Aging is associated with modifications of T-cell phenotype and function, leading to impaired activation in response to both new and recall antigens. It is not known if T-cell activation results in elimination of a number of the CD4 molecules from the cell surface, as is the case with CD3/T-cell receptor complexes, or how aging influences the process. The T cells of young and elderly donors with reduced expression of CD4 were examined to see whether these cells exhibit other phenotypic features suggesting their active state. It was found that T lymphocytes expressing CD4 can be divided into 2 semidiscrete subpopulations: the major (CD4+) population, in which the level of expression of CD4 is constant and high, and a minor population (CD4lo), in which the expression of CD4 can be up to an order of magnitude lower than on the CD4+ cells. The proportion of CD4locells is age dependent and highly variable in the apparently healthy human population, with the expression of CD4 ranging from around 10% of all peripheral blood lymphocytes in the young to more than 30% in the elderly. Lowered expression of CD4 is correlated with a reduced expression of CD3, as well as with a decreased amount of CD28 and CD95Fas. Activation of CD4lo cells is suggested by their expression of CD25 and increased amounts of HLA-DR. Phenotypic characteristics of the CD4lo T-cell subpopulation suggest that it might be formed by (perhaps chronically) activated, temporarily apoptosis-resistant cells, possibly accumulating in the elderly.
Introduction
The CD4 antigen, a major coreceptor necessary for initiation of the T-lymphocyte activation sequence, is found on a major subpopulation of helper T cells. Cross-linking of the CD4 by the HLA class II molecule leads to activation of associated p56lck(lck) tyrosine kinase. Lck-dependent phosphorylation of the CD3ζ chain of the CD3/T-cell receptor (TCR) complex (simultaneously triggered by the antigenic epitope presented by the class II molecule) is thus at the beginning of the cascade of events leading to lymphocyte activation.1-6
CD4 expression is usually believed to be constant on the surface of human peripheral blood T lymphocytes; about 90 000 to 100 000 molecules are estimated to be expressed on a single T cell.7-9 However, it is known that many surface receptors undergo internalization upon binding by appropriate ligands and that the receptor-ligand complexes are eventually dissociated and/or digested by cellular proteolytic mechanisms. Also, some observations speak of the diminution, or even abolition, of the expression of CD4 on the surface of human peripheral blood T cells stimulated with the phorbol ester phorbolmyristate acetate, owing to down-regulation of both transcription and translation.10Until recently, neither the exact number of ligated receptors on the surface of an activated T cell nor their fate was a subject of closer study, mostly owing to lack of appropriate methodology. An appropriate method, involving quantitative flow-cytometric determination of the number of receptors on resting and stimulated T cells, has recently been described and has allowed for thorough studies of activation-related changes of a number of CD3-TCR complexes.11-13 It was shown that T-cell stimulation leads inadvertently to the reduction (detectable by flow cytometry) of a number of the CD3-TCR molecules, predominantly owing to their internalization.11 Depending on the presence or absence of costimulatory signals, the number of CD3-TCR complexes required for activation was established to be at very specific thresholds: about 1500 and 8000 per cell, respectively.12
It is thus conceivable that other T-cell surface receptors involved in activation follow the same fate. This should particularly be the case for the T-lymphocyte molecules that, along with the CD3-TCR complex, directly participate in the earliest stages of antigenic stimulation, such as the CD4 and CD8 antigens. It was recently shown that contact between murine CD8+ T cells and antigen-presenting cells (APCs) loaded with a peptide recognized by the CD3-TCR complex leads to some major histocompatibility (MHC) class I molecules' being picked up from the APCs and ultimately internalized by the lymphocytes concomitantly with the internalization of the CD3-TCR complex itself.14 The authors of that study did not address the question of the fate of the CD8 molecule in the process; however, knowing the role of CD8 in class I recognition and binding, one can speculate that CD8 could also be incorporated in the internalized complex. If this is a general phenomenon, a subpopulation of activated CD4+ cells, in which some of the CD4 is “spent” or exhausted simultaneously with the CD3-TCR, should be detectable even in normal people exposed to environmental antigens at the time of study. So far, unlike what occurs in the CD3-TCR complex, possible changes of the actual level of CD4 expression on single T cells have not been a subject of much study. Even the most recent papers, some of which appeared after publication of the original observations of modulation of the CD3-TCR complex expression, indicate that the coefficient of variation (CV) for CD4 fluorescence intensity was very low.7 8 Therefore, the authors of these papers do not consider the possibility of modulation of the expression of CD4 on the T-cell surface.
Aging is accompanied by progressive changes in the function of the immune system, ultimately leading to the decreased response to both primary and recall antigens. At least some gerontologists believe that the CD4+ T cell, acting as a central coordinator of the immune response, is among the most affected elements of the aging immune system.15,16 Not much is known about the fate of the functionally important receptors, such as CD3 and CD4, in T cells in the elderly. However, at least in the case of the CD28 molecule, its elimination from senescent CD4+ cells is quite well documented.17-19 On the other hand, there are suggestions of a chronic activation of at least a subpopulation of CD4+ cells in the elderly.18-20
In this work, we tried to answer the question of whether such a modulation of the CD4 expression can take place under physiological conditions (eg, when the T cells of a healthy individual are responding to environmental antigens), by looking for a subpopulation of peripheral blood T lymphocytes showing lowered expression of this antigen.
Materials and methods
Subjects
Peripheral venous blood was obtained from 55 healthy people of both sexes (42% men; average age, 51.56 ± 26.18 years; age range, 23-93 years). Twenty-four people from the group under study were classified as young (average age, 28.7 ± 8.9 years; 54% men); 10 were considered middle-aged (average age, 43.2 ± 2.7 years; 50% men); and 21 participants qualified as elderly (average age, 84.6 ± 6.3 years; 38% men). The people (aged above 69 years) included in the elderly group conformed to the health criteria of the Senieur protocol.21
Cell preparation and antibody staining
Fifty microliters of total blood was stained for 30 minutes at 4°C with one, or a mixture, of the antibodies listed below, processed semiautomatically by means of a Q-prep (Coulter, Miami, FL), and analyzed. To exclude methodological bias, this protocol was compared with 2 other methods of preparation of the same material. In the first method, antibody-treated blood samples were manually lysed with Tris-NH4Cl solution and analyzed. In the second method, samples were diluted 1:1 in phosphate-buffered saline (PBS); peripheral blood mononuclear cells (PBMCs) were purified by Histopaque (Sigma-Aldrich Chemie, Deisenhofen, Germany) flotation; and 100 000 cells stained with the same antibodies for 30 minutes on ice were analyzed after washing 3 times with PBS containing 0.1% bovine serum albumin. The second method was used in some comparisons and was then used exclusively in the experiments applying Quantibrite beads (Becton Dickinson, San Jose, CA) for receptor enumeration (see below). Cell samples prepared with any of these methods did not differ significantly in the proportion of T cells with lowered CD4 expression (not shown).
