Stem cell factor (SCF) binds the receptor tyrosine kinase c-Kit and is critical for normal hematopoiesis. Substitution of valine for aspartic acid 816 (D816V) constitutively actives human c-Kit, and this mutation is found in patients with mastocytosis, leukemia, and germ cell tumors. Immortalized murine progenitor cells (MIHCs) transduced with wild-type c-Kit proliferate in response to SCF, whereas cells expressing D816V c-Kit (MIHC-D816V) are factor-independent and tumorigenic. However, the mechanisms mediating transformation by D816V c-Kit are unknown. The objective of this study was to identify signaling components that contribute to D816V c-Kit–mediated transformation. SCF stimulates association of p85PI3K with phosphorylated tyrosine 721 of wild-type c-Kit. Phosphatidylinositol 3 kinase (PI3K) subsequently contributes to the activation of Akt and Jnks. In contrast, these studies demonstrated that the D816V c-Kit mutant was constitutively associated with phosphorylated p85PI3K, and, downstream of PI3K, Jnk 1 and Jnk 2 were activated but Akt was not. Interestingly, Erks 1 and 2 were not constitutively activated by D816V c-Kit. Thus, D816V c-Kit maintains the activity of PI3K but not of all signaling pathways activated by wild-type c-Kit. Further, all pathways downstream of PI3K are not constitutively active in MIHC-D816V cells. Studies with a PI3K inhibitor and D816V/Y721F c-Kit, a mutant incapable of recruiting PI3K, indicate that constitutive activation of PI3K through direct recruitment by D816V c-Kit plays a role in factor-independent growth of MIHC and is critical for tumorigenicity.
Introduction
c-Kit, also known as stem cell factor receptor or steel factor receptor, is a type 3 receptor tyrosine kinase (RTK) belonging to the platelet-derived growth factor receptor subfamily. The c-kit gene maps to the white spotting locus (W) in the mouse.1,2 The absence of functional c-Kit or its ligand, stem cell factor (SCF), causes perinatal lethality. Mutations resulting in reduced expression or function of either c-Kit or SCF result in macrocytic anemia, aberrations in pigmentation, decreased fertility, mast cell deficiency, reduction in gastrointestinal motility, and impairment of learning functions in the hippocampal region of the brain.3-6 Thus, c-Kit plays an important role in hematopoiesis, melanogenesis, and germ cell development and in the function of the gastrointestinal tract and the brain.
Diverse cellular responses associated with c-Kit include differentiation, proliferation, growth, survival, adhesion, and chemotaxis. These pleiotropic functions are mediated through the activation of multiple signal transduction pathways.7These include Src family kinases,8-13 Janus kinases, and signal transducer and activator of transcription (JAK/STAT) family members,14-20 phosphatidylinositol 3 kinase (PI3K),21-30 the Ras/Raf/Map kinase cascade,10,30-38 and phospholipase C.26,28 32 Because many of these proteins are the products of proto-oncogenes, alterations in regulation of these pathways could result in cellular transformation.
In the past decade, several gain-of-function c-Kit mutations have been identified. Many of these mutations are clustered in either the c-Kit juxtamembrane region or the second catalytic domain.39-42Factor-dependent cell lines expressing activated c-Kit mutants survive and proliferate in the absence of exogenous growth factors.43-45 Gain-of-function mutations in c-Kit have been implicated in human disease. Mutations in the juxtamembrane region are found in many human gastrointestinal stromal tumors.46Many patients with mastocytosis have a point mutation in codon 816 encoding a residue in the second catalytic domain of c-Kit.47-49 Some patients with germ cell tumors also have this mutation.50 Equivalent mutations have been identified in mast cell leukemia lines derived from rats and humans and in a murine mastocytoma cell line. Substitution of aspartic acid 814 in murine c-Kit (the equivalent of amino acid 816 in human c-Kit) with valine (D814V) or tyrosine (D814Y) results in constitutive increases in kinase activity and in alterations in substrate preference of this RTK.39,44,45,51,52 Cell lines expressing D816V human c-Kit or D814V murine c-Kit are tumorigenic in mice.43-45
Previous studies have demonstrated that D816V c-Kit is constitutively phosphorylated on tyrosine residues and that degradation of Shp1 is enhanced in cells expressing this mutant.39,44,45,51,52However, nothing is known about the signaling pathways mediating transformation by D816V c-Kit. To understand these events, we used immortalized murine myeloid progenitor cells (MIHC).53,54These cells are normally dependent on murine granulocyte-macrophage colony-stimulating factor (mGM-CSF) for survival and growth. Expression of wild-type c-Kit rendered them capable of growth in human SCF (huSCF), whereas D816V c-Kit led to factor-independent growth and tumorigenicity.43 We have now compared the signaling pathways constitutively activated by D816V Kit with those rapidly stimulated by SCF binding to wild-type c-Kit. We report that the 85-kd regulatory subunit of PI3K (p85PI3K) is constitutively tyrosine phosphorylated and associated with D816V Kit. The resultant activation of PI3K contributes substantially to factor-independent growth and plays a pivotal role in D816V c-Kit–mediated tumorigenicity.
Materials and methods
c-Kit expression constructs and generation of MIHC infectants
Wild-type human c-Kit cDNA encoding the isoform containing the juxtamembrane GNNK tetrapeptide sequence55 and cDNA encoding the constitutively active D816V mutant form39 were cloned into the retroviral expression vector pRUFneo as described previously.43 56AccIII and ApaI were used to excise a 666-bp fragment from human c-Kit cDNA in pcDNA3 that contained an A-to-T mutation at residue 2183, resulting in the substitution of phenylalanine for tyrosine at position 721 (Y721F). This fragment was subcloned into the corresponding site of the wild-type c-Kit cDNA in pRUFneo. The D816V/Y721F double mutant was generated by excising a 360-bp fragment from the D816V c-Kit construct with EspI and subcloning it into the corresponding site of the Y721F construct. Orientation of the insert was determined by polymerase chain reaction using primers 5'-GATGTGGGCAAGACTTCT-3' (sense) and 5'-AACTTAGAATCGACCGGC-3' (antisense). Both Y721F constructs were validated by sequencing across the manipulated regions.
The MIHC cell line was generated as described previously.43,53 In brief, murine fetal liver was infected with pRUF(CT3Myb) and maintained in mGM-CSF. In initial experiments, MIHCs were infected with wild-type or D816V c-Kit through coculture with the virus packaging line, psi2, transfected with pRUFneo containing the appropriate c-Kit construct. The derivation of these lines has been described in detail previously.43 In brief, infected MIHCs were initially selected using 1 mg/mL G418, and cells expressing wild-type c-Kit were then selected for surface expression of human c-Kit using a FACStarPLUS cell sorter (Becton Dickinson, Mountain View, CA). MIHCs transduced with wild-type human c-Kit (MIHC-Kit) were grown in Dulbecco modified Eagle medium (DMEM)–15% fetal calf serum (FCS) supplemented with huSCF (100 ng/mL). After initial selection in G418 (1 mg/mL), MIHCs expressing D816V c-Kit (MIHC-D816V) were selected for factor-independent growth through culture in DMEM–15% FCS. MIHC-Kit and MIHC-D816V cell lines were derived from mixed-cell populations and were not the result of single-cell cloning.
