final intracellular loop of band 3.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/1/10.1182_blood.v99.1.342/6/m_h80121934002.jpeg?Expires=1765922424&Signature=EMBHp-0biGAiC7As7MFAsBtZ30MGncpQducGJitfrywqzqStK-MVYVnY7NHnHvw6Tvh-ZGaqGAc4jgc1OLx2oX~phxr0qxRP60Ygu2IKWm-DJIoBwPNnQDaZEkrAAZANq4Pr6XONLMkzV5Q3wE16jJgyRFBcHvjUv68j5vHDkhM5BfWWHF8xUS-dtbGSbqKbZbhVmtas31QDOv~6lUDv~gHmA8VECQoRNNvXQEToyJjAurFICV6~MEmbLowrfuJIjmjKkV9jSbjxvFef~tjCT5EZhnZcSOeX45SK6JIrQdZL8qXSa8ov-McVzwLH1e21N1ucUllzw-2-tch4xg0M2A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Anion transport studies of band 3 Walton. / (A) DIDS titration of sulfate influx into erythrocytes. The number of DIDS binding sites was determined by titration of the influx of [35S]sulfate into erythrocytes, as described.518 Control erythrocytes (▾); B1 erythrocytes (○); B2 erythrocytes (●). Three replicate measurements were taken for each point. The results show the mean and the error bars the SD of the 3 replicate measurements. (B) Chloride influx intoXenopus oocytes. Oocytes were injected with 1.5 ng kidney band 3 cRNA (K) or kidney band 3 Walton cRNA (KW) with or without 0.15 ng glycophorin A cRNA (A). After 24 hours, Cl− influx over 1 hour was measured individually on 13 to 15 oocytes, in the presence or absence of 2 mM 4,4′-dinitrostilbene-2,2′-disulfonate. Control oocytes were injected with water. The results show the mean stilbene disulfonate–sensitive Cl− influx for each cRNA, and the error bars indicate the SEM for each sample.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/99/1/10.1182_blood.v99.1.342/6/m_h80121934003.jpeg?Expires=1765922424&Signature=vUKk7Dr9sfDkqpgSWNi~VVmMFg~vc53S2ioq-LC-udCIS1nb0FkEQ46beDaEF7iqDgTlLomFh6c5PcoRkPxq7DaHglvjn6qiRrN4qkyigUWMaDJ4nPo2MLUzdudKDShzs-m3ZDV~VC70rzE0U8HO0WEwNZp-qE~axNd5b~4kJXZMJP57NeExBOPY44m5NDGeCJuiCAsfxZTn8nK31c~-Bfj2Nl82u7e8dZoul~CgLmkxpju49kTLQdExSQfAyFJBf8E45~y2YAY1OanvT0Gp4-ZPH4Cg2AyFszMU46nnOFaDKd4CjCY1B4DCU1aOheZnWfV9DpmplKe-Ts~caIG22g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

