Abstract
Acquired immunodeficiency syndrome–related non-Hodgkin lymphomas (AIDS-NHL) are thought to arise because of loss of Epstein-Barr Virus (EBV)-specific cellular immunity. Here, an investigation was done to determine whether cellular immunity to EBV is lost because of physical loss or dysfunction of EBV-specific cytotoxic T cells. Data on EBV-specific cellular immunity were correlated with EBV load. For comparison, individuals who progressed to AIDS with opportunistic infections (AIDS-OI) and long-term asymptomatics (LTAs) were studied. The number of virus-specific T cells was detected using tetrameric HLA–EBV-peptide complexes; function of these EBV-specific T cells was determined using the interferon-γ (IFN-γ) Elispot assay. It was observed that EBV-specific CD8+ T cells were present in normal numbers in human immunodeficiency virus (HIV)-infected individuals. However, their functional capacity was decreased compared with HIV− individuals. In AIDS-NHL patients, EBV-specific T cells were not physically lost in the course of HIV-1 infection but showed progressive loss of their capability to produce IFN-γ in response to EBV peptides. This loss of function correlated with lower CD4+ T-cell numbers and was accompanied by increasing EBV load. In HIV-1–infected LTA individuals, in whom CD4+T-cell numbers were maintained, and progressors to AIDS-OI, IFN-γ–producing EBV-specific T cells were stable and EBV load remained stable or decreased in the course of HIV infection, suggestive of immune control. Our data indicate that functional loss of EBV-specific CD8+ T cells with a concomitant increase in EBV load may play a role in the pathogenesis of AIDS-NHL.
Introduction
Epstein-Barr virus (EBV) is a widespread human gamma herpesvirus. Primary infection with EBV usually occurs asymptomatically,1 whereafter the virus persists for life in a latent form in B lymphocytes.2 The initial expansion and reactivation of these latently infected B lymphocytes is controlled by specific CD8+ major histocompatibility complex (MHC) class I–restricted cytotoxic T-lymphocyte (CTL) responses.3 Because reactivation may result in lytic antigen expression,4 CTL control during this stage of reactivation may involve lytic antigen-specific effector T cells.5
During immunodeficiency, a higher rate of reactivation of EBV infection may lead to uncontrolled lymphoproliferation.6 In human immunodeficiency virus (HIV)-infected individuals, the incidence of non-Hodgkin lymphomas (NHLs) is considerably increased (5%-10%), and most of these lymphomas (75%) are EBV+.7These acquired immunodeficiency syndrome (AIDS)-related diffuse large cell NHLs are therefore thought to arise because of loss of EBV-specific T-cell immunity.8 9
Until now, only few cross-sectional studies have been performed to study EBV-specific immunity in AIDS-NHL patients. Some studies show decreased EBV-specific cytotoxic T-cell activity in patients with AIDS and AIDS-related complex.10 In contrast, other studies by Carmichael et al and Geretti et al comparing EBV- with HIV-specific T-cell responses showed sustained EBV-specific CTL responses with declining HIV-1–specific CTL in advanced HIV-1 infection, suggesting selective loss of HIV-1–specific CTL.11,12 We previously reported a longitudinal study into the kinetics of HIV-1– and EBV-specific CTL responses in HIV-infected individuals using limiting dilution analysis to determine the number of CTL precursors (CTLp). This study revealed that loss of HIV-specific CTLs is not necessarily paralleled by loss of EBV-specific T-cell responses. In patients with AIDS-NHL, diagnosis of NHL was found to be preceded by a decrease in EBV CTLp and an increase in the number of infected B cells as measured in an in vitro EBV transformation assay.8
The underlying cause for this loss in EBV-specific CTLp is unknown. Recently, new techniques using MHC class I–peptide tetrameric complexes have been developed for enumerating antigen-specific CD8+ T cells.13 This method demonstrated a much higher frequency of antigen-specific circulating T cells than previously estimated by limiting dilution analysis. Moreover, interferon (IFN)-γ Elispot assays have been shown to enable enumeration of functional EBV-specific T cells at the peptide level.14 Combination of these new methods allowed us to investigate at the peptide level whether EBV