To compare the effect of the Y721F mutation on factor-independent proliferation and tumorigenicity of D816V c-Kit, MIHCs were infected with wild-type, Y721F, D816V, or D816V/Y721F c-Kit, selected in 1 mg/mL G418, and maintained in DMEM–15% FCS supplemented with mGM-CSF. All cell lines represent bulk populations of cells and were not derived from single-cell clones. In addition, data obtained with MIHCs infected with wild-type and D816V c-Kit selected through these means were identical to data obtained with MIHC-Kit selected using c-Kit expression and to MIHC-D816V cells selected using factor-independent growth.
Immunoprecipitation and immunoblotting
Cells were pelleted, washed once with ice-cold PBS, and lysed at 4 × 107/mL with ice-cold lysis buffer (1% Triton X-100, 10 mM Tris base, 50 mM NaCl, 5 mM EDTA, 5 mM tetrasodium pyrophosphate, 5 mM NaF, 5 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, pH 7.6). The protein concentration of the clarified lysates was determined using BCA (Pierce, Rockford, IL). One milligram cell lysate was immunoprecipitated as previously described.11 Immunoblotting was also performed as described.11
Cytokines, antibodies, and inhibitors
Murine GM-CSF and huSCF were purchased from PeproTech (Rocky Hill, NJ). In some experiments, mGM-CSF was produced in insect cells from baculovirus constructs kindly provided by Dr Andrew Hapel (John Curtin School of Medical Research, Canberra, Australia). The monoclonal antibody SR-1 was used for immunoprecipitation of c-Kit (a kind gift from Virginia Broudy, University of Washington). Polyclonal antibodies against Jnks (Jnks 1 and 2), Akt, Erk1, Erk2, c-Kit, and monoclonal antibodies to phosphorylated Erks 1 and 2 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies specific for phosphorylated Jnks and Akt were purchased from Promega (Madison, WI) and New England Biolabs (Beverly, MA), respectively. Antibody specific for p85 and antiphosphotyrosine monoclonal antibody used for immunoblotting (4G10) were obtained from Upstate BioTechnologies (Lake Placid, NY). The monoclonal antibody PY20 was used for immunoprecipitating phosphotyrosyl proteins (Leinco Technologies, St Louis, MO). The PI3K inhibitor wortmannin was obtained from Sigma (St Louis, MO).
PI3K assay
One milligram protein from clarified cell lysate was immunoprecipitated with SR-1, a c-Kit–specific monoclonal antibody, as described above. The immunocomplex was divided in half, and the portion to be used in the PI3K assay was washed 3 times with cold lysis buffer, twice with lithium HCl-Tris buffer (0.5 M LiCl, 100 mM Tris-HCl, pH 7.4) and twice with NTE buffer (100 mM NaCl, 10 mM Tris-HCl, 1 mM EDTA). Sonicated phospholipids containing phosphatidylinositol, phosphatidylserine, and phosphatidylcholine were added to the immunocomplex and incubated for 10 minutes on ice, and then reaction buffer (50 mM HEPES, pH 7.5, 20 mM MgCl2, 50 mM adenosine triphosphate (ATP), and 100 mM NaCl) with 370 μBq (10 μCi) [γ-32P]-ATP added to each tube. The kinase reaction was incubated for 20 minutes at room temperature and terminated with 1 N HCl. Phosphorylated lipids were extracted with methanol:chloroform (1:1). The organic phase was spotted on a thin-layer chromatography (TLC) plate. The TLC plate was precoated with 1.3% potassium oxalate and baked at 110°C for 3 hours before use. Chromatography was performed in a sealed glass chamber using solvent containing chloroform, methanol, and 2 M ammonia (9:7:2, vol/vol). PI3K activity was visualized with autoradiography and quantitated using a PhosphorImager Storm 860 (Molecular Dynamics, Sunnyvale, CA). In parallel with these studies, the other half of the c-Kit immunoprecipitates were resolved with sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), transferred to Immobilon, and immunoblotted with antisera specific for c-Kit. The relative level of c-Kit expression was quantitated using a densitometer. To calculate the amount of PI3K activity per equivalent amount of c-Kit, PI3K activity was divided by the amount of c-Kit in each immunoprecipitate.
Cell proliferation assays
For tritiated thymidine incorporation assays, cells were washed twice with DMEM and resuspended in DMEM plus 15% FCS at 105 cells/mL. Then 104 cells (100 μL) were seeded in each well of a 96-well microtiter plate. Cells were preincubated in the presence or absence of wortmannin at indicated concentrations for 4 hours before the addition of growth factors. This was followed by the addition of 100 μL media with or without huSCF at 100 ng/mL−1. Cells were incubated for 72 hours, and then 74 μBq (2 μCi) 3H-thymidine was added to each well. The plate was incubated for 5 hours and harvested with a cell harvester (Mach II M, Wallac, Gaithersburg, MD), and incorporation of3H-thymidine was measured by liquid scintillation counting. Inhibition of cell proliferation by wortmannin was calculated using untreated cells as control. Cell proliferation was also measured using the CellTitre Assay system (Promega) and was performed according to the manufacturer's instructions. Triplicate 0.1 mL cultures of MIHC, 5 × 103/well, were set up in 96-well microtiter trays in DMEM–20% FCS with or without 400 U/mL GM-CSF. Cell density was determined at time 0 and after 2, 4, and 6 days of culture.
Colony and tumorigenicity assays
Colony and tumorigenicity assays were carried out as previously described.43 Briefly, parental and Kit-expressing MIHCs were tested for colony formation by plating (in triplicate) 5 × 103 cells/mL culture containing 1.4% methylcellulose, 20% FCS, and 1% bovine serum albumin in Iscoves modified Dulbecco medium in the presence or absence of 400 U/mL GM-CSF. Colonies consisting of more than 50 cells were counted after 1 week. Statistical analysis involved the use of a Student ttest. Tumorigenicity was tested by injecting aliquots of 2 × 106 cells in serum-free DMEM subcutaneously into the flank of 8-week-old syngeneic (CBA strain) female mice. Mice were monitored for tumor development and killed for autopsy when tumors measured 5 to 10 mm in diameter.
Results
Expression of D816V c-Kit in MIHC results in factor-independent growth and constitutive tyrosine phosphorylation of multiple proteins, including c-Kit
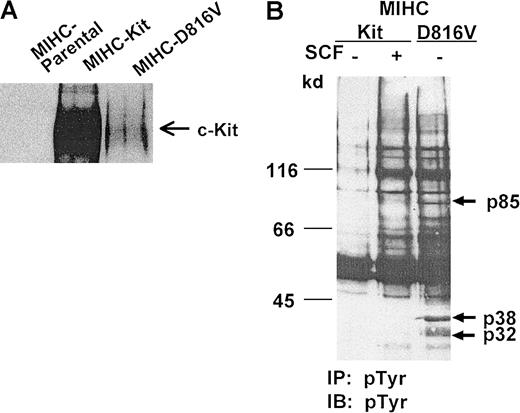
To study signaling pathways of D816V c-Kit in progenitor cells, we used MIHCs transduced with wild-type c-Kit or the D816V c-Kit mutant.43 The immunoblot in Figure1A demonstrates that MIHC infected with wild-type or D816V c-Kit express human c-Kit protein. In contrast, as expected, the MIHC parental cell line does not express human c-Kit. The low level of expression of the mutant c-Kit protein in MIHC is consistent with earlier observations by flow cytometry and immunohistochemistry43 and likely results, at least in part, from an increase in the rate of degradation of this activated RTK.51
Expression of D816V c-Kit in MIHC results in constitutive tyrosine phosphorylation of multiple proteins.
