Abstract
Current information on Waldenström macroglobulinemia (WM) is based on retrospective or single-institution studies of patients requiring therapy. Between 1992 and 1998, 231 patients with WM were enrolled in a prospective observational multicenter clinical trial. Of these, 182 patients with symptomatic or progressive disease were treated with 4 to 8 cycles of therapy with a purine nucleoside analogue, fludarabine (FAMP; 30 mg/m2 of body-surface area daily for 5 days every 28 days). A serum β2-microglobulin (β2M) level below 3 mg/L and a hemoglobin level of at least 120 g/L (12 g/dL) at presentation predicted a lower likelihood of requiring therapy. The overall rate of response to FAMP therapy was 36% (95% confidence interval, 29%-44%), with 2% complete remissions. Patients who were 70 years old or older had a substantially lower likelihood of response (odds ratio, 0.34; P = .004) than younger patients. On multivariate analysis, a serum β2M level of 3 mg/L or higher, hemoglobin level below 120 g/L, and serum IgM level below 40 g/L [4 g/dL] were significant adverse prognostic factors for survival. We developed a simple staging system for WM by using these variables and identified 4 distinct subsets of patients with estimated 5-year overall survival rates of 87%, 64%, 53%, and 22%, and 5-year progression-free survival rates of 83%, 55%, 33%, and 12%. Prognosis in WM is highly variable and serum β2M was the dominant predictor of a need for therapy and of survival. FAMP has activity against WM. Our staging system may provide guidance for a risk-based approach to the treatment of WM.
Introduction
The term Waldenström macroglobulinemia (WM) was originally coined to describe a clinical syndrome characterized by high levels of macroglobulin (IgM) and symptoms related to hyperviscosity or lymphocytoid tissue infiltration.1 Histologically, the disease overlaps with lymphoplasmacytoid lymphoma or immunocytoma in the Kiel classification and lymphoplasmacytic lymphoma in the World Health Organization classification.2 Although most patients present with symptomatic disease, WM is often diagnosed during the asymptomatic stage.3 Care of patients with WM remains a challenge because current information on its natural history, therapy, and prognosis is limited to data derived from small retrospective or single-institution studies.3-5 Moreover, these studies focused largely on patients who required therapy. The decision to initiate therapy is therefore generally individualized. Survival and disease progression after therapy are difficult to predict, and no standard prognostic models exist.3 Low-dose chlorambucil is the mainstay of therapy but rarely produces complete remissions (CRs).6,7 Purine analogs such as fludarabine phosphate (FAMP) and 2-chlorodeoxyadenosine (2-CDA) have shown remarkable activity in low-grade lymphoproliferative disorders.8 In small preliminary studies, promising clinical activity of both FAMP and 2-CDA in WM was observed.8-15
In 1992, the Southwest Oncology Group (SWOG) began a prospective clinical trial to examine the natural history of WM and evaluate the clinical activity of FAMP in untreated patients as well as those who had previously undergone alkylating agent–based therapy. We present the results of this first large intergroup clinical trial in patients with WM. We also describe a simple prognostic model predictive of the need for therapy and of survival.
Patients and methods
Eligibility and study design
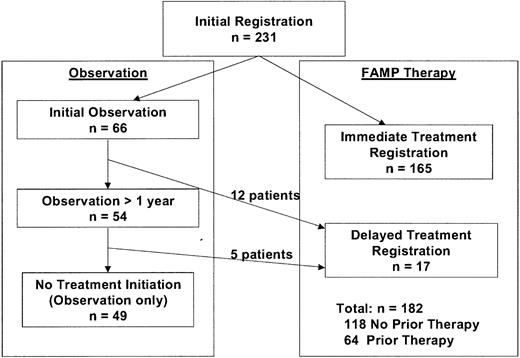
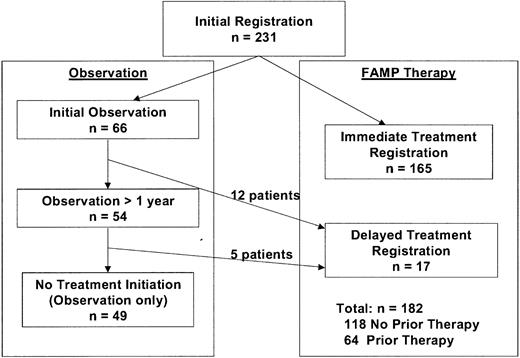
The study schema is shown in Figure1. All patients had a diagnosis of WM based on demonstration of an IgM monoclonal gammopathy with monoclonal light-chain components in the serum or urine and the presence of monoclonal lymphocytic infiltration in the bone marrow, lymph nodes, spleen, or liver.16 Other eligibility criteria were performance status score of 0 to 2 according to SWOG criteria and adequate hematologic function (granulocyte level ≥ 1.5 × 109/L [1500/μL] and platelet count ≥ 100 × 109/L [100 000/μL]) unless explained by marrow involvement or autoimmune suppression. Patients who had undergone chemotherapy within 4 weeks of enrollment, patients with a history of other malignant disease, and pregnant or lactating women were excluded from the study.
