Abstract
The importance of dendritic cells (DCs) for the initiation and regulation of immune responses not only to foreign organisms but also to the self has raised considerable interest in the qualitative and quantitative analysis of these cells in various human diseases.Plasmodium falciparum malaria is characterized by the poor induction of long-lasting protective immune responses. This study, therefore, investigated the percentage of peripheral blood DCs as lineage marker–negative and HLA-DR+ or CD83+cells in healthy children and in children suffering from acute malaria in Kilifi, Kenya. Comparable percentages of CD83+ DCs were found in peripheral blood of healthy children and children with malaria. However, the percentage of HLA-DR+ peripheral blood DCs was significantly reduced in children with malaria. The results suggest that a proportion of peripheral blood DCs may be functionally impaired due to the low expression of HLA-DR on their surface.
Introduction
Dendritic cells (DCs) are central for the induction of immune responses to pathogens infecting the human host because they transport antigen from the periphery to lymphoid tissue in response to inflammatory signals. Here, they activate naive T cells and boost memory responses.1 It is therefore not surprising that many pathogens have evolved mechanisms to subvert the function of this important cell. In endemic areas, infection with Plasmodium falciparum results in a wide range of outcomes from asymptomatic infection to severe disease and death. Acquisition of immunity is through exposure and therefore age-related and occurs only after several years of constant exposure to mild disease and probably never to asymptomatic infection.2,3 Among other mechanisms of immune evasion, P falciparum–infected erythrocytes (iRBCs) modulate the maturation and function of monocyte-derived DCs in vitro.4 Given the central role of DCs in the induction of immune responses, we analyzed the number of circulating peripheral blood DCs ex vivo in children with malaria and in healthy children in Kilifi, Kenya.5
Study design
Study population
The study was performed at the district hospital in Kilifi, Kenya, between July and September 1999. Children suffering from mild malaria were treated in the outpatient department, whereas children suffering from severe malaria who were prostrated, hypoglycemic, in respiratory distress, or severely anemic were admitted to the high-dependency ward.6 Children who also suffered from diseases other than malaria were excluded from analysis. A control group of afebrile children with negative blood films were recruited into the study during a cross-sectional survey. The study was approved by the National Ethical Committee, Kenya.
Analysis of blood samples
Blood (2 mL) was obtained from each child and a differential blood count performed. Interleukin-10 (IL-10) and tumor necrosis factor-α (TNF-α) in plasma were measured in triplicate by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's recommendations (R & D Systems, Abingdon, United Kingdom). The sensitivity of the assay was 15 pg/mL for TNF-α and for IL-10. Peripheral blood mononuclear cells (PBMCs) were separated and diluted to 1 × 107/mL. A proportion of cells was incubated for 24 hours in RPMI supplemented with 10% human AB serum, 2 mM glutamine, 50 μg/mL kanamycin, and 10 mM HEPES to induce surface expression of CD83.5,7 For the detection HLA-DR+ or CD83+ DCs, cells were incubated in duplicate with a cocktail of lineage-specific monoclonal antibodies against CD3 (UCHT1), CD19 (TÜK4), CD14 (HD37), CD16 (DJ130c), and CD56 (MOC1) for 30 minutes at 4°C, and bound antibodies were detected using R-phycoerythrin (RPE)–conjugated goat anti–mouse immunoglobulin antibody. Cells were incubated for 10 minutes in 10% mouse serum before addition of the fluorescein isothiocyanate (FITC)–conjugated isotype control, anti–HLA-DR (CR3/43), or anti-CD83 antibody (HB-15e, Pharmingen, Oxford, United Kingdom).5 For detection of CD34+progenitor cells, cells were stained with anti-CD45 (T29/33, 2B11) and FITC-conjugated anti-CD34 antibodies (BIRMA-K3).8 If not stated otherwise, antibodies were purchased from Dako (Ely, United Kingdom). At least 50 000 events were analyzed by flow cytometry (Epics XL, Beckmann-Coulter, High Wycombe, United Kingdom). Samples that had fewer than 0.02% positive events were regarded as being below the detection limit.