(A) Human c-Kit protein is expressed in MIHCs infected with wild-type or D816V c-Kit. Equivalent amounts of protein from clarified lysates of parental MIHCs or MIHCs infected with the indicated human c-Kit construct were immunoprecipitated with antibody specific for c-Kit, resolved with SDS-PAGE, transferred to Immobilon, and immunoblotted with antiserum specific for c-Kit. (B) Comparison of phosphotyrosyl proteins in MIHCs expressing wild-type or D816V c-Kit. Cells were incubated for 10 minutes with or without huSCF (100 ng/mL) as indicated, lysed, and immunoprecipitated (IP) with antibody specific for phosphotyrosine (PY-20). Samples were resolved using SDS-PAGE, transferred to Immobilon, and immunoblotted (IB) with antibody specific for phosphotyrosine (4G10). Phosphotyrosyl proteins were visualized with enhanced chemiluminescence (ECL).
Expression of D816V c-Kit in MIHC results in constitutive tyrosine phosphorylation of multiple proteins.
(A) Human c-Kit protein is expressed in MIHCs infected with wild-type or D816V c-Kit. Equivalent amounts of protein from clarified lysates of parental MIHCs or MIHCs infected with the indicated human c-Kit construct were immunoprecipitated with antibody specific for c-Kit, resolved with SDS-PAGE, transferred to Immobilon, and immunoblotted with antiserum specific for c-Kit. (B) Comparison of phosphotyrosyl proteins in MIHCs expressing wild-type or D816V c-Kit. Cells were incubated for 10 minutes with or without huSCF (100 ng/mL) as indicated, lysed, and immunoprecipitated (IP) with antibody specific for phosphotyrosine (PY-20). Samples were resolved using SDS-PAGE, transferred to Immobilon, and immunoblotted (IB) with antibody specific for phosphotyrosine (4G10). Phosphotyrosyl proteins were visualized with enhanced chemiluminescence (ECL).
Previous studies have shown that MIHC transduced with wild-type human c-Kit (MIHC-Kit) survive and proliferate in response to huSCF, whereas MIHC expressing D816V-Kit (MIHC-D816V) are capable of factor-independent growth (data not shown, 43). To determine whether the expression of D816V c-Kit induced constitutive activation of signaling pathways in MIHCs, we compared phosphotyrosyl proteins in cells expressing wild-type or D816V c-Kit. As expected, huSCF induced increases in tyrosine phosphorylation of numerous proteins in MIHC-Kit cells (Figure 1B). The level of constitutive tyrosine phosphorylation in MIHC-D816V cells was dramatically increased compared to that in unstimulated MIHC-Kit cells. Many of the phosphotyrosyl proteins in unstimulated MIHC-D816V cells comigrated with those induced by huSCF stimulation of MIHC-Kit cells. However, phosphotyrosyl proteins of 32 kd and 38 kd (designated by arrows in Figure 1B) were unique to MIHC-D816V cells. These results suggest that the expression of D816V c-Kit in MIHC may result in constitutive activation of signaling pathways similar to wild-type c-Kit and in some pathways that are potentially distinct.
One of the phosphotyrosyl proteins observed after stimulation of wild-type c-Kit with SCF measured approximately 145 kd, and a constitutively phosphorylated protein of the same size was also observed in cells expressing the D816V c-Kit mutant. One candidate protein of this size is the SCF receptor, c-Kit. Reprobing the blot in Figure 1B with c-Kit–specific antiserum indicated that a portion of the 145-kd band was c-Kit (data not shown). Additionally, immunoprecipitation of D816V c-Kit and subsequent immunoblotting with antibody specific for phosphotyrosine demonstrate that D816V c-Kit is constitutively phosphorylated on tyrosine (data not shown). These data are consistent with those of other laboratories.39,40,44,51 52
The 85-kd regulatory subunit of PI3K associates with D816V c-Kit and is constitutively phosphorylated on tyrosine residues
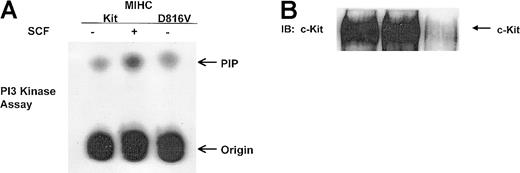
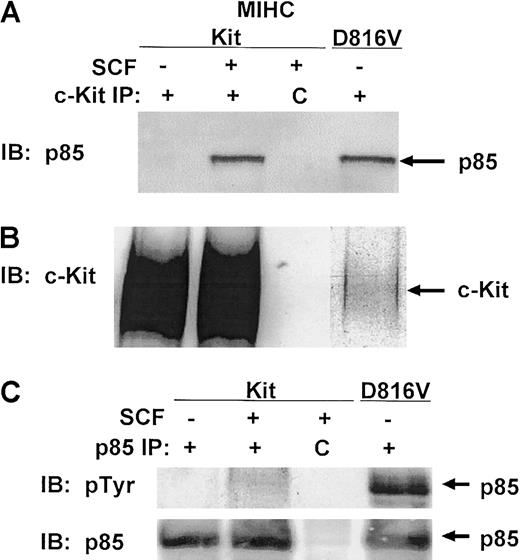
PI3K has been implicated in SCF-mediated adhesion, survival, proliferation, and differentiation in a variety of hematopoietic cell lineages.13,21,23-25,29 30 SCF induces the association of p85PI3K with wild-type c-Kit, and this induces tyrosine phosphorylation of p85PI3K in some cell lineages. Because one protein constitutively phosphorylated in MIHC-D816V cells measured approximately 85 kd (designated by the arrow in Figure 1B), we examined whether p85PI3K was constitutively associated with D816V c-Kit. Despite the low level of D816V c-Kit protein expressed in the MIHC-D816V cells (Figure 2B), p85PI3K was readily detectable in the D816V c-Kit complex (Figure 2A). Thus, p85PI3K was constitutively associated with D816V c-Kit, whereas p85PI3K associated with wild-type c-Kit only after stimulation with huSCF. Although low levels of D816V c-Kit protein were expressed in MIHC-D816V cells (Figure 2B), similar amounts of p85PI3K were associated with D816V and activated wild-type c-Kit. This suggests enhanced recruitment of p85PI3K to the D816V c-Kit receptor complex.
The 85-kd regulatory subunit of PI3K is constitutively associated with D816V c-Kit.