In the absence of symptoms or advanced tumor mass, patients were registered for observation after baseline studies were completed. Patients were eligible for registration for therapy with FAMP if WM-related symptoms thought to require therapy developed or they had an marked tumor mass defined as lymphadenopathy (> 2 cm), palpable hepatomegaly, splenomegaly, or considerable (> 50%) bone marrow infiltration. Patients were also eligible for therapy if they had evidence of disease progression defined as an increase in serum IgM level of at least 50% or a decrease in hemoglobin level of more than 20 g/L (2 g/dL) or in platelet count of more than 50 × 109/L (50 000/μL). In cases in which the indications for therapy were clinically unclear, investigators were encouraged to observe the patients to obtain information on disease evolution. Patients meeting the criteria for therapy at initial registration were eligible for immediate reregistration for therapy. Patients were stratified at the time of treatment according to whether they had previously received therapy for WM.
Treatment
FAMP was administered intravenously at a dose of 30 mg/m2 of body-surface area daily for 5 days. In the absence of disease progression, cycles were repeated every 28 days for at least 4 cycles. Patients who had a response to treatment received 4 additional cycles of therapy or 2 cycles beyond the maximum response level, whichever occurred earlier.
Laboratory monitoring
Laboratory evaluations at baseline included a complete blood count; serum and urine electrophoresis, immunoelectrophoresis, or immunofixation studies; quantitative immunoglobulin assessments; measurements of serum viscosity, β2-microglobulin (β2M), and lactate dehydrogenase (LDH); a bone marrow biopsy; and computed tomographic (CT) scanning of the abdomen. Patients registered to receive observation alone were monitored monthly for 3 months and then every 3 months. Follow-up monitoring after completion of FAMP therapy was done every 3 months. Bone marrow examinations and radiographic studies (if indicated) were repeated at 6-month intervals.
Response criteria
CR required the disappearance of all monoclonal protein in the serum or urine on immunofixation studies and resolution of measurable tumor mass lesions and marrow involvement for at least 2 months. Remission (R) required at least a 75% reduction in IgM levels together with at least a 50% reduction in tumor mass lesions and a decrease in marrow lymphocytosis to below 25%. Partial remission (PR) was defined as a greater than 50% reduction in IgM level and tumor mass lesions. All responses had to be confirmed by a second assessment 4 weeks later. Progression was defined as an increase in measurable tumor marker lesions of more than 25% after 2 cycles of FAMP.
Statistical analysis
Overall survival (OS) from initial or treatment registration was calculated as time from registration to the last date of contact or the date of death. Progression-free survival (PFS) was calculated as time from treatment registration to the date of progression, death, or last contact. Time to treatment was calculated as the time from initial registration to date of treatment registration (for patients who did not register concurrently to receive the treatment step). Survival curves were estimated by using the product limit method and compared by using the log rank test.17,18 Proportions were compared with the χ2 test. Univariate and multivariate analyses were done with the Cox regression model for survival and with logistic regression for binary end points.19 20
To construct a prognostic model for patients with WM, the following pretreatment factors were analyzed by using univariate Cox proportional hazards regression: previous therapy, age at registration, patient sex, hemoglobin, serum IgM, serum IgG, serum IgA, serum β2M, C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum viscosity, LDH, albumin, calcium, hepatomegaly, splenomegaly, current lymphadenopathy, cryoglobulinemia, M-component protein level, SWOG performance status score, percentage of marrow involvement, platelet count, and duration of disease. Factors for which P values below 0.1 were observed in the univariate analysis were included in the multivariate stepwise model.