Statistical analysis
Parameters were compared between groups of children using a Mann-Whitney U test and correlated within groups of children using the Spearman rank correlation test. The agreement of the 2 detection methods for peripheral blood DCs was examined according to a method by Altman.9
Results and discussion
Circulating peripheral blood DCs were readily detected in the blood of healthy control children both as a population of HLA-DR+ DCs immediately after purification of PBMCs and as CD83+ DCs after a period of culture (Figure1). Both detection methods gave a similar overall distribution with a median percentage of 1.15% (interquartile range [IQR] 0.53-1.8) for HLA-DR+ DCs and a median percentage of 1.1% (IQR 0.48-1.8) for CD83+ DCs. Within the group of healthy children, we found good agreement between the 2 methods with a mean individual difference of 0.1% (SD 1%).9 The percentage of HLA-DR+ and CD83+ peripheral blood DCs correlated with each other (Spearman rho = 0.557, P < .01) but not with the age of the child (CD83+, rho = 0.085; HLA-DR+, rho = −0.015) or with white blood cells (CD83+, rho = 0.0.07; HLA-DR+, rho = 0.215; Table1). These results imply that both methods detected an overlapping population of DCs rather than distinct subsets. The methods did not distinguish between myeloid and plasmacytoid DCs because both populations constitutively express HLA-DR and, after a period of in vitro culture, CD83.10 11
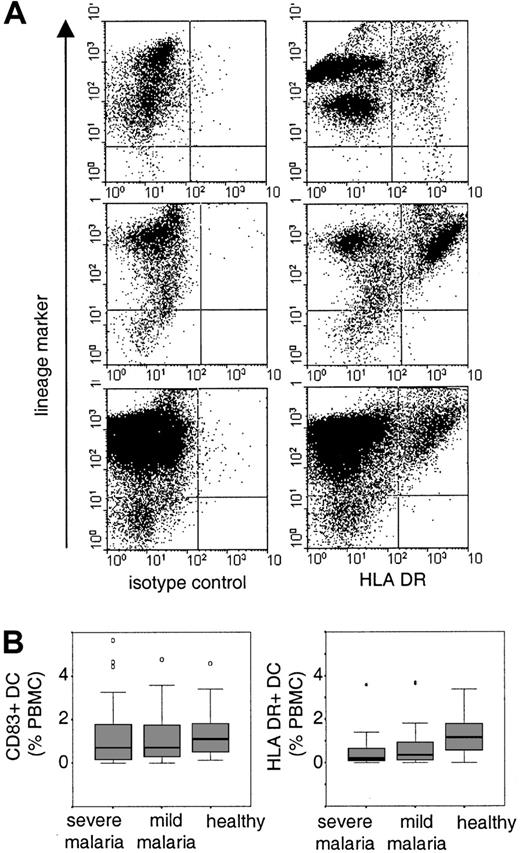
Identification and distribution of peripheral blood DCs.
(A) PBMCs were gated and at least 50 000 events were acquired per sample. B cells, T cells, monocytes, and natural killer cells were detected with a cocktail of lineage-specific antibodies (lineage-marker) in FL-2. DCs were detected as lineage marker–negative cells with an isotype control antibody or anti–HLA-DR antibody in FL-1. Shown are examples of HLA-DR+ DCs in one healthy child (upper panel) and in 2 children suffering from severe malaria (middle and lower panels). (B) The boxplots indicate median and 25% and 75% percentiles for the percentage of CD83+ cells or HLA-DR+ DCs in healthy children and in children with severe or mild malaria. Outliers are indicated by open circles and extreme values are indicated by stars.
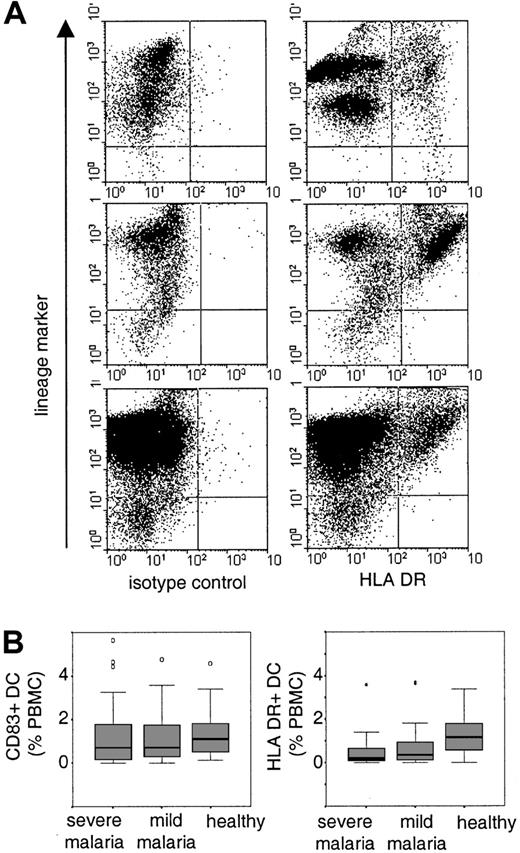
Identification and distribution of peripheral blood DCs.