(A) p85PI3K coimmunoprecipitates with D816V c-Kit. Indicated cells were incubated in the presence or absence of huSCF for 10 minutes and lysed. Equivalent amounts of protein from the clarified lysates were immunoprecipitated with antisera specific for c-Kit or a matched control antibody, idicated by C. Immunoprecipitates were washed, proteins were resolved with SDS-PAGE and transferred to Immobilon, and the lower portion of the membrane was immunoblotted (IB) with antisera specific for p85PI3K. Proteins were visualized with ECL. (B) Comparison of the expression levels of wild-type and D816V c-Kit in MIHCs. The upper portion of the membrane from panel A was immunoblotted with antisera specific for human c-Kit. (C) The 85-kd regulatory subunit of PI3K is constitutively phosphorylated in cells expressing D816V c-Kit. Indicated cells were incubated in the presence or absence of huSCF for 10 minutes and lysed. Equivalent amounts of protein from the clarified lysates were immunoprecipitated with antisera specific for p85PI3K or a matched control antibody, indicated by C. Immunoprecipitates were washed, and proteins were resolved with SDS-PAGE and transferred to Immobilon. The membrane was immunoblotted (IB) first with antisera specific for phosphotyrosine (upper panel), then stripped and reprobed with antibody specific for p85PI3K (lower panel).
The 85-kd regulatory subunit of PI3K is constitutively associated with D816V c-Kit.
(A) p85PI3K coimmunoprecipitates with D816V c-Kit. Indicated cells were incubated in the presence or absence of huSCF for 10 minutes and lysed. Equivalent amounts of protein from the clarified lysates were immunoprecipitated with antisera specific for c-Kit or a matched control antibody, idicated by C. Immunoprecipitates were washed, proteins were resolved with SDS-PAGE and transferred to Immobilon, and the lower portion of the membrane was immunoblotted (IB) with antisera specific for p85PI3K. Proteins were visualized with ECL. (B) Comparison of the expression levels of wild-type and D816V c-Kit in MIHCs. The upper portion of the membrane from panel A was immunoblotted with antisera specific for human c-Kit. (C) The 85-kd regulatory subunit of PI3K is constitutively phosphorylated in cells expressing D816V c-Kit. Indicated cells were incubated in the presence or absence of huSCF for 10 minutes and lysed. Equivalent amounts of protein from the clarified lysates were immunoprecipitated with antisera specific for p85PI3K or a matched control antibody, indicated by C. Immunoprecipitates were washed, and proteins were resolved with SDS-PAGE and transferred to Immobilon. The membrane was immunoblotted (IB) first with antisera specific for phosphotyrosine (upper panel), then stripped and reprobed with antibody specific for p85PI3K (lower panel).
Constitutive association of p85PI3K and D816V c-Kit could lead to increases in tyrosine phosphorylation of p85PI3K. Indeed, MIHC-D816V cells contained an 85-kd protein constitutively phosphorylated on tyrosine residues (Figure 1B). Therefore, we were interested in determining whether p85PI3K was constitutively phosphorylated. Figure 2C (upper panel) demonstrates a heavily phosphorylated 85-kd protein in p85PI3Kimmunoprecipitates from unstimulated MIHC-D816V cells, whereas stimulation of MIHC-Kit cells did not induce a significant increase in tyrosine phosphorylation of p85PI3K. Immunoblotting with antibody specific for p85PI3K demonstrated that the phosphotyrosyl protein in MIHC-D816V cells was p85PI3K(Figure 2C, lower panel). Identical results were obtained with another myeloid progenitor cell line (FDCP1 cells) infected with the D816V human c-Kit mutant (data not shown).
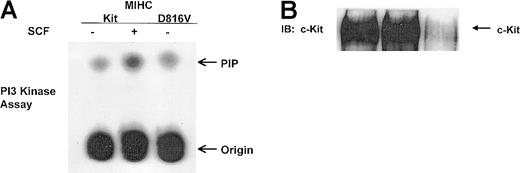
D816V c-Kit is constitutively associated with higher levels of PI3K activity than wild-type c-Kit
To assess whether constitutive association of p85PI3Kwith D816V c-Kit led to an increase in the receptor-associated PI3K activity, assays for PI3K activity were performed on the wild-type and mutant c-Kit receptor complexes. Cells were treated with or without huSCF and lysed, and clarified lysates were immunoprecipitated with antibody specific for c-Kit. Immunoprecipitates were divided in half and either used for PI3K assays (Figure3A) or assessed for expression of c-Kit protein by immunoblotting (Figure 3B). Stimulation with huSCF induced a 5-fold increase in the PI3K activity associated with c-Kit as compared to unstimulated controls, whereas the PI3K activity associated with D816V c-Kit was 1.5-fold greater than that associated with unstimulated wild-type c-Kit (Figure 3A). When the receptor-associated PI3K activity was normalized for the amount of c-Kit protein in each immunoprecipitate, there was greater than 10-fold more activity in the D816V c-Kit receptor complex than the same relative amount of unstimulated wild-type c-Kit. These data demonstrate that D816V c-Kit is constitutively associated with higher levels of PI3K activity than unstimulated wild-type c-Kit.
D816V c-Kit is constitutively associated with higher levels of PI3K activity than wild-type c-Kit.
Indicated cells were incubated in the presence or absence of huSCF for 10 minutes and lysed. Equivalent amounts of protein from the clarified lysates were immunoprecipitated with antisera specific for c-Kit and divided in half. One portion of the immunoprecipitate was used to assess c-Kit–associated PI3K activity (shown in panel A) as described in “Materials and methods.” The other portion of the c-Kit immunoprecipitate was resolved with SDS-PAGE, transferred to Immobilon, and immunoblotted (IB) with antisera specific for c-Kit (shown in panel B).
D816V c-Kit is constitutively associated with higher levels of PI3K activity than wild-type c-Kit.
Indicated cells were incubated in the presence or absence of huSCF for 10 minutes and lysed. Equivalent amounts of protein from the clarified lysates were immunoprecipitated with antisera specific for c-Kit and divided in half. One portion of the immunoprecipitate was used to assess c-Kit–associated PI3K activity (shown in panel A) as described in “Materials and methods.” The other portion of the c-Kit immunoprecipitate was resolved with SDS-PAGE, transferred to Immobilon, and immunoblotted (IB) with antisera specific for c-Kit (shown in panel B).
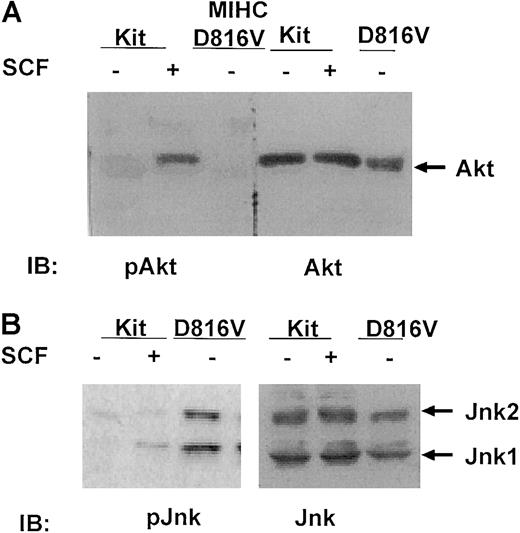
Jnk1 and Jnk2 are constitutively activated in cells expressing D816V c-Kit, but Akt is not
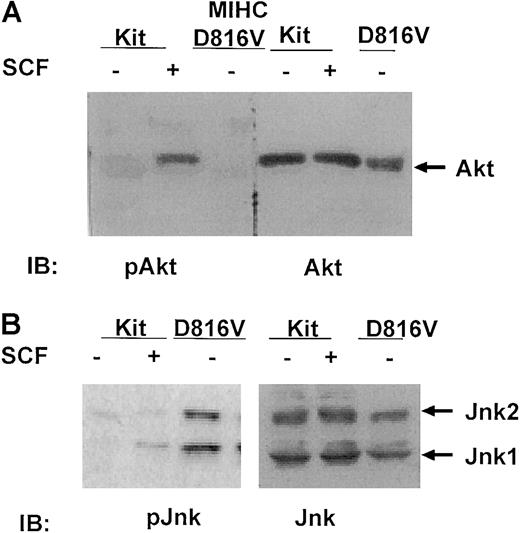
One downstream target for the PI3K pathway is the serine kinase Akt. To determine whether constitutive activation of PI3K by D816V c-Kit resulted in constitutive activation of Akt, lysates from MIHC-Kit or MIHC-D816V cells were immunoblotted with antibodies specific for phosphorylated or total Akt. Similar to the findings of Blume-Jensen et al,21 activation of wild-type c-Kit by SCF induced increases in serine phosphorylation of Akt (Figure4A, left panel). Surprisingly, Akt was not constitutively phosphorylated in cells expressing the D816V c-Kit mutant, though the amount of total Akt in the lysates was similar to that found in MIHC-Kit cells (Figure 4A, right panel).