To make sure that the apparent variation in CD4 expression was not due to a specific antibody, we compared different clones of anti-CD4 antibodies supplied by different manufacturers and conjugated to either fluorescein isothicyanate (FITC), phycoerythrin, (PE) or Cychrome (Pharmingen, San Diego, CA). The antibodies were not repurified prior to use. The following anti-CD4 monoclonal antibodies were used in the study: (1) PE–anti-CD4 (Leu-3A) (Becton Dickinson) clone SK3, immunoglobulin (Ig)–G1; (2) FITC–anti-CD4 (Dako, Glostrup, Denmark) clone MT310, IgG1; (3) PE–anti-CD4 (Sigma-Aldrich Chemie) clone Q4120, IgG1; (4) PE–anti-CD4 (Pharmingen) clone RPA-T4, IgG1; and (5) Cychrome–anti-CD4 (Pharmingen) clone RPA-T4, IgG1.
If not mentioned otherwise, the results presented in the paper were obtained with the use of the PE-conjugated Leu-3A antibody. The proportion of CD4lo cells in an individual's T lymphocytes did not depend significantly on the type and manufacturer of anti-CD4 antibody conjugate used, although the Leu-3A antibody usually gave slightly lower results than the other products. Specifically, the mean percentages of CD4lo lymphocytes in the CD4+population were 23.0% ± 5.4% (n = 13), 18.8% ± 13.5% (n = 31), and 25.7% ± 10.5% (n = 11) for FITC–anti-CD4, PE–anti-CD4 (Becton Dickinson), and Cychrome–anti-CD4, respectively. Other antibodies used in this project were the following: (1) FITC–anti-CD3 (Dako); (2) FITC–anti-CD14 (Pharmingen); (3) FITC–anti-CD28 (Pharmingen); (4) PE–anti-CD95 (Pharmingen); (5) PE–anti-CD152 (Pharmingen); (6) PE–anti-CD45RA (Dako); (7) PE–anti-HLA-DR (Becton Dickinson); (8) PE–anti-CD25 (Pharmingen); (9) Cychrome–anti-CD16 (Pharmingen).
Except for CD14 and CD28, cell samples were always stained with anti-CD4 and anti-CD3 plus an additional third-color antibody, in order to clearly distinguish the CD3+CD4+ T lymphocytes and characterize the CD4lo cells within the T-cell pool only.
The usual concentration of each antibody was 0.5 μg/105cells. To examine the concentration dependency of the observed effect, PE–anti-CD4 antibody concentrations of 1.0 and 2.0 μg/105 cells were used in 3 experiments each involving young, middle-aged, and elderly donors, along with the usual concentration. Changes of the antibody concentration from 0.5 to 2 μg/105 cells did not result in significant changes of the CD4lo percentage (not shown).
Irrelevant antibodies of the same class, conjugated to the corresponding fluorochrome and manufactured by the same company as the relevant antibodies, were used as isotype controls in each experiment.
Flow cytometry
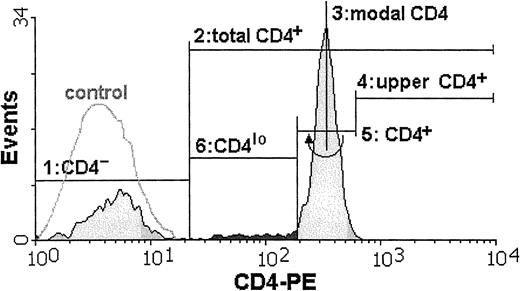
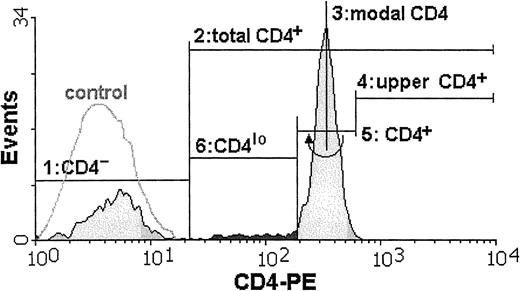
Cytometric analysis of all samples was performed by means of an Epics XL (Coulter, Hialeah, FL) at the Department of Histology and Immunology, Medical University of Gdansk, or a FACSCalibur (Becton Dickinson, Sunnyvale, CA) at the Hematology Clinic, Medical University of Gdansk, or a FACSort (Becton Dickinson, Sunnyvale, CA) at the Voivodial Hospital for Infectious Diseases in Gdansk. To check for the possibility of instrument bias, data from most of the samples were acquired on at least 2 instruments. Wherever the CD4lo population was seen, it could be discerned by analysis on any of the 3 instruments, and the differences in its proportion in the total CD4 population were negligible. Data from 20 000 mononuclear cells were acquired from each sample and stored as list modes. Off-line analysis was done by means of dedicated software (WinMDI versions 2.7 through 2.9, Joseph Trotter, Scripps, CA) and consisted of estimation of the percentages of distinct subpopulations and of the mean fluorescence intensities (MFIs). The CD4losubpopulation usually formed a “shoulder” attached to the main CD4+ population and not a separate peak. Thus, a set of rules was adopted in order to consistently define the limits of this cell group: First, a lower limit of the total CD3+CD4+ population was set by cutting off the CD4− cells as those with the fluorescence not different from that of appropriate isotype control (steps 1 and 2 in Figure 1). Then, the modal value for the entire population of lymphocytes bearing CD4 antigen was read (step 3), and the position of marker cutting off 0.2% of cells with the highest CD4 fluorescence was found (step 4). This marker position was then mirrored on the other side of the modal value and served as a boundary between the CD4+ and CD4lo cells (steps 5 and 6). We subtracted 0.2% from the proportion of CD4lo cells in the CD4+ population to account for the cells belonging to the lower end of the CD4+ group. In one example, the proportion of CD4lo cells in the total CD4+ population is 7.8% (Figure 1).
The proportion of T lymphocytes exhibiting the CD4lo phenotype can be found among anti-CD4–stained peripheral blood lymphocytes.