Potential prognostic factors were examined as both continuous variables and dichotomous groups. Only dichotomous variables were used to construct the prognostic models, since the goal was to use easily interpreted groupings. Recursive partitioning methods were used to calculate the best cut point (based on the log rank statistic) for continuous variables that were significant in the univariate models.21 Recursive partitioning methods are based on the raw data and compute cut points that provide the greatest separation in the outcome of interest. Because clinically relevant cut points for possible prognostic factors in WM have not been not defined and are often borrowed from accepted myeloma or lymphoma models, we used adaptive cut points when different from the standard (for example, age in years, 65 [standard] versus 70 [most significant]).
Results
Clinical characteristics
Between November 1992 and November 1998, 231 eligible patients with WM were enrolled in the study, 182 of whom required therapy with FAMP (Figure 2). Of these 182 patients, 118 had received no previous therapy and 64 had undergone treatment previously. Clinical and laboratory features at initial presentation in these cohorts are shown in Table 1 and Table 2, respectively. Even with the use of CT scanning of the abdomen at baseline, splenomegaly and lymphadenopathy were detected in only 17% and 19%, respectively, of the patients in these 2 groups. Serum β2M was elevated at least 3 mg/L in 50% of the patients. Most of the patients (66%) were entered into the study within a year of diagnosis. Among the previously treated patients, the earlier therapy consisted of administration of alkylating agents in all except 4 patients (one who had received plasmapheresis, 1 dexamethasone, and 2 of whom had a response to cladribine).
Analysis of cohort not requiring therapy
One hundred sixty-five patients were reregistered immediately (or within 2 weeks) for the treatment step because of symptomatic or progressive disease. Fifty-four patients were observed without therapy for more than a year (median follow-up, 44 months; range, 16-88 months). A comparison of clinical features in this observation cohort with those in the cohort requiring therapy immediately or within a year revealed a significantly (all P < .05) lower incidence of anemia (hemoglobin level < 120 g/L [12 g/dL]), thrombocytopenia (platelet count < 150 × 109/L [150 000/μL]), lymphadenopathy, hepatosplenomegaly, hypoalbuminemia (albumin level, < 35 g/L [3.5 g/dL]), and serum hyperviscosity. In addition, the cohort not requiring therapy also had a lower proportion of patients with elevated levels of serum IgM (≥ 40 g/L [4 g/dL]), CRP (≥ 1.0 mg/L), and β2M (≥ 3 mg/L; all P < .05; Tables 1 and2).
Analysis of factors predicting requirement for therapy
On multivariate analysis, hemoglobin and serum β2M levels at presentation were the only significant predictors of an eventual need for therapy in the 66 patients who did not immediately register for therapy. Serum β2M levels below 3 mg/L and hemoglobin levels of at least 120 g/L identified a subset of patients with the lowest probability of requiring therapy. Thirty-seven of the 66 patients who did not immediately register for therapy were in this subset, and only 5 of these 37 eventually registered for therapy. Patients with serum β2M levels of 3 mg/L or higher or hemoglobin levels below 120 g/L were overall 2.4 times more likely to require therapy (P = .02).
Response to FAMP
Response was assessed in all 182 eligible patients with a median follow-up of 30 months. The overall confirmed response rate to FAMP therapy was 36% (95% confidence interval [CI], 29%-44%), with 4 CRs (2%), 25 remissions (14%), and 34 PRs (19%) (Table3). Unconfirmed partial responses (failure to have a second assessment 4 weeks after the initial response) were observed in 3 additional patients (2%). Response to therapy was not significantly greater in the cohort with no previous therapy than in those previously given alkylating agent–based treatment (38% [95% CI, 29%-48%] versus 33% [95% CI, 22%-46%]; P = .62). CR was achieved in only 4 patients (4%) with no previous therapy, and no CRs were attained in previously treated patients. Most responses occurred within 3 to 6 months of initiation of therapy; however, delayed responses occurring more than 6 months and more than 1 year, respectively, after initiation of therapy were observed in 17% and 5% of patients with a response (data not shown).
Factors predicting response to FAMP
Age at the time of registration was the only significant variable predicting response to treatment (≥ PR), both as a continuous and a dichotomized variable. Patients older than 70 years had a substantially lower response rate (hazard ratio (HR), 0.34; P = 0.004) than younger patients. Age was also the only significant variable predicting response in the cohort with no previous therapy (HR, 0.33,P = 0.02). No variables predicting CR or near CR (R and CR) were identified.