Jnks are constitutively phosphorylated in MIHC expressing D816V c-Kit, but Akt is not.
(A) Akt is not activated in MIHC-D816V cells. Indicated cells were incubated in the presence or absence of huSCF for 10 minutes and lysed. Equivalent amounts of protein from the clarified lysates were resolved with SDS-PAGE, transferred to Immobilon, and immunoblotted (IB) with antisera specific for either phosphorylated or total Akt. Proteins were visualized with ECL. (B) Jnks are constitutively activated in MIHC-D816V cells. Equivalent amounts of protein from clarified lysates were resolved with SDS-PAGE, transferred to Immobilon, and immunoblotted (IB) with antisera specific for either phosphorylated or total Jnks. Proteins were visualized with ECL.
Jnks are constitutively phosphorylated in MIHC expressing D816V c-Kit, but Akt is not.
(A) Akt is not activated in MIHC-D816V cells. Indicated cells were incubated in the presence or absence of huSCF for 10 minutes and lysed. Equivalent amounts of protein from the clarified lysates were resolved with SDS-PAGE, transferred to Immobilon, and immunoblotted (IB) with antisera specific for either phosphorylated or total Akt. Proteins were visualized with ECL. (B) Jnks are constitutively activated in MIHC-D816V cells. Equivalent amounts of protein from clarified lysates were resolved with SDS-PAGE, transferred to Immobilon, and immunoblotted (IB) with antisera specific for either phosphorylated or total Jnks. Proteins were visualized with ECL.
Another family of signaling components activated by SCF, downstream of the PI3K pathway, are Jnks.13,57,58 Structure–function studies with mutant forms of c-Kit and dominant-negative Jnk mutants have demonstrated that this family of serine–threonine kinases plays a role in SCF-mediated growth of mast cells.13 To determine whether Jnks were constitutively activated in cells expressing D816V c-Kit, lysates from MIHC-Kit or MIHC-D816V cells were immunoblotted with antibodies specific for either phosphorylated or total Jnks (both are specific for Jnks 1 and 2). These studies indicate that stimulation of MIHC-Kit cells with SCF induced increases in the phosphorylation of Jnk1, whereas both Jnk1 and Jnk2 were constitutively phosphorylated in MIHC-D816V cells (Figure 4B, left panel). Total Jnk protein levels in these 2 cell lines were similar (Figure 4B, right panel). These data demonstrate that Jnk 1 and Jnk 2, but not Akt, are constitutively activated in cells expressing D816V c-Kit.
Erk1 and Erk2 are not constitutively active in cells expressing D816V c-Kit
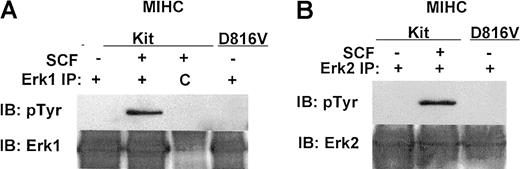
Previous studies have shown that wild-type c-Kit activates extracellular signal-regulated protein kinases (Erks).10,22,30 32-38 To determine whether signaling pathways other than PI3K are constitutively activated by D816V c-Kit, we compared phosphorylation of Erk 1 and Erk 2 in cells expressing D816V c-Kit with huSCF-induced activation in MIHC-Kit cells. Although huSCF induced increases in tyrosine (Figure5A,B) and serine–threonine phosphorylation (data not shown) of both Erk1 and Erk2 in cells expressing wild-type c-Kit, no constitutive phosphorylation of Erk1 or Erk2 was detected in MIHC-D816V cells (Figure 5A,B). These results demonstrate that Erk1 and Erk2 are not constitutively activated in cells expressing D816V c-Kit. Thus, all the signaling pathways used by wild-type c-Kit are not constitutively activated by D816V c-Kit.
Erk1 and Erk2 are not constitutively phosphorylated in MIHCs expressing D816V c-Kit.
Cells were incubated in the presence or absence of huSCF for 10 minutes and lysed. Equivalent amounts of protein from the clarified lysates were immunoprecipitated (IP) with antisera specific for Erk1 or Erk2 (panels A and B, respectively) or with a matched control antibody, indicated by C. Immunoprecipitates were washed, and proteins were resolved with SDS-PAGE, transferred to Immobilon, and immunoblotted (IB) with antisera specific for phosphotyrosine (A and B, upper panels). Proteins were visualized with ECL. Blots were then stripped and reprobed with antisera specific for Erk1 (A, lower panel) or Erk2 (B, lower panel).
Erk1 and Erk2 are not constitutively phosphorylated in MIHCs expressing D816V c-Kit.
Cells were incubated in the presence or absence of huSCF for 10 minutes and lysed. Equivalent amounts of protein from the clarified lysates were immunoprecipitated (IP) with antisera specific for Erk1 or Erk2 (panels A and B, respectively) or with a matched control antibody, indicated by C. Immunoprecipitates were washed, and proteins were resolved with SDS-PAGE, transferred to Immobilon, and immunoblotted (IB) with antisera specific for phosphotyrosine (A and B, upper panels). Proteins were visualized with ECL. Blots were then stripped and reprobed with antisera specific for Erk1 (A, lower panel) or Erk2 (B, lower panel).