(A) PBMCs were gated and at least 50 000 events were acquired per sample. B cells, T cells, monocytes, and natural killer cells were detected with a cocktail of lineage-specific antibodies (lineage-marker) in FL-2. DCs were detected as lineage marker–negative cells with an isotype control antibody or anti–HLA-DR antibody in FL-1. Shown are examples of HLA-DR+ DCs in one healthy child (upper panel) and in 2 children suffering from severe malaria (middle and lower panels). (B) The boxplots indicate median and 25% and 75% percentiles for the percentage of CD83+ cells or HLA-DR+ DCs in healthy children and in children with severe or mild malaria. Outliers are indicated by open circles and extreme values are indicated by stars.
We found no evidence for a difference between the percentages of CD83+ DCs in children with mild or severe malaria and healthy children (median and IQR: severe malaria 0.7%, 0.15-1.8,P = .153; mild malaria 0.7, 0.26-1.8,P = .276; Figure 1B). This observation is surprising because it has been reported that infectious diseases in both humans and mice induce the rapid migration of DCs into lymphoid tissue.5 12-15 During acute malaria, DCs may either not migrate into the spleen or they may be subjected to a higher turnover, which, in its steady state, would indicate normal percentages of circulating blood DCs. The latter hypothesis would be compatible with the observation that children with malaria were more likely to have detectable levels of circulating CD34+ progenitor cells (severe malaria, 77%; mild malaria, 82%; healthy children, 53%; Pearson χ2 test on 2 df = 11.93,P = .002).
However, the percentages of HLA-DR+ DCs were significantly lower in children with severe and mild malaria compared to healthy children (median and IQR: severe malaria 0.19%, 0.08-0.68,P < .0001; mild malaria 0.36, 0.07-1,P < .002; Figure 1B). This difference was independent of age (rho = 0.004) and parameters of malarial disease (Table 1) such as granulocyte counts (rho = −0.24), parasitemia (rho = 0.007), or the plasma concentration of IL-10 (rho = 0.044) and TNF-α (rho = −0.075). Furthermore, the mean fluorescence intensity of HLA-DR+ DC was reduced in children with malaria (median and IQR: malaria 89, 69-107; controls 249, 223-392;P < .0001). Given that CD83+ and HLA-DR+ DCs are an overlapping population, we suggest that a proportion of peripheral blood DCs in children with malaria is functionally impaired. This observation is consistent with our in vitro studies demonstrating that adhesion of infected erythrocytes to DCs modulates their maturation and function that may have consequences for the initiation and maintenance of immune responses.4Studies both in human falciparum malaria and in mouse malaria have shown that the induction of primary immune responses during acute malarial disease can be impaired.16-19 By contrast, antibody responses to a diverse number of parasite antigens are readily induced although only a subset of these seem to be associated with protection from disease.20-22 However, antibody responses to both nonvariant and variant-specific targets of the infecting parasite can be short-lived, indicating that here maintenance of humoral immune response may be disturbed.23 24
Clearly, our observation of altered DC phenotype in the peripheral blood of children with acute malaria warrants further investigation. Considering the high surface expression of CD36 on plasmacytoid DCs10 and the ability of iRBCs to bind to CD36, it will be of particular interest to differentiate quantitatively and qualitatively whether all DC subsets are affected similarly during acute malaria and after the onset of treatment. Such studies may contribute to the understanding of mechanisms of immune evasion by iRBCs.
This study is published with permission of the director of the Kenyan Medical Research Institute.
Supported by the Sir E. P. Abraham Trust, University of Oxford (B.C.U.) and the Kenyan Medical Research Institute. K.M. is a Wellcome Trust Senior Clinical Research Fellow.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Britta C. Urban, Weatherall Institute of Molecular Medicine, John Radcliffe Hospital, University of Oxford, Oxford OX3 9DS, United Kingdom; e-mail: burban@hammer.imm.ox.ac.uk.