The study by Bellamy et al1 has numerous critical limitations. First, the authors incorrectly assume that abnormal localization of immature precursors (ALIPs) can be identified in the bone marrow clot section, which has no bony trabeculae. It should be pointed out that ALIPs are collections of immature myeloid precursors in the central, intertrabecular region of the bone marrow trephine biopsy section.2,3 Moreover, this is a feature of microarchitecture disorganization in the bone marrow biopsy sections in myelodysplastic syndromes (MDSs), which also show disorganized erythroid and megakaryocytic precursors in the trabecular region.3,4 In a normal human bone marrow biopsy, myeloid precursors are near the trabecular region, and erythroid and megakaryocytic precursors are in the intertrabecular region.5 This physiologic cell distribution may be reversed in the bone marrow biopsy of cases with MDS.4 The data presented by Bellamy et al1 show only myeloid and monocytoid blasts in a clot section without bony trabeculae, which is typical of blasts seen in a bone marrow aspirate. Moreover, it is reported that most myeloid blasts and myeloid leukemia cell lines express vascular endothelial growth factors (VEGFs) and their receptors.6 7 This simply indicates that, like acute myeloid leukemia blasts, immature myeloid cells in MDS do express VEGF and this is not specific to displaced/ectopic blasts or ALIP.
Second, the authors show absence of VEGF in a healthy human bone marrow clot section, but their data highlight only the absence of VEGF in mature myeloid cells (neutrophils). Like normal bone marrow, Figure 4 in Bellamy et al clearly demonstrates absence of VEGF in all mature myeloid cells in cases with MDS. Therefore, we do not know whether healthy human bone marrow blasts differ from “dislocated myeloid precursors” (Bellamy et al1), and they do not have autocrine or paracrine promotion by VEGF.
Third, their data show that cases with refractory anemia (RA, or low-risk MDS) were 100% (8 of 8) positive for VEGF, and only 72% (16 of 22) of cases with refractory anemia with excess of blasts (RAEB) and RAEB in transformation (RAEBt) show positivity for VEGF. According to our experience3,4 and that of others,2 only 30% of cases with RA show presence of ALIP and more than 95% of cases with RAEB/RAEBt (high-risk MDS) do have ALIP in their bone marrow biopsies. Bellamy et al1 do not clarify how many RA and RAEB/RAEBt cases were with ALIP and without ALIP, why RAEB/RAEBt cases with ALIP do not demonstrate positivity for VEGF, and what is the explanation for VEGF positivity in RA cases without ALIP.
Fourth, the authors do not mention anything about the international prognostic scoring system (IPSS) in MDS.8 This scoring system is used by most physicians to make a therapeutic decision in cases with MDS. It will be interesting to know about any correlation between VEGF expression and IPSS risk categories.
Finally, most investigators will agree that lymph-node fine-needle aspirates do not give enough information about lymph node architecture. Likewise, bone marrow clot sections are not suitable to start assuming about architectural disorganization in MDS. To get the full picture about ALIP in MDS and the autocrine or paracrine promotion by VEGF or other growth factors, authors should justify their conclusions on the basis of bone marrow biopsy examinations.
Expression of VEGF in myelodysplasia
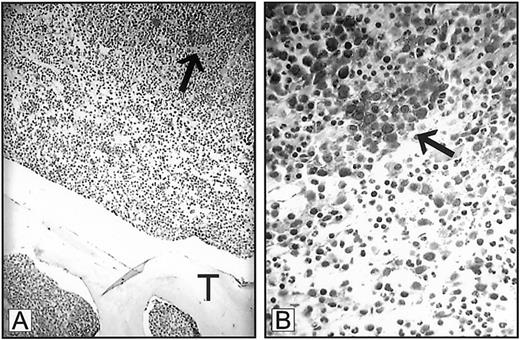
Dr Mangi interprets our data as indicating that the means by which we diagnose ALIP was based solely on findings derived from bone marrow clot sections.1-1 The definition of ALIP as originally proposed by Tricot et al1-2 1-3 resides upon resolving the spatial relationship between myeloblast clusters to bone trabeculae within trephine biopsies, as we described in “Discussion.”1(pp1432-1433) Indeed, we stress that the displacement of myeloid precursor clusters from their paratrabecular locale represents an adverse prognostic feature in MDS that is associated with an imminent risk for leukemic transformation. We do not, however, base a diagnosis of ALIP on bone marrow clot sections alone. All myeloblast clusters identified in marrow clot sections were confirmed by trephine biopsy prior to immunohistochemical staining. Our use of bone marrow clot sections to examine VEGF and receptor expression was predicated on the superior staining characteristics of bone marrow clot sections as compared to cores under the conditions used for staining in this study. Nevertheless, Mangi's letter now prompts us to include a figure showing VEGF expression in ALIP in a bone marrow trephine biopsy. Although the level of staining is not as optimal as that observed in the clot sections provided in the original manuscript, VEGF expression is clearly demonstrated (Figure1-1).
Expression of VEGF in ALIP.
Bone marrow trephine biopsy from a patient with myelodysplastic syndrome demonstrating expression of VEGF in myeloblast clusters (arrows). T indicates trabecular bone. Panel A, 250 × magnification; panel B, 1000 × magnification.
Expression of VEGF in ALIP.
Bone marrow trephine biopsy from a patient with myelodysplastic syndrome demonstrating expression of VEGF in myeloblast clusters (arrows). T indicates trabecular bone. Panel A, 250 × magnification; panel B, 1000 × magnification.
Dr Mangi incorrectly states that we conclude that the VEGF/receptor profile is specific to ALIP. We do not state that the observed staining characteristics are specific to ALIP and, indeed, a similar pattern is observed in AML, an observation that we report from our own data in the manuscript. But acquisition of the VEGF autocrine responsive phenotype may represent an early event in AML evolution that might also facilitate homotypic myeloblast adhesion.
Although we report the characteristic staining pattern for VEGF/receptor in isolated myelomonocytic precursors in 8 of 8 patients with refractory anemia, we do not describe ALIP in this low-risk FAB category, as stated by Dr Mangi. We fully agree with Dr Mangi that the International Prognostic Scoring System (IPSS) in MDS is a useful scoring system based upon clinical and pathologic features at disease presentation.1-4 Our series of patients was not restricted to bone marrow specimens obtained at diagnosis and includes patients with proliferative chronic myelomonocytic leukemia (CMML), which are excluded from the IPSS.
An important aspect of our study overlooked by Dr Mangi is that, while we and others have demonstrated the expression of VEGF in hematopoietic malignancies,1-5-1-8 this is one of the first reports to characterize the functional relevance as it relates to potential autocrine stimulation of tumor growth and survival. Such translational investigations are critical to the development of novel therapies targeting the VEGF-receptor axis and to thereby offer the potential to impact leukemia progenitor growth and development.