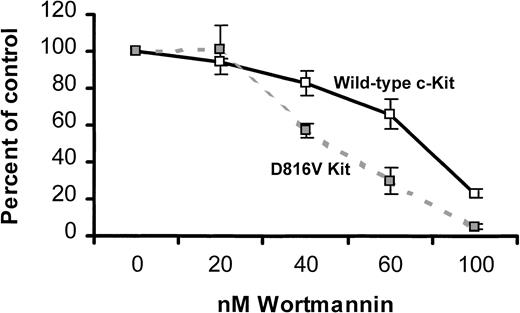
Factor-independent growth of MIHC expressing D816V c-Kit is more sensitive to a PI3K inhibitor than SCF-induced growth of cells expressing wild-type c-Kit
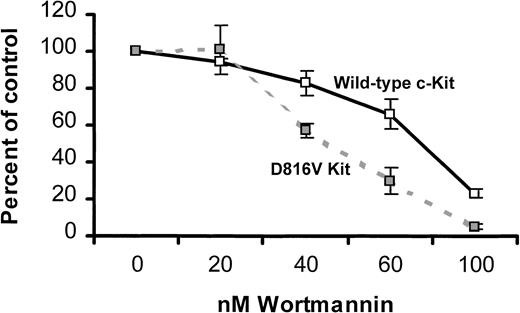
Because activated PI3K was constitutively associated with D816V c-Kit, we were interested in defining the role of PI3K in the factor-independent proliferation mediated by D816V c-Kit. One approach to this question was to compare the effect of a PI3K inhibitor on growth of MIHCs expressing either wild-type or D816V c-Kit. Cells were preincubated with the indicated concentrations of wortmannin, cultured in either media alone (MIHC-D816V cells) or huSCF (MIHC-Kit cells), and assessed for tritiated thymidine incorporation. At 40, 60, and 100 nM of wortmannin, factor-independent growth of MIHC-D816V cells was inhibited to a greater extent than SCF-induced growth of MIHC-Kit cells (Figure 6). Identical results were obtained with LY2940002, another PI3K inhibitor (data not shown). These data suggest that factor-independent growth mediated by D816V c-Kit is more dependent on PI3K than SCF-induced growth mediated by wild-type c-Kit.
Factor-independent proliferation of MIHCs expressing D816V c-Kit is more sensitive to a PI3K inhibitor than SCF-induced proliferation of cells expressing wild-type c-Kit.
Cells were incubated for 4 hours at the indicated concentration of wortmannin and then cultured for 72 hours in growth media alone (MIHC-D816V) or supplemented with 100 ng/mL huSCF, as indicated (MIHC-Kit). Samples were pulsed with 3H-thymidine and harvested 4 hours later as described in “Materials and methods.” Mean percentage control and standard deviation were calculated using the 3H-thymidine incorporation of triplicate samples in the presence and absence of wortmannin. These data are representative of the results of 4 independent experiments.
Factor-independent proliferation of MIHCs expressing D816V c-Kit is more sensitive to a PI3K inhibitor than SCF-induced proliferation of cells expressing wild-type c-Kit.
Cells were incubated for 4 hours at the indicated concentration of wortmannin and then cultured for 72 hours in growth media alone (MIHC-D816V) or supplemented with 100 ng/mL huSCF, as indicated (MIHC-Kit). Samples were pulsed with 3H-thymidine and harvested 4 hours later as described in “Materials and methods.” Mean percentage control and standard deviation were calculated using the 3H-thymidine incorporation of triplicate samples in the presence and absence of wortmannin. These data are representative of the results of 4 independent experiments.
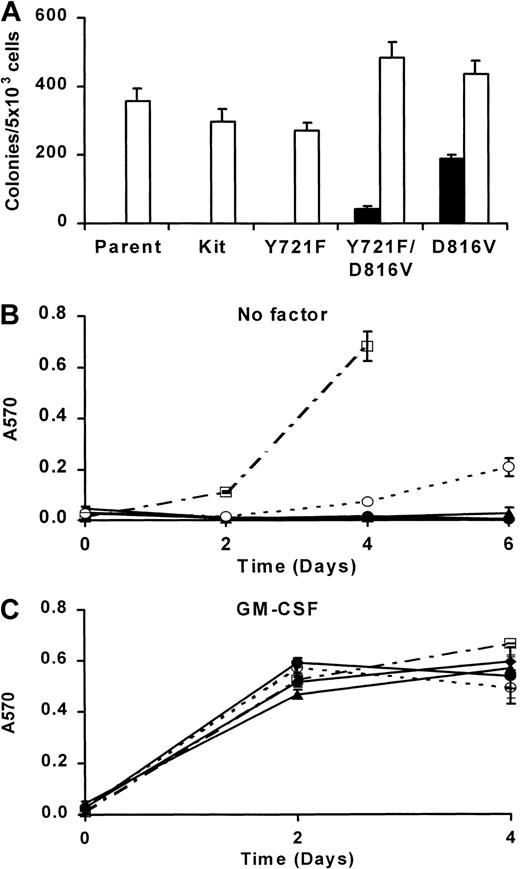
Mutation of the PI3K binding site reduces factor-independent growth of MIHCs expressing D816V c-Kit
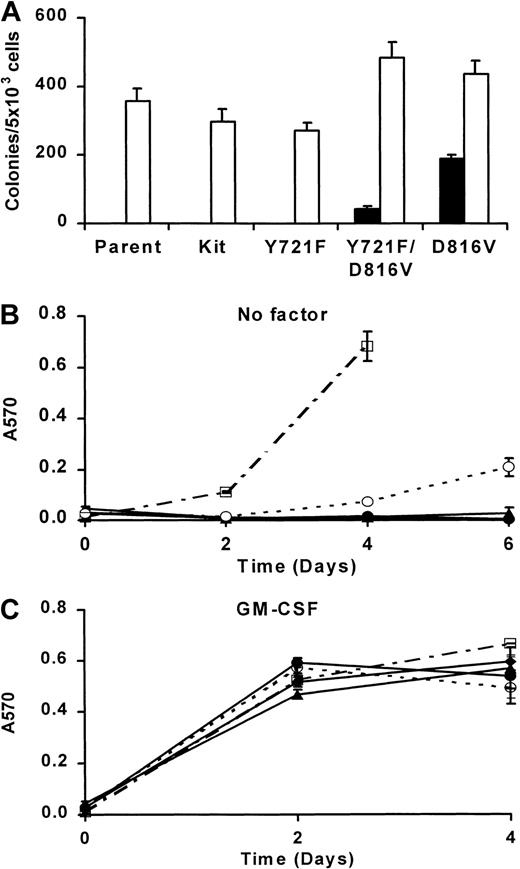
Low concentrations of wortmannin used in these experiments suggest that growth inhibition results from the specific inhibition of PI3K activity. However, it is possible that wortmannin inhibits other signaling components involved in cell growth or survival. To further examine the role of PI3K in D816V c-Kit–mediated transformation of MIHC, the direct association of PI3K and D816V c-Kit was eliminated by the substitution of phenylalanine for tyrosine 721, the binding site for p85PI3K. The corresponding mutation has been shown to eliminate direct recruitment of PI3K to murine wild-type c-Kit.29 Similarly, mutation of tyrosine 721 abrogated the capacity of D816V c-Kit to associate with p85PI3K (data not shown). MIHCs were infected with D816V/Y721F, D816V, Y721F, or wild-type c-Kit, selected using G418 resistance, and maintained in mGM-CSF. In contrast, MIHC-D816V cells used in the previous experiments had been selected using G418 resistance and factor-independent growth. Cell surface expression of normal and mutant c-Kit was examined by flow cytometry. These studies indicated that MIHCs infected with either D816V or D816V/Y721F c-Kit express similar amounts of the receptor on the cell surface, albeit at lower levels than wild-type c-Kit (data not shown).
MIHCs expressing c-Kit, or the c-Kit mutants, were next assessed for their capacity for factor-independent growth in semisolid medium and liquid culture. Figure 7A shows the number of colonies formed after 1 week in methylcellulose culture in the presence or absence of mGM-CSF. As expected, MIHC-D816V cells were able to form colonies in the absence of exogenous factor. Expression of the D816V/Y721Fc-Kit mutant, lacking the PI3K recruitment site, in MIHCs resulted in significantly fewer colonies in the absence of factor than D816V c-Kit (P < .00001). MIHCs expressing these mutant receptors did not differ in their ability to form colonies in the presence of mGM-CSF (P > .1). Parental MIHCs and MIHCs expressing wild-type or Y721F c-Kit were unable to form colonies in the absence of factor even though they formed colonies in mGM-CSF.