Toxicity of FAMP therapy
Toxicity had been assessed for 175 of the 182 patients at the time of the analysis described here. The principal toxic effect of FAMP was myelosuppression (Table 4), from which most patients recovered rapidly. Seven patients (4%) died as a result of possible treatment-related toxicity consisting of infection-related complications.
OS and PFS
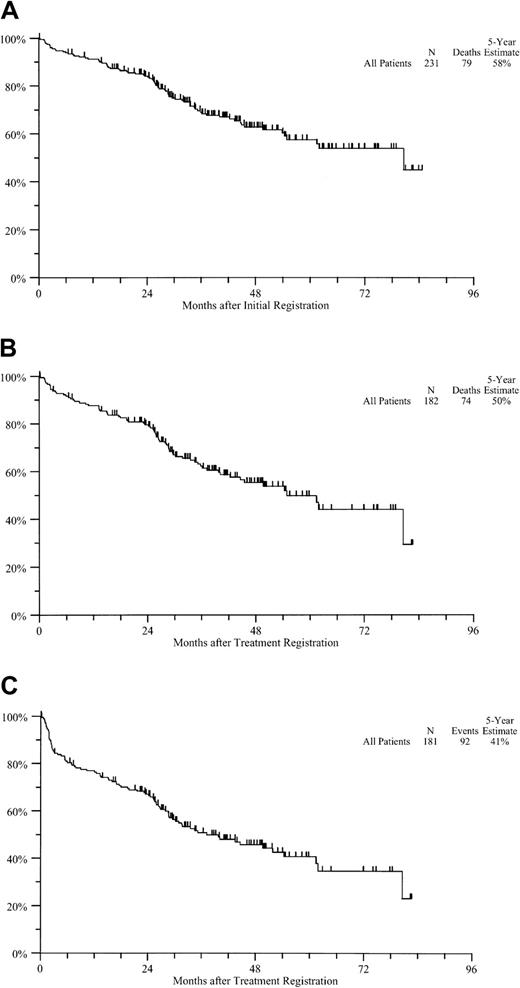
With a median follow-up of 41 months, 152 of 231 patients are alive, for an estimated 5-year OS rate of 58% (Figure3A). Estimated rates of 5-year OS and PFS from treatment registration in the treated cohort were 50% and 41%, respectively (Figure 3B and 3C). The 5-year rates of OS and PFS in the cohort with no previous therapy were 62% and 49%, respectively, whereas those in the previously treated patients were 36% and 30%, respectively.
Survival from registration.
(A) OS from initial registration. (B) OS from treatment registration. (C) PFS from treatment registration.
Survival from registration.
(A) OS from initial registration. (B) OS from treatment registration. (C) PFS from treatment registration.
Analysis of prognostic factors
Predictors of OS in the entire cohort.
In univariate analysis, higher age and serum β2M, as well as lower pretreatment hemoglobin and albumin levels, were significant adverse predictors of OS in the entire cohort as continuous variables. When tested as dichotomized variables, age 70 years or older, previous therapy, disease duration greater than a year, CRP level of 1 mg/L or higher, hemoglobin level below 120 g/L, serum albumin level below 35 g/L, and β2M level of 3 mg/L or higher emerged as significant variables (Table 5). In the multivariate stepwise model, the only significant variables for adverse OS were pretreatment hemoglobin level below 120 g/L, serum IgM level below 40 g/L, serum β2M level of 3 mg/L or higher, and previous therapy.
The adverse prognostic effect of low serum IgM (< 40 g/L) was surprising because the patients in the cohort not requiring therapy also had lower IgM levels (Table 2). To examine the interaction between serum IgM and β2M, we evaluated each of the 4 groups (low and high β2M and IgM) individually. In the group with high β2M levels (≥ 3 mg/L), there was a large survival difference between patients in the low-IgM group (< 40 g/L) and those in the high-IgM group (5-year OS, 51% versus 0%). However, in the low-β2M group, there was little difference in survival according to whether the serum IgM level was low or high (5-year OS, 76% and 77%, respectively). Therefore, the significance of IgM in the multivariate stepwise model appeared to depend on the large survival difference between patients with low IgM levels and those with high IgM levels in the high-β2M group and was lost when this group was excluded.