The cell suspension is stained with FITC–anti-CD3 and PE–anti-CD4 monoclonal antibodies and analyzed by FACS. Only CD3+lymphocytes are included in subsequent analysis. The flow cytometry histogram contains the overlay of the fluorescence intensity of the isotype control (white, control) and the fluorescence distribution for the cells stained with PE-conjugated anti-CD4 antibody (shaded). Boundaries of the CD4lo subpopulation (darkly shaded) are found as follows: 1, a marker is set to delineate the CD4−population (closely overlapping the isotype control); 2, a marker containing all CD4+ cells (total CD4+) is stretched from the higher end of the first (CD4−) marker; 3, a position of the modal value for the CD4-PE fluorescence is found; 4 and 5, an “upper” half of the fluorescence peak containing the CD4+ cells is delimited, and mirrored toward the lower fluorescence values to cover all of the CD4+ population; 6, the CD4lo subpopulation is assigned the position between the beginnings of the second (“total” CD4+) and fifth (“true” CD4+) markers.
The proportion of T lymphocytes exhibiting the CD4lo phenotype can be found among anti-CD4–stained peripheral blood lymphocytes.
The cell suspension is stained with FITC–anti-CD3 and PE–anti-CD4 monoclonal antibodies and analyzed by FACS. Only CD3+lymphocytes are included in subsequent analysis. The flow cytometry histogram contains the overlay of the fluorescence intensity of the isotype control (white, control) and the fluorescence distribution for the cells stained with PE-conjugated anti-CD4 antibody (shaded). Boundaries of the CD4lo subpopulation (darkly shaded) are found as follows: 1, a marker is set to delineate the CD4−population (closely overlapping the isotype control); 2, a marker containing all CD4+ cells (total CD4+) is stretched from the higher end of the first (CD4−) marker; 3, a position of the modal value for the CD4-PE fluorescence is found; 4 and 5, an “upper” half of the fluorescence peak containing the CD4+ cells is delimited, and mirrored toward the lower fluorescence values to cover all of the CD4+ population; 6, the CD4lo subpopulation is assigned the position between the beginnings of the second (“total” CD4+) and fifth (“true” CD4+) markers.
Quantification of the numbers of CD4 molecules
Quantification of the numbers of CD4 molecules on the surface of cells belonging to different populations of CD4-bearing T lymphocytes was performed by means of the Cellquest Quantitative Analysis data acquisition module (Becton Dickinson, San Jose, CA) with the acquisition of signal from Quantibrite calibration beads prior to the acquisition of data from the cells. The anti-CD4 antibody used for this purpose was always PE conjugated (Leu-3A), and the cells were simultaneously stained with FITC–anti-CD3. Data analysis was performed by means of the QuantiCALC program (Verity Software, Topsham, ME), which transforms the values of fluorescence recorded from individual cells into absolute numbers of detected molecules, using a calibration curve generated by simultaneous analysis of the beads.
Cell culture
To investigate possible modifications of the level of CD4 expression on T cells owing to in vitro stimulation, peripheral blood lymphocytes of 4 healthy individuals were stimulated with plastic-immobilized anti-CD3 (OKT-3) (Ortho Biotech, Raritan, NJ) in 24-well plastic culture plates at 10 ng/well. Immediately before use, the antibody-containing wells were washed with 3 changes of complete culture medium (RPMI 1640 supplemented with 10% fetal calf serum, 2 mM L-glutamine, and antibiotics [penicillin/streptomycin mix, Sigma Chemical, St Louis, MO]). Cells were suspended in the same medium at 106/mL, and 1 mL cell suspension was placed in each well. Plates were then incubated for 48 hours in a mixture of 5% CO2 and 95% air at 37°C, collected, and stained with anti-CD4 and anti-CD3 antibodies. In a separate experiment, cells were cultured for 8 days before collecting, antibody staining, and analysis; in this experiment, cells were also stained with the anti-CD28 antibody.
Statistics
The results are shown as representative 2-dimensional flow cytometry histograms (dot plots and density plots) or as averages in bar graph form. Statistical information includes arithmetic means, SDs, SEMs, and 95% confidence intervals (CIs), as indicated in Table 1 and in the figure legends. The statistical significance of the differences was determined by means of paired or unpaired Student t test as necessary, after confirmation of the normality of data distribution. Correlation between the proportion of CD4lo cells in the total CD4+ lymphocyte population and the age of individuals was done by means of Pearson R coefficient.
Results
The proportion of human T lymphocytes with lower-than-average expression of the CD4 increases in the elderly
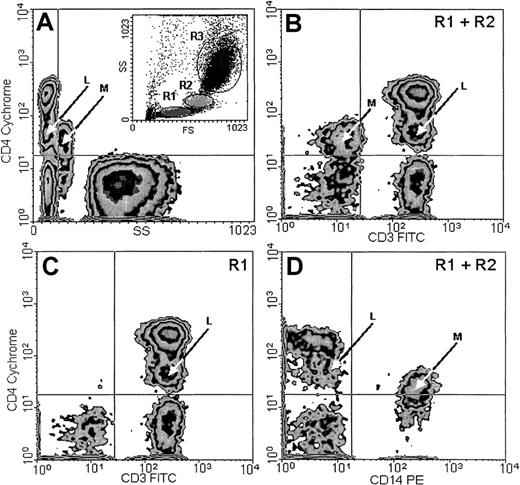
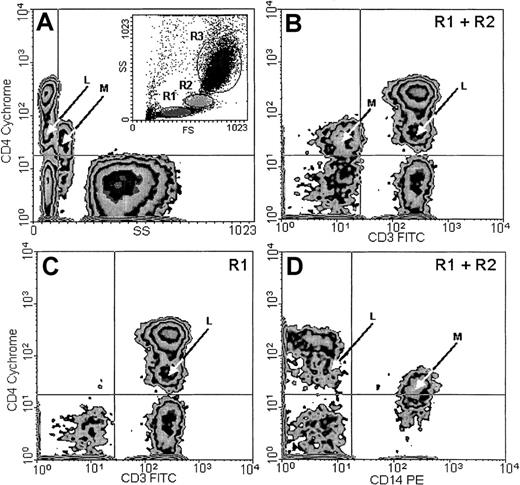
In addition to the typical, major CD4+ population with small CV, found within the lymphocyte window on the basis of low forward scatter (FS) and side scatter (SS) parameters (Figure2A, inset R1), a smaller population, which we designated CD4lo, could be detected among the T lymphocytes of every person examined. This population presented a CD4-staining intensity intermediate between the isotype control or CD4− cells and the normal CD4+ cells. The CV of CD4 fluorescence in this population was higher than in the CD4+ cells. CD4lo lymphocytes could be distinguished from the monocytes by their lower FS and SS (Figure 2A) and their positive CD3 (Figure 2B-C) and negative CD14 (Figure 2D) expression. The monocytes present in the mixture consistently showed a CD3−CD14+ phenotype in addition to high SS and low CD4 expression detectable on their surfaces (Figure 2). For the subjects as a group, the mean percentage of CD4lo cells was 18.0% ± 3.38% (mean ± 95% CI, n = 55) of the total CD4+ population, with a range of 1.8% to 46.1%. The corresponding proportion of the CD4lo lymphocytes among the total peripheral blood lymphocytes (PBLs) was 9.2% ± 1.95% (range, 1.4% to 27.9%). Thus, both in total PBLs and in the CD4+population, the proportion of CD4lo cells greatly varied among individuals. However, the proportion was quite stable if one individual was tested more than once. Thus, cells were obtained from 4 different donors aged 34 to 65 years at least twice at monthly intervals; for one of these donors, the analysis was also performed after another month. The results from each individual varied about 6% to 15% (1.9% vs 2.2% vs 2.0%; 3.1% vs 3.3%; 4.5% vs 4.2%; and 16.3% vs 18.9%). We did not observe significant correlation between the number or proportion of cells with the CD4lo phenotype and those with the CD4+ phenotype (r2 = 0.16,P > .1). Considering possible causes of this variability, we analyzed the proportion of CD4lo lymphocytes in relation to the sex and age of donors. We found no difference in the percentages of the CD4lo cells in women (26.5% ± 5.9%, n = 34) and men (20.84 ± 4.62%, n = 21, P = .15) in the group under study.