Mutation of the binding site for PI3K reduces factor-independent growth of MIHC-D816V.
(A) Factor-independent colony formation of MIHC-D816V/Y721F is reduced in comparison with MIHC-D816V. Parental MIHCs and MIHCs expressing wild-type c-Kit, Y721F c-Kit, D816V/Y721F c-Kit, or D816V c-Kit were tested for the ability to form colonies in methylcellulose culture in the presence (■) and absence (▪) of GM-CSF. Colonies comprising more than 50 cells were scored after 1 week. Data shown are means ± SEM derived from 2 independent experiments, each carried out in triplicate. (B) Factor-independent growth of MIHC-D816V/Y721F is reduced in liquid culture compared with MIHC-D816V. MIHCs infected with the indicated c-Kit mutant seeded at a density of 5 × 104/mL in media without exogenous growth factors on day 0 were assessed for the total number of cells on days 2, 4, and 6 by CellTitre Assay. Mean ± SEM is shown from a representative experiment of 3. Because of overgrowth, several points on day 6 are not shown in panels B and C. ♦ indicates parent; ●, wild-type Kit; ▴, Y721F Kit; ○, D816V/Y721F Kit; ■, D816V Kit. (C) GM-CSF–induced growth is not altered in MIHCs expressing c-Kit mutants. Proliferation assays were performed as described in panel B, except in the presence of murine GM-CSF.
Mutation of the binding site for PI3K reduces factor-independent growth of MIHC-D816V.
(A) Factor-independent colony formation of MIHC-D816V/Y721F is reduced in comparison with MIHC-D816V. Parental MIHCs and MIHCs expressing wild-type c-Kit, Y721F c-Kit, D816V/Y721F c-Kit, or D816V c-Kit were tested for the ability to form colonies in methylcellulose culture in the presence (■) and absence (▪) of GM-CSF. Colonies comprising more than 50 cells were scored after 1 week. Data shown are means ± SEM derived from 2 independent experiments, each carried out in triplicate. (B) Factor-independent growth of MIHC-D816V/Y721F is reduced in liquid culture compared with MIHC-D816V. MIHCs infected with the indicated c-Kit mutant seeded at a density of 5 × 104/mL in media without exogenous growth factors on day 0 were assessed for the total number of cells on days 2, 4, and 6 by CellTitre Assay. Mean ± SEM is shown from a representative experiment of 3. Because of overgrowth, several points on day 6 are not shown in panels B and C. ♦ indicates parent; ●, wild-type Kit; ▴, Y721F Kit; ○, D816V/Y721F Kit; ■, D816V Kit. (C) GM-CSF–induced growth is not altered in MIHCs expressing c-Kit mutants. Proliferation assays were performed as described in panel B, except in the presence of murine GM-CSF.
In liquid culture, MIHC-D816V cells were able to proliferate in the absence of factor (Figure 7B). Mutation of tyrosine 721 in D816V c-Kit decreased factor-independent growth but did not abolish it. This was consistent with the results of the colony assays. Parental MIHCs or MIHCs expressing wild-type c-Kit or Y721F c-Kit did not grow in the absence of factor. All cell lines grew to a similar extent in mGM-CSF (Figure 7C). These results show that the lack of direct PI3K recruitment reduced, but did not abolish, factor-independent survival and growth, and they suggest that constitutive recruitment of PI3K directly by D816V c-Kit is not the sole mechanism mediating factor independence.
Mutation of the PI3K binding site eliminates tumorigenicity of MIHCs expressing D816V c-Kit
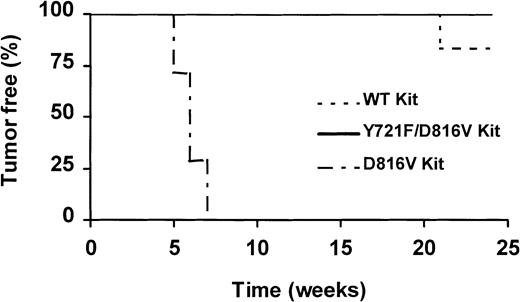
To examine the role of direct PI3K recruitment by D816V c-Kit in the tumorigenicity of this mutant, we assessed the capacity of MIHC expressing D816V/Y721F c-Kit to form tumors in mice. Aliquots of 2 × 106 cells of either parental MIHCs or MIHCs expressing wild-type c-Kit, Y721F c-Kit, D816V/Y721F c-Kit, or D816V c-Kit were injected subcutaneously into syngeneic (CBA) mice. Mice from each group were monitored for the development of tumors for up to 24 weeks. All mice injected with D816V c-Kit–expressing cells developed tumors 5 to 7 weeks after the injection date (Figure8). Cells derived from the tumors were capable of growth in the absence of factor in vitro. One of the 6 mice injected with MIHC wild-type c-Kit developed a tumor after 21 weeks. No tumors were identified in the other groups, including MIHC-D816V/Y721F, over the 24-week period. On termination of the experiment, no weight differences were observed, and dissection revealed no evidence of tumors or other abnormalities in any of the mice. These results demonstrate that although D816V/Y721F c-Kit can confer some factor-independent growth in vitro, MIHCs expressing this mutant do not form tumors in vivo.
Mutation of the binding site for PI3K abolished tumor formation induced by MIHC-D816V.
Syngeneic (CBA) mice were injected subcutaneously with 2 × 106 MIHCs expressing D816V c-Kit (n = 7), D816V/Y721F c-Kit (n = 7), wild-type c-Kit (n = 6), Y721F c-Kit, or parental MIHC (n = 6; not shown). All cells were maintained in GM-CSF before injection. The experiment was terminated after 24 weeks with no tumors identified in mice injected with MIHC parent, MIHC-Y721F, or MIHC-D816V/Y721F.
Mutation of the binding site for PI3K abolished tumor formation induced by MIHC-D816V.
Syngeneic (CBA) mice were injected subcutaneously with 2 × 106 MIHCs expressing D816V c-Kit (n = 7), D816V/Y721F c-Kit (n = 7), wild-type c-Kit (n = 6), Y721F c-Kit, or parental MIHC (n = 6; not shown). All cells were maintained in GM-CSF before injection. The experiment was terminated after 24 weeks with no tumors identified in mice injected with MIHC parent, MIHC-Y721F, or MIHC-D816V/Y721F.
Discussion
Mutation of aspartic acid 816 activates human c-Kit and generates an oncogenic form of this RTK. Expression of either D816V human c-Kit or the comparable mutation in murine c-Kit (D814V or D814Y) in factor-dependent hematopoietic cell lines results in factor-independent survival and proliferation (data not shown).43-45 In addition, cell lines expressing this activated c-Kit mutant form tumors when injected into mice.43-45 Previous studies have shown that both D814Y and D814V murine c-Kit are constitutively phosphorylated on tyrosine residues.39,44,45,51,52 Mutant c-Kit protein is rapidly degraded and, concomitant with this, degradation of the protein tyrosine phosphatase Shp1 is also accelerated.51 52 However, little else is known about the signaling pathways activated by D816V c-Kit, and the signaling pathways mediating cellular transformation by this oncogenic mutant are unknown.