Diagnostic criteria for WM in previous studies were variable and somewhat arbitrary.3 Specifically, existing data do not allow establishment of a clear cut-off point for serum IgM level and percentage of marrow involvement. Pathological evaluation in WM is complicated by the low concordance rate among pathologists for the diagnosis of lymphoplasmacytic or lymphoplasmacytoid disorders in the Revised European-American Lymphoma classification. The eligibility criteria in this study therefore did not specify a minimal percentage of marrow involvement and IgM level. However, the broad inclusion criteria used in the study did raise the possibility of inclusion of patients who might otherwise be considered to have IgM–monoclonal gammopathy of undetermined significance (MGUS). In this data set, we found only 5 asymptomatic patients with serum IgM levels below 30 g/L, marrow lymphocytosis below 20%, and no lymphadenopathy and hepatosplenomegaly, a condition that could be classified as IgM-MGUS.5 These patients did not require therapy, and their exclusion did not significantly affect any survival analysis.
Predictors of survival and disease progression in patients treated with FAMP.
In the univariate analysis of patients treated with FAMP, age 70 years or older, previous treatment, disease duration greater than a year, CRP of 1.0 mg/L or higher, serum IgM level below 40 g/L, and serum β2M level of 3 mg/L or higher were significant variables predicting OS (Table 5). Of these, serum IgM and β2M remained the only significant variables for OS in the cohorts with and without previous therapy (data not shown). Serum IgM level below 40 g/L and β2M level of 3 mg/L or higher were also significant variables for PFS in the univariate analysis of patients treated with FAMP. Of these, only serum β2M remained a significant variable for PFS in both previously treated and untreated cohorts (data not shown). In the multivariate stepwise model for patients treated with FAMP, only serum β2M level higher than 3 mg/L and IgM level below 40 g/L were significant variables for PFS and OS.
Development of risk models
Prognostic models that predict survival may not predict the risk of disease progression in treated patients (for example, the Rai classification in chronic lymphocytic leukemia [CLL]).22Therefore, we examined risk models in the entire cohort and in the treated patients separately.
Risk model in the entire cohort.
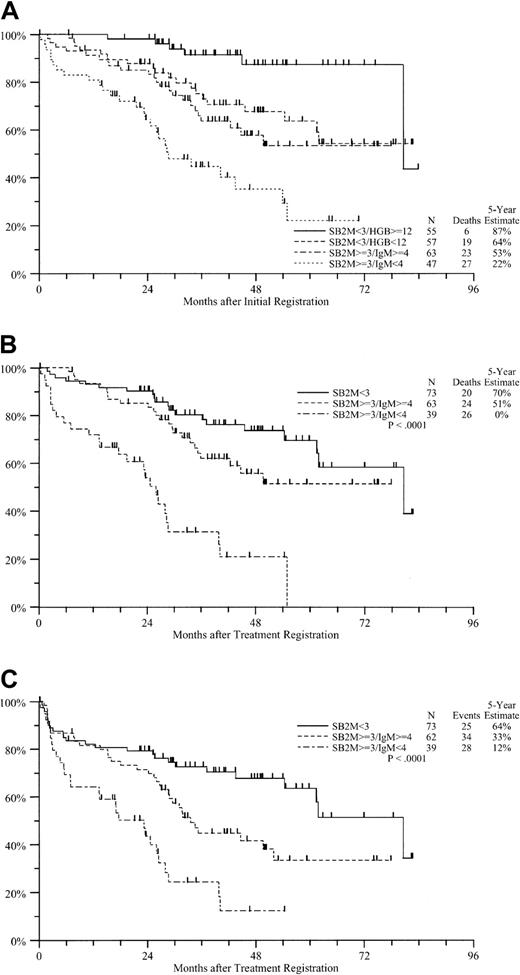
Serum β2M level emerged as the most dominant prognostic variable in this study. Therefore, we developed a simple risk model for WM based on initial serum β2M level and subclassified based on IgM and hemoglobin. This model identified 4 subsets, each including about 25% of all patients. The estimated 5-year OS rates in the 4 cohorts were 87%, 64%, 53%, and 22%, respectively (Figure4A).
Survival according to prognostic group.
(A) OS from initial registration according to prognostic group. (B) OS from treatment registration according to prognostic group. (C) PFS from treatment registration according to prognostic group.
Survival according to prognostic group.