CD4lo cells.
CD4lo cells form a distinct subpopulation of small T lymphocytes. Peripheral blood from an 85-year-old individual was stained with Cychrome–anti-CD4, FITC–anti-CD3, and PE–anti-CD14 monoclonal antibodies and analyzed by flow cytometry after gating the lymphocyte (A, inset, R1), monocyte (R2), and granulocyte (R3) populations. CD4lo lymphocytes (arrows marked L) show low side scatter (SS; A); expression of CD3 similar to that of other CD4+ lymphocytes (B-C); and no expression of CD14 (D). Contrarily, monocytes (arrows marked M) with low CD4 expression (B) show higher SS (A); no CD3 (B); and strong CD14 expression (D). Panels B and D contain data from gates R1 and R2 (including both lymphocytes and monocytes), and panel C contains data from from gate R1 (lymphocytes only).
CD4lo cells.
CD4lo cells form a distinct subpopulation of small T lymphocytes. Peripheral blood from an 85-year-old individual was stained with Cychrome–anti-CD4, FITC–anti-CD3, and PE–anti-CD14 monoclonal antibodies and analyzed by flow cytometry after gating the lymphocyte (A, inset, R1), monocyte (R2), and granulocyte (R3) populations. CD4lo lymphocytes (arrows marked L) show low side scatter (SS; A); expression of CD3 similar to that of other CD4+ lymphocytes (B-C); and no expression of CD14 (D). Contrarily, monocytes (arrows marked M) with low CD4 expression (B) show higher SS (A); no CD3 (B); and strong CD14 expression (D). Panels B and D contain data from gates R1 and R2 (including both lymphocytes and monocytes), and panel C contains data from from gate R1 (lymphocytes only).
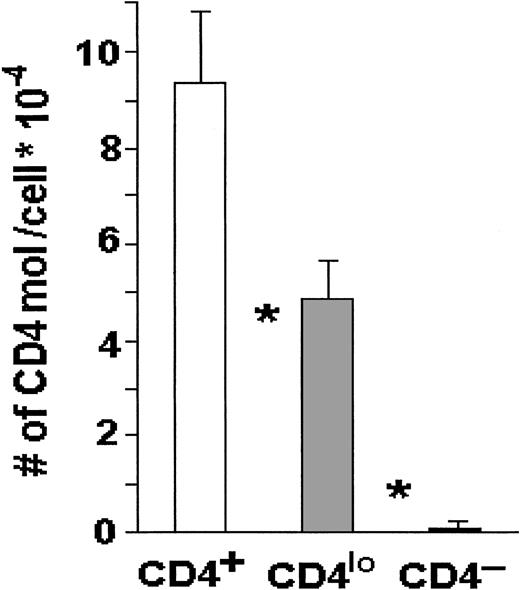
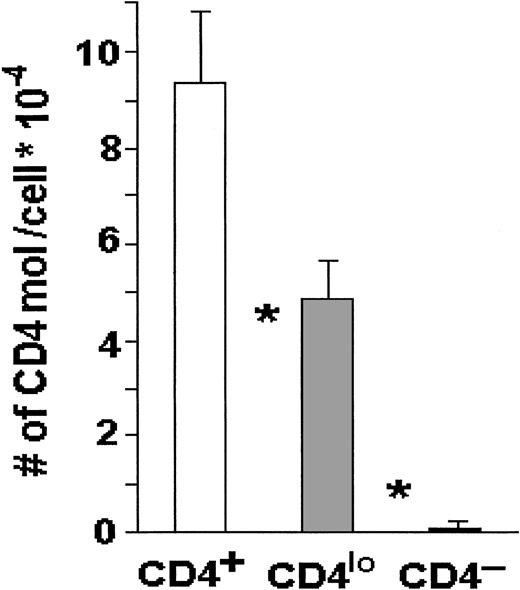
When the absolute numbers of CD4 receptors were compared on CD4lo and CD4+ T cells by means of Quantibrite beads, the numbers on the major CD4+ populations were about 95 000 CD4 molecules per lymphocyte, whereas CD4lo cells expressed on average fewer than 50 000 CD4 molecules each, and this difference was highly statistically significant (Figure3).
CD4 molecules on CD4+ and CD4locells.
The number of CD4 molecules is significantly higher on the surface of CD4+ than on the surface of CD4lo T cells. Peripheral blood lymphocytes from 17 healthy people were stained with FITC–anti-CD3 and PE–anti-CD4, and the absolute numbers of CD4 molecules per cell were determined with the use of Quantibrite beads as described in “Materials and methods.” The results are shown as mean numbers of CD4 molecules per CD3+CD4+(CD4+), CD3+CD4lo(CD4lo), or CD3+CD4−(CD4−) cell (bars); vertical lines show SDs. Asterisks indicate highly significant differences between the numbers of CD4 molecules on CD4+ and CD4lo cells (P < .0000001) and between CD4lo and CD4− cells (P < .000000001).
CD4 molecules on CD4+ and CD4locells.
The number of CD4 molecules is significantly higher on the surface of CD4+ than on the surface of CD4lo T cells. Peripheral blood lymphocytes from 17 healthy people were stained with FITC–anti-CD3 and PE–anti-CD4, and the absolute numbers of CD4 molecules per cell were determined with the use of Quantibrite beads as described in “Materials and methods.” The results are shown as mean numbers of CD4 molecules per CD3+CD4+(CD4+), CD3+CD4lo(CD4lo), or CD3+CD4−(CD4−) cell (bars); vertical lines show SDs. Asterisks indicate highly significant differences between the numbers of CD4 molecules on CD4+ and CD4lo cells (P < .0000001) and between CD4lo and CD4− cells (P < .000000001).
Compared with the young, the elderly group significantly showed an almost 2-fold higher proportion of CD4lo T cells. Interestingly, the middle-aged group did not show such a tendency (Table 1). Next, we performed a regression analysis on the whole set of data, including proportions of CD4lo T cells among CD4 lymphocytes and among PBLs on one side and the age of each donor on the other. We found a significant positive correlation between age and the proportion of CD4lo cells among CD4+ cells (r = 0.42,P < .005) and among PBLs (r = 0.49,P < .05). We then tested for a possible relation between an increased proportion of CD4lo lymphocytes and a decreased proportion of the CD4+ T cells in the elderly. In the group under study, no such correlation could be demonstrated between the age of donor and the proportion of the CD4+cells in the PBL (r = −0.16, P > .2), indicating no decrease in the proportion of CD4+ cells in our elderly group.
In principle, anti-CD4 antibody molecules could, like any other immunoglobulin, be picked up and bound nonspecifically by the non-CD4 cells having enough Fc receptors on their surface, especially some of the CD16+ natural killer (NK) cells. To exclude the possibility that CD4lo cells belong to FcR+cells (say, of NK or CD8+ phenotype), we stained the cells with anti-CD4, anti-CD3, and either anti-CD8 or anti-CD16. We found that the CD4loCD3+ cells bore neither CD8 nor CD16 on their surface (not shown). Also, CD4lo lymphocytes did not bind more isotype control antibodies than the rest of the CD4 population. We concluded from these experiments that the binding of anti-CD4 to the CD4lo cells is specific and does not depend on the binding of antibody via its Fc portion.
Modulation of expression of activation-related antigens indicates the activated status of CD4lo T cells
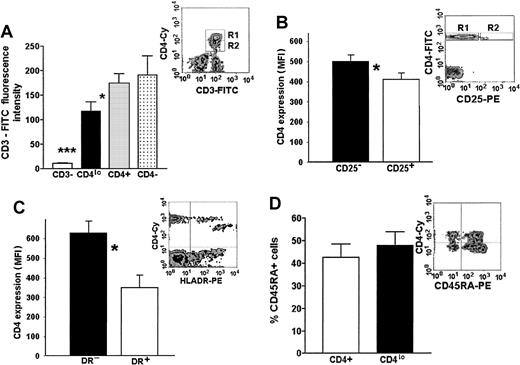
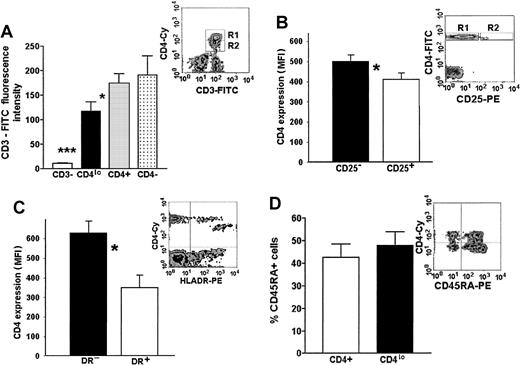
Our hypothesis of an activation-related association between a decrease of CD3 and CD4 expression is based on the data relating T-cell activation to a loss of some surface CD3.3 11-13 To this end, we compared the levels of CD3 expression on T cells showing different levels of expression or a complete lack of CD4, as well as on non–T cells from the PBMC pool. A representative flow cytometry data plot and a graph of MFIs of FITC–anti-CD3 on the CD3+CD4− (presumably CD8+lymphocytes), CD3+CD4lo, and CD3+CD4+ cells as well as the MFI of CD3− PBLs (presumably a mixture of B and NK cells) are shown in Figure 4A. Although the level of CD3 expression on CD4− (CD8+) lymphocytes and CD4+ T cells did not differ significantly, the MFI of FITC–anti-CD3 bound to CD4lo cells was, on average, 1.2 to 2.1 times lower than that seen on the CD4+ lymphocytes, yet very significantly higher than that of CD3−CD4− cells (Figure 4A).
Phenotypic features of CD4lo cells.
CD4lo cells show phenotypic features of activated T lymphocytes. (A) CD3 expression on CD4lo cells is lower than on CD4+ and CD4− T cells. Bar graph of mean fluorescence intensities (mean ± SEM, n = 27) of peripheral blood lymphocytes stained with Cychrome–anti-CD4 and FITC–anti-CD3 and analyzed after setting up the regions as in a representative density plot (A inset, R1-CD4+, R2-CD4lo). Statistically significant (paired ttest) differences were observed between the FITC-CD3 fluorescence intensities of CD4lo cells (▪) and CD4+lymphocytes (░; *P = .022) and between CD4loand CD3− cells (■; ***P = .00004). (B-C) Lowered expression of CD4 is associated with high expression of activation antigens CD25 and HLA-DR (B and C insets show representative density plots). Bar graph in panel B shows statistically significant difference between the expression of CD4 on cells from the R2 region of the inset expressing CD25 (□) and the CD25− T cells from region R1 (▪) (*mean ± SEM, n = 5, P = .0001). Panel C shows a similar difference between HLA-DR+ (■) and HLA-DR− cells (▪; *mean ± SEM, n = 5,P = .0001). (D) The proportion of CD4lo and CD4+ cells expressing CD45RA antigen was similar (bar graph), but the intensity of its expression was higher on CD4lo cells (inset).
Phenotypic features of CD4lo cells.
CD4lo cells show phenotypic features of activated T lymphocytes. (A) CD3 expression on CD4lo cells is lower than on CD4+ and CD4− T cells. Bar graph of mean fluorescence intensities (mean ± SEM, n = 27) of peripheral blood lymphocytes stained with Cychrome–anti-CD4 and FITC–anti-CD3 and analyzed after setting up the regions as in a representative density plot (A inset, R1-CD4+, R2-CD4lo). Statistically significant (paired ttest) differences were observed between the FITC-CD3 fluorescence intensities of CD4lo cells (▪) and CD4+lymphocytes (░; *P = .022) and between CD4loand CD3− cells (■; ***P = .00004). (B-C) Lowered expression of CD4 is associated with high expression of activation antigens CD25 and HLA-DR (B and C insets show representative density plots). Bar graph in panel B shows statistically significant difference between the expression of CD4 on cells from the R2 region of the inset expressing CD25 (□) and the CD25− T cells from region R1 (▪) (*mean ± SEM, n = 5, P = .0001). Panel C shows a similar difference between HLA-DR+ (■) and HLA-DR− cells (▪; *mean ± SEM, n = 5,P = .0001). (D) The proportion of CD4lo and CD4+ cells expressing CD45RA antigen was similar (bar graph), but the intensity of its expression was higher on CD4lo cells (inset).