SCF induces association of the SH2 domain of p85PI3K with tyrosine 721 of human c-Kit (tyrosine 719 of murine c-Kit) and increases in receptor-associated PI3K activity. Some, but not all, laboratories have also reported that SCF induces small increases in tyrosine phosphorylation of p85PI3K.22,26-30Mutation of this docking site abrogates SCF-induced PI3K activation and results in reductions in SCF-induced survival, growth, differentiation and adhesion.21,24,25,30 Alterations in the trafficking of Y719F murine c-Kit have also been reported.23 The important role of PI3K in SCF-mediated responses led us to examine the role of this pathway in D816V c-Kit signaling. We have shown that p85PI3K is more heavily phosphorylated in cells expressing D816V c-Kit than in SCF-stimulated cells expressing wild-type c-Kit (Figure 2). D816V c-Kit is constitutively associated with p85PI3K and the level of receptor-associated PI3K activity is 1.5-fold greater than that associated with unstimulated, wild-type c-Kit (Figure 2, 3). When normalized for the equivalent amount of c-Kit protein, D816V c-Kit is associated with more than 10-fold more PI3K activity than wild type c-Kit. This appears to occur primarily through an increase in recruitment of p85PI3K to the c-Kit receptor complex. One explanation for the enhanced recruitment and phosphorylation of PI3K by D816V c-Kit may be the accelerated degradation of Shp1.52 Recent reports indicate that Shp1 regulates PI3K phosphorylation and activity.59 60
We have assessed the role of constitutive recruitment of PI3K directly by D816V c-Kit in factor-independent growth and tumorigenicity. MIHCs expressing D816V/Y721F c-Kit, a mutant unable to associate with p85PI3K, did not form tumors when injected into mice (Figure 8). In addition, the factor-independent growth of cells expressing this mutant was reduced, but not eliminated, in colony and proliferation assays (Figure 7). Residual factor-independent growth might have been caused by the indirect activation of PI3K.61 Although these studies cannot rule out the possibility that other signaling components associated with tyrosine 721 are involved in factor-independent growth mediated by D816V c-Kit, the inhibition of PI3K activity with wortmannin reduced factor-independent growth of cells expressing D816V c-Kit to a greater extent than SCF-induced growth of MIHC c-Kit cells (Figure 6). In total, these studies demonstrate that PI3K plays a role in factor-independent growth of myeloid progenitor cells expressing D816V c-Kit, though signaling pathways in addition to PI3K may also be involved. Our data further demonstrate that PI3K is required for the tumorigenicity of D816V c-Kit in the MIHC murine myeloid progenitor cell line.
SCF-induced activation of PI3K through wild-type c-Kit results in the activation of Akt.21 The important role of Akt in cell survival, in conjunction with the enhanced survival of MIHC-D816V cells reported by Ferrao et al,43 led us to examine the activation of Akt in cells expressing D816V c-Kit. As expected, SCF activated Akt in cells expressing wild-type c-Kit. Surprisingly, Akt was not constitutively activated in cells expressing D816V c-Kit, despite the constitutive activation of PI3K (Figure 4A). These findings suggest that the enhanced survival of MIHCs expressing D816V c-Kit is independent of Akt. Several recent papers have suggested the existence of Akt-independent pathways that transmit survival signals in a cell-specific manner.62-66 Alternatively, Akt may be transiently activated by D816V c-Kit, but the activity may be down-regulated over time.
Previous studies have found that SCF activates Jnks and that this plays a role in SCF-mediated growth of mast cells.13,57,58 In agreement with these findings, we show SCF-induced activation of Jnk1 in MIHCs expressing wild-type c-Kit. Because PI3K contributes to SCF-induced activation of Jnks, we examined Jnk activity in MIHC-D816V cells.13 Interestingly, both Jnk1 and Jnk2 were heavily phosphorylated in cells expressing D816V c-Kit (Figure 4B). Constitutive activation of Jnks has been implicated in the transformation by Met, the epidermal growth factor receptor, Bcr-Abl, and Src.67-70 Thus, constitutive activation of PI3K by the D816V c-Kit mutant may promote cellular transformation through the activation of Jnk family members.
Wild-type c-Kit activates multiple signal transduction pathways, including Src family members, PI3K, the JAK/STAT pathway, and the Map kinase cascade. Although Erk1 and Erk2 are downstream of the PI3K pathway in some receptor-mediated signaling pathways, there is strong evidence that the PI3K pathway is independent of Erk1 and Erk2 in c-Kit–mediated signal transduction.13,30,58 Our data demonstrate that the D816V c-Kit mutant constitutively activates PI3K but not all pathways used by wild-type c-Kit. SCF stimulation of wild-type c-Kit induced phosphorylation of both Erk1 and Erk2; however, neither of these Map kinase family members was constitutively phosphorylated in cells expressing D816V c-Kit (Figure 5). Wild-type c-Kit activates the Ras-Raf-MAP kinase cascade through multiple mechanisms, including the recruitment of Shc, Grb2, and Shp2 to the receptor complex. Recent studies by Lennartsson et al10demonstrate a critical role for autophosphorylated tyrosine 568 of human c-Kit in Src-family–mediated phosphorylation of Shc and subsequent activation of Erks. One explanation for the absence of constitutive Erk activation by D816V c-Kit could be decreased recruitment of the adaptor molecules required for activation of this pathway. Indeed, the D814Y mutation alters the autophosphorylation sites of murine c-Kit and substrate specificity in vitro.52 Alternatively, D816V c-Kit may transiently activate the Map kinase cascade but may not maintain this activity over time. Other differences in signaling pathways constitutively activated by D816V c-Kit, compared to those transiently activated by wild-type c-Kit, were also observed. Phosphotyrosyl proteins of 32 kd and 38 kd were present in cells expressing D816V c-Kit but not in SCF-stimulated cells expressing wild-type c-Kit (Figure 1B). The identity of these proteins is unknown but is under investigation.
In summary, we demonstrate that the D816V c-Kit mutant constitutively associates with PI3K and activates the PI3K pathway. We also show that the PI3K pathway is critical in D816V c-Kit–mediated tumorigenicity and contributes to factor-independent proliferation of MIHCs expressing this mutant. Because this mutation is found with high frequency in patients with mastocytosis and core binding factor leukemias, these findings may provide insight into designing strategies for treatment of these diseases.
We thank Dr Douglas Lowy for review of this manuscript and for many helpful discussions. We also thank Dr Paul Randazzo for assistance with PI3K assays. We thank Drs Thomas Gonda, Andrew Hapel, and Virginia Broudy, respectively, for the kind gifts of retroviral constructs (pRufCT3myb and pRufneo), mGM-CSF baculovirus constructs, and SR-1 antibody.
Supported in part by grant 960549 from the National Health and Medical Research Council of Australia. S.Y. is the recipient of an Australian Postgraduate Research Scholarship.
R.C. and S.Y. contributed equally to this work.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Diana Linnekin, Basic Research Laboratory, Division of Basic Sciences, Bldg 567, Rm 226, National Cancer Institute-Frederick, Frederick, MD 21702; e-maildlinnekin@mail.ncifcrf.gov.