(A) OS from initial registration according to prognostic group. (B) OS from treatment registration according to prognostic group. (C) PFS from treatment registration according to prognostic group.
Risk model in patients treated with FAMP.
We used 2 significant prognostic variables, serum β2M and IgM, to identify 3 distinct groups among the treated patients. Patients with β2M levels below 3 mg/L had the highest survival rates (Figure 4B and4C). Patients with high β2M levels (≥ 3 mg/L) could be subdivided according to serum IgM level into 2 distinct groups, with 5-year OS rates of 51% and 20%, respectively, and 5-year PFS rates of 33% and 12%, respectively (Figure 3B and 3C). Notably, these subsets did not differ with respect to response to FAMP therapy (data not shown).
Development of a staging system
Because the 2 risk models were remarkably similar, we combined them to develop a novel staging system for WM (Table6). According to this model, patients with stage A disease (low risk) have an excellent prognosis and the lowest probability of requiring therapy, and they may include a subset with “smoldering macroglobulinemia.” Patients with stages B to D generally require therapy but have a highly variable outcome after FAMP therapy. Those with stage B or C disease (intermediate risk) have similar OS but differ in durability of response after therapy (Table6). In particular, patients with stage D disease (high risk) fare poorly. These patients also have a significantly higher incidence of anemia, hypoalbuminemia, lymphadenopathy, and hepatosplenomegaly than other patients (data not shown).
Discussion
Management decisions in WM, a clinical syndrome with a highly variable prognosis, are complicated by the lack of a standard prognostic model predicting the need for therapy and patient survival. In this study, we analyzed data from the first large prospective multicenter study of WM to describe the clinical response to a purine analog (FAMP) and develop a simple staging system predictive of both the need for therapy and the OS and PFS of patients. The clinical characteristics and laboratory data at study entry in our patients were comparable to those in previously reported investigations.3 5 However, this study was the first in which several laboratory characteristics, including serum β2M, CRP, and LDH, were analyzed in a large cohort of patients with WM.
This was also the first study in which patients with asymptomatic or early macroglobulinemia were followed prospectively, and it therefore provides insights into predictors of the need for therapy in such patients. We found that low serum levels of β2M and hemoglobin values of 120 g/L or higher identified a subset of patients with an excellent prognosis and a low probability of requiring therapy. This subset (termed stage A) may therefore include patients with smoldering macroglobulinemia. Identification of factors predicting prognosis and the need for therapy is important because early treatment of other low-grade lymphoproliferative disorders (such as CLL) has not increased survival. It is notable that many patients registered in this study required therapy within 2 weeks of initial registration (Figure 2). However, it was unlikely that the study had a bias toward enrollment of symptomatic patients from referral centers because participating institutions received separate credit for registration for the observation step, and accrual for the study occurred nationwide.
The clinical activity of FAMP we observed in patients with WM who had previously been given alkylating agents was similar to that occurring in earlier small or retrospective studies.11,13,14However, a lower proportion of previously untreated patients had a response than was observed in one small study.15 It should be stressed, however, that none of these studies are directly comparable with respect to clinical activity. Possible reasons for the difference include a small sample size (19 patients in one study) and differences in patient populations or response criteria. For example, the age distribution of patients or the need to confirm response after 4 weeks in the current study may have affected the observed response rate. Because most treated patients had a follow-up exceeding 1 year, however, it is unlikely that a failure to record delayed or mixed responses (for example, improvement in splenomegaly without a reduction in paraprotein levels) accounts for the observed results.
Several other therapeutic approaches have been used in patients with WM, including administration of another purine nucleoside analog, 2-CDA; alkylating agents, used either singly or in combination23; steroids; α-interferon; anti-CD20 monoclonal antibody; and high-dose therapy.9,10,12,16 24-26 However, investigations of other therapies have the same limitations as previous studies of FAMP treatment, including the retrospective nature of several studies, small sample size, short follow-up periods, and possible patient selection and referral bias. Thus, on the basis of existing data, it is difficult to compare response rates among these studies or to conclude that one approach is superior to another. In particular, these data should not be interpreted as suggesting a superiority of 2-CDA over FAMP, since 2-CDA has not yet been tested in a similar clinical setting. In addition, in view of the prognostic heterogeneity of WM (illustrated in our patients) and the absence of any controlled data, the net impact of any of the current approaches on survival of patients with WM cannot currently be ascertained. It is notable that a common feature in all studies of WM (including ours) is a low CR rate. Achieving a higher proportion of CRs may be an important surrogate end point for future research.