The majority (on average, more than 52.6% ± 8.9%) of the CD4lo cells display high levels of expression of CD25 antigen (Figure 4B). Contrarily, its expression can be seen only on a small proportion (around 18.4% ± 3.4%) of other CD4+cells and is significantly lower (average MFI, 29.0 vs 495.2 for CD4lo).
The expression of HLA-DR, believed to be present only on the activated and not on the resting CD4+ T cells, could be seen both in the CD4+ and CD4lo populations. However, similarly to the pattern of CD25 expression, all of the CD4lo lymphocytes expressed HLA-DR and the level of expression of this antigen was higher in this population than in the CD4+ population (Figure 4C). Monocytes (known to express high amounts of HLA-DR) were eliminated from the analysis by simultaneous staining with the anti-CD3 antibody and analyzing only the CD3+ population in addition to the usual gating based on forward and side scatter.
It is believed that the expression of CD45RO delineates T cells that are activated. The proportion of naive (CD45RO−RA+) to memory-activated (CD45RO+RA−) cells did not differ significantly between the CD4lo and the CD4+lymphocytes; interestingly, CD4lo cells seem to contain a subpopulation showing a very high expression of the CD45RA, which is absent from the rest of the CD4+ cells (Figure 4D).
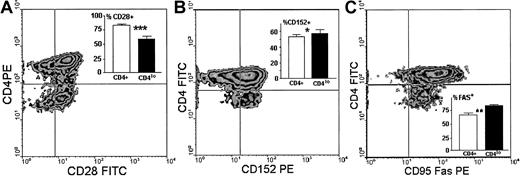
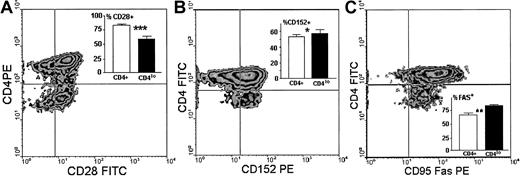
We then compared the expression of 3 other surface antigens known either to directly participate in antigenic stimulation of T cells (CD28, CD152) or to be involved in termination of this response (CD95). We found that unlike the CD4+ population, in which on average 83.6% cells expressed CD28, only 59.5% ± 4.9% of CD4lo cells were CD28+ (Figure5A), and the difference in the proportion of CD4+CD28+ and CD4loCD28+ cells was strongly significant. The amount of CD28 expressed on single CD4lo T cells was minimally lower than that seen on CD4+ lymphocytes (Figure5A). Reciprocally, the percentage of CD4lo lymphocytes bearing CD152 was higher than that observed in the population showing high expression of CD4 (Figure 5B). The actual difference, although statistically significant, was not big. There was no difference between CD4+ and CD4lo cells in the levels of expression of CD152. Finally, the proportion of CD4lo cells expressing the CD95 receptor was significantly higher than was observed for the CD4+ population. However, the level of expression of CD95 on CD4lo cells was lower than on CD4+lymphocytes, with the MFI values at 43.7 ± 4.2 compared with 76.1 ± 8.4 (Figure 5C).
Surface expression of antigens participating in stimulation.
Surface expression of antigens participating in stimulation differentiates CD4lo lymphocytes from the CD4+population. Panels show representative density plots of expression of CD4 versus CD28 (A), CD152 (B), and CD95 (C), and insets illustrate the comparison and statistical evaluation (mean ± SD) of the proportions of cells showing respective phenotypes within the CD4+ (■) and CD4lo (▪) populations. Samples from 5 people were analyzed in each group. Asterisks indicate the significance of observed differences at P = .0001 (A, ***, n = 16), P = .043 (B, *, n = 10), andP = .005 (C, **, n = 12).
Surface expression of antigens participating in stimulation.
Surface expression of antigens participating in stimulation differentiates CD4lo lymphocytes from the CD4+population. Panels show representative density plots of expression of CD4 versus CD28 (A), CD152 (B), and CD95 (C), and insets illustrate the comparison and statistical evaluation (mean ± SD) of the proportions of cells showing respective phenotypes within the CD4+ (■) and CD4lo (▪) populations. Samples from 5 people were analyzed in each group. Asterisks indicate the significance of observed differences at P = .0001 (A, ***, n = 16), P = .043 (B, *, n = 10), andP = .005 (C, **, n = 12).
Stimulation in vitro leads to increased proportion of CD4lo T cells
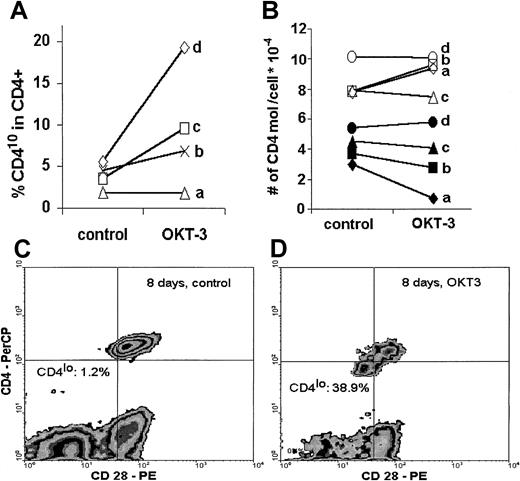
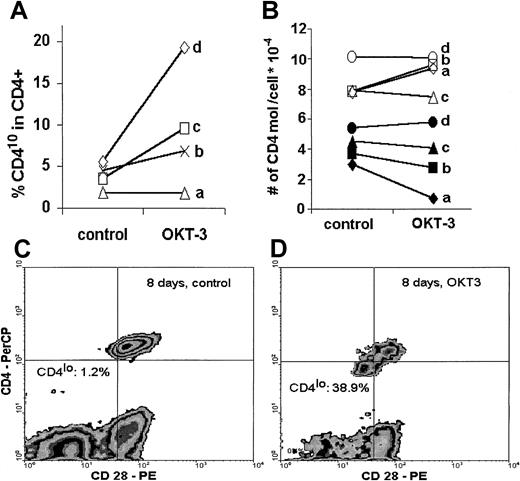
When human PBLs were stimulated in vitro with an optimal concentration of immobilized anti-CD3 antibody, the proportion of CD4lo cells among CD4+ T lymphocytes increased after only 48 hours of stimulation in 3 of 4 individuals tested. Interestingly, this increase was proportional to the age of the donor, with the highest percentage of CD4lo cells exhibited by the oldest person (60 years old) (Figure 6A). In a separate experiment, when stimulation with immobilized anti-CD3 was prolonged to 8 days, the proportion of CD4lo cells increased from 1.2% in unstimulated control to 38.9% in the anti-CD3–treated sample (Figure 6C-D). We then asked if stimulation led to any change in the number of CD4 molecules on the surface of predefined CD4lo and CD4+ populations with the use of quantitative calibration with Quantibrite beads. No clear tendency could be seen in either population. In fact, in 2 young donors (donors a and b), there was an increase in the number of CD4 molecules on stimulated CD4+ cells with a simultaneous (but proportionally smaller) decrease in the CD4lo population. On the other hand, the number of CD4 molecules on the surface of both CD4lo and CD4+ cells of a middle-aged (donor c) and an elderly individual (donor d) did not change upon stimulation (Figure 6B).
Effect of in vitro stimulation.
In vitro stimulation leads to an increased proportion of CD4lo cells and to decreased expression of CD4. Peripheral blood lymphocytes of 4 healthy individuals (donor a, 34 years; donor b, 39 years; donor c, 47 years; and donor d, 60 years old) were stimulated in vitro with immobilized anti-CD3 (OKT-3) as described in “Materials and methods”; stained with anti-CD4, anti-CD3, and anti-CD28 antibodies; and analyzed by flow cytometry. (A) Lymphocytes were stimulated for 48 hours, and the proportion of CD4lo cells was compared in the control and OKT-3–treated CD3+CD4+ populations. (B) The number of CD4 molecules was determined by means of Quantibrite beads on CD4lo (black symbols) and CD4+ (open symbols) T cells before (control) and after 48 hours' stimulation with anti-CD3 (OKT-3). (C) (D) In a separate experiment, peripheral blood lymphocytes of a healthy 47-year-old man were stimulated with OKT-3 for 8 days, stained with anti-CD4 and anti-CD28, and analyzed. The proportion of the CD4lo cells showing simultaneous decrease of CD28 expression was greatly increased compared with the CD4+population (compare Figure 5A).
Effect of in vitro stimulation.
In vitro stimulation leads to an increased proportion of CD4lo cells and to decreased expression of CD4. Peripheral blood lymphocytes of 4 healthy individuals (donor a, 34 years; donor b, 39 years; donor c, 47 years; and donor d, 60 years old) were stimulated in vitro with immobilized anti-CD3 (OKT-3) as described in “Materials and methods”; stained with anti-CD4, anti-CD3, and anti-CD28 antibodies; and analyzed by flow cytometry. (A) Lymphocytes were stimulated for 48 hours, and the proportion of CD4lo cells was compared in the control and OKT-3–treated CD3+CD4+ populations. (B) The number of CD4 molecules was determined by means of Quantibrite beads on CD4lo (black symbols) and CD4+ (open symbols) T cells before (control) and after 48 hours' stimulation with anti-CD3 (OKT-3). (C) (D) In a separate experiment, peripheral blood lymphocytes of a healthy 47-year-old man were stimulated with OKT-3 for 8 days, stained with anti-CD4 and anti-CD28, and analyzed. The proportion of the CD4lo cells showing simultaneous decrease of CD28 expression was greatly increased compared with the CD4+population (compare Figure 5A).
Discussion
We have found a population of human peripheral blood lymphocytes bearing significantly lower amounts of CD4 than the major CD4+ population. Usually, these CD4lo cells seen in the cytometric histogram form a “shoulder” or a “tail” between the CD4+ and the CD4− cells; only rarely do they form a distinct subpopulation. Their presence (albeit in our opinion quite common) is not very conspicuous on a typical dot plot; the enhancement of cytometric image by the creation of a contour or density plot helps one to visualize these cells. Analysis of light-scatter properties of CD4lo cells as well as their expression of CD3 and lack of CD14 or CD16 antigens confirms their T-cell lineage. Further characterization of CD4lo cells has determined that they express other typical T-cell antigens, frequently (including CD28, CD152, CD25, HLA-DR, and Fas) in a proportion different from what is observed in the CD4+ population. This unique phenotype sets the CD4lo T cells apart from the major CD4+ population. Our attempt to quantify the numbers of CD4 molecules on the surface of CD4+ and CD4lo T cells further confirmed these observations, showing an almost 2-fold decrease in the number of CD4 molecules on the postulated subpopulation (about 49 000 per cell assigned to the CD4lo population vs about 95 000 for the cells from the major CD4+ pool). The latter value is in good agreement with the reported numbers of CD4 on the surface of resting T cells.7-9
To make sure we were not chasing after an artifact, we applied extensive variations to the methodology, including the use of 4 different clones of anti-CD4 antibody from different manufacturers, different conjugates of these clones, changes in the antibody concentration, and the use of 3 different methods of sample preparation and staining. We did not detect significant differences in the proportion of CD4lo T cells with any of these variations in methodology. We did not attempt to specifically block the Fc receptors on the analyzed cells. However, CD4lo T cells did not differ from the rest of the CD4+ population (or from other lymphocytes) in the level of nonspecific staining with the PE-conjugated isotype control containing irrelevant antibodies (not shown). Thus, we can assume that the level of FcR expression on CD4lo cells does not differ from that on other CD4+ cells and has no influence on their lower staining with anti-CD4.
To understand the genesis of CD4lo cells, we hypothesized that CD4 could either become down-regulated on these cells owing to activation or be in a different stage (perhaps the terminal stage) of differentiation. The proportion of CD4lo cells in the peripheral blood varied greatly among apparently healthy individuals, from typically only a few percentage points to (in rare cases) more than 40% of all T cells expressing CD4. Why, then, have CD4lo cells not been described before? First, except for the works of Lanzavecchia's group,12,13 the possibility of even partial modulation of the level of expression of CD4 on T cells is not considered, even in the most recent papers on the subject.7,8,22 One of these recent papers actually assumes absolute constancy of the numbers of CD4 molecules on T cells and uses this constant as a calibration gauge for estimation of the numbers of CD38 molecules.8 Second, in healthy young subjects (being studied for the normal values of the CD4 expression), the percentages of CD4lo cells are usually low (not exceeding 10% to 15% of all CD4+ cells) and are seen in cytometric histograms mostly in the form of an inconspicuous shoulder at the main CD4+ population. They stand out more clearly in the analysis of material from elderly people, who are usually excluded from non–aging-oriented studies. The papers cited above do not characterize their subjects other than as “a small sample of healthy donors”7 or as “healthy HIV-1 uninfected men”8 and do not give the number of individuals studied or their ages. Neither of the just-mentioned papers contains plots of actual flow-cytometric data; thus, it is hard to predict if the CD4lo cells could be seen in their specimens.
In our study, the percentage of CD4lo lymphocytes was positively correlated with the donor's age. This suggests that the physiological level of CD4 expression on the T lymphocytes may be dependent on age and possibly the functional status of the cell. There are currently only a very few papers that approach the problem of age-dependent changes of CD4 receptor density, and all of them look at the changes in the total pool of CD4-bearing T cells.23-25Also, different authors report either an increase,23 a decrease,24 or no change25 in the CD4 density. In one instance, a decrease in average expression of CD4 is described in late-passage (the in vitro approximation of aging) human T-cell clones.26
Few lines of reasoning indicate that activation plays a role in the genesis of the CD4lo T cells. The first one comes from analogy to the recently shown activation-related decrease of the CD3-TCR complex on the T-cell surface.3,11-13 In our study, the expression of CD3 on the CD4lo lymphocytes was significantly lower than on the other CD4+ T cells. This observation may indicate that the lower level of expression of CD4 is characteristic for activated T cells, which express lower amounts of CD3. The hypothesis of activated status of the CD4lo cells is also supported by our observation of higher frequency of activation-related antigens CD25 and HLA-DR on their surface. To further validate our hypothesis, we examined the CD4 expression on T cells stimulated with immobilized anti-CD3. We were able to show an increased proportion of CD4lo T cells after just 48 hours; this tendency was strengthened after 8 days of culture, when the proportion reached almost 40%. Interestingly, the numbers of CD4 molecules did not seem to change significantly within any of the 2 CD4-bearing populations upon stimulation. This may indicate that the process of activation-dependent down-regulation of the CD4 molecule is rapid and involves a quasi-standard number of molecules that have to be eliminated from the surface during effective stimulation, similar to what happens to the CD3-TCR complex.3 11-13
The frequency of CD4lo cells is similar in the memory-activated CD45RO+ and naive CD45RA+population; in fact, they tend to express more CD45RA than the rest of the CD4+ lymphocytes. This may reflect the reversion of the CD45RO+ phenotype to the CD45RA+ after the acute phase of stimulation is over27 and suggests that the CD4lo population in fact contains T cells at later stages of activation.
In the CD4lo population, the percentage of CD28+ cells was significantly lower than in the main CD4+ pool, and the level of expression of the CD28 molecule was marginally lower than on the CD4+ lymphocytes. This in turn may suggest that the process of activation leading to a lowered expression of CD4 (and CD3) on CD4lo cells eliminates (or, at least, reduces) the CD28 antigen. In fact, significant down-regulation of both CD28 messenger RNA and surface expression in activated T cells upon ligation by the B7 molecule has been shown to last up to 48 hours following stimulation.28 As we show here, in anti-CD3–stimulated PBMCs, a population of CD3+CD4lo cells with visibly lower (albeit not null) expression of CD28 could be clearly discerned (Figure 6D). This observation seems to be in line with the earlier reports.
Alternatively, CD4lo, CD3lo, and CD28lo cells could form a stable subpopulation of T cells originally endowed with this phenotype and having as-yet-unknown functional properties. Whatever its genesis, this population—in our opinion—accumulates with age, similar to the accumulation of the T cells expressing high amounts of surface P-glycoprotein and increased amounts of MHC class I antigens in the spleens of old mice.29,30 An age-related increase of the proportion of CD28− T cells is well documented, although it was not previously associated with lower expression of CD3 or CD4 on these cells.18,19 This down-regulation of CD28 is probably related to impaired induction of the CD28 gene transcription.18
Thus, CD4lo phenotype can be due either to down-regulation at the transcription or translation level, to reduction from the resting state by activation–related shedding and/or internalization, or to intrinsically lower expression of the molecule on a separate population of T cells. The last explanation seems the least possible though, in light of our observations showing stimulation-dependent increase in the proportion of CD4lo cells in vitro. The discovery of membrane “rafts” as specific sites in the T-cell membranes important for initiation of signal transduction may offer some clues to understanding the apparent concerted down-regulation of surface molecules. It was recently suggested that T-cell activation is accompanied by dimerization (or even oligomerization) of some (MHC-II–ligated) CD4 molecules.31 This observation may have something in common with the existence of rafts containing CD3-TCR complex, CD4, CD28, and LAT.32 It is conceivable that the ultimate fate of all transmembrane molecules of such a raft after ligation of at least some of them and propagation of the activating signal into the cytoplasm is common and leads to their simultaneous disappearance from the cell surface.
In any case, CD4lo cells would form an activated subpopulation of the CD4 T cells that were not (or not yet) able to rebuild their CD4 surface complement. It is a current paradigm that activation promptly leads to an increased expression of CD95 on the T-cell surface. Our finding of a uniformly intermediate level of CD95 on the CD4lo lymphocytes may thus be attributed to their prolonged (rather than recent) activated state. Decreased CD95 may confer some level of protection from the too-early onset of apoptosis, before the activated lymphocyte completes its task, or it may be an indication of decreased ability of the immune system to eliminate “used” T cells at the end of an immune response, especially in the elderly. The proportion of CD4lo cells found in any single individual would then tell about the donor's prolonged contact with T-cell–activating antigens, even if the person did not manifest any pathological symptoms at the time of investigation. If our theory is correct, it should be expected that situations leading to chronic activation and eventually functional exhaustion of the CD4 cells should lead to the accumulation of CD4lo lymphocytes. Our recent observations suggest that this may be the case for rheumatoid arthritis. T cells of rheumatoid arthritis patients are believed to undergo chronic activation and precocious proliferative senescence.33 Chronic activation involves multiple rounds of cell division, leading, inter alia, to telomere shortening. This was recently exemplified in the paper showing that clonally expanded CD8+CD28− T cells have shorter telomeres than their CD28+counterparts.34 It will be of interest to see if such a difference in telomere length exists between the CD4+ and CD4lo T cells. Along the same line, we should expect lower frequency of TCR rearrangement excision circles if the CD4lo population is indeed chronically activated.33 Further analysis of functional properties of these cells, including their ability to make and react to cytokines or to proliferate, is necessary for better understanding of their role in the pathophysiology of the immune system.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Jacek M. Witkowski, Department of Pathophysiology, Medical University of Gdansk Debinki 7 80-211 Gdansk, Poland; e-mail: jawit@amedec.amg.gda.pl.