Few studies have analyzed prognostic factors in patients with WM.27-31All studies that did include such an analysis were retrospective. Of the 3 larger studies, one found hypoalbuminemia, age, and cytopenias to be independent predictors,28 whereas another identified anemia, age, weight loss, and cryoglobulinemia as prognostic factors.29 The third found that age, albumin level, and the number of cytopenias were prognostically important.31 In our study, age, anemia, and hypoalbuminemia were significant factors in the univariate analysis, but serum β2M level, which was not tested in other multivariate models, emerged as the dominant prognostic determinant. Indeed, in one of these studies, serum β2M measurements were available for a small subset of patients and β2M level was found to be a significant prognostic variable.
An important finding of our study is identification of serum β2M level as a dominant prognostic variable in patients with WM. Serum β2M is also a dominant prognostic determinant in both myeloma and CLL.22,32 Currently, serum β2M is thought of as a measure of tumor bulk. However, it is also possible that there is a direct biologic effect on the tumor33 or the host immune response. A surprising finding was that among patients with high serum β2M levels, those with higher serum IgM values did better than those with low serum IgM levels. One possible explanation for this finding is that a high serum IgM level may reflect the differentiated nature (and thereby the low proliferative potential) of the malignant clone. It is notable that independent analysis of another large data set including diverse therapies also suggested that patients with higher M-protein or lower serum β2M levels have higher survival rates (S. V. Rajkumar, personal communication, February 2001).
Similarities among various risk models allowed us to develop a simple staging system predictive of both the need for therapy and survival in patients with WM. A principal and unique strength of this analysis is that it is based on a large prospective data set with a uniform follow-up. The prognostic variables (hemoglobin and β2M levels) used here were, when measurements were available, also significant in data sets of patients treated with other therapies,31suggesting that our proposed staging system may be broadly applicable and useful in guiding clinical research on WM. Our finding of a poor prognosis in the subset of patients with high β2M and low IgM levels (stage D disease) must be confirmed by analysis of an independent data set.
Our data have several implications for the care of patients with WM. Measurement of serum β2M should be incorporated into the initial work-up for WM. Our study provides evidence that patients with asymptomatic WM can be observed safely without therapy. In particular, patients with stage A disease have an excellent prognosis and may not require therapy for prolonged periods. However, observation of these patients must be considered an active intervention, analogous to “watchful waiting” in low-grade lymphoma. Our data also show that FAMP has clinical activity in WM, both in untreated patients and in patients previously given alkylating agent–based therapy. In particular, our study was the first large prospective investigation in which a purine analog was tested as front-line therapy in WM. However, existing data do not support a preference for either alkylating agents or purine analogs (FAMP or 2-CDA) as initial therapy. Because the proportion of patients achieving CRs after any of the current approaches remains low, patients should be treated, when possible, in the context of hypothesis-driven clinical studies. Our proposed staging system may allow guidance for a risk-adapted approach to testing newer strategies for treating WM. Moreover, additional molecular characterization of the clinical subsets of patients we identified may provide important insights into the pathogenesis and biologic diversity of this distinct clinical syndrome.
We thank Jeana Cromer, Monica Yee, Patricia O'Kane, and Diana Lowry for invaluable assistance with data monitoring; Mike LeBlanc for discussions about statistical analysis; and all participating physicians and clinical research associates for their interest and participation in this study. This paper is dedicated to the memory of Dr Sidney Salmon, who led the myeloma committee at SWOG for the entire 10 years that it took to complete this study.
Supported in part by the following Public Health Service Cooperative Agreement grants awarded by the National Cancer Institute, Department of Health and Human Services: CA38926, CA32102, CA20319, CA22433, CA46441, CA58416, CA37981, CA13650, CA66636, CA21115, CA13612, CA35261, CA12644, CA76462, CA35192, CA45450, CA04919, CA35090, CA58686, CA45807, CA42777, CA45377, CA46282, CA04920, CA58861, CA76447, CA67663, CA46113, CA45560, CA63850, CA14028, CA35431, CA35176, CA27057, CA76132, and CA12213.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Southwest Oncology Group (SWOG-9003), Operations Office, 14980 Omicron Dr, San Antonio, TX 78245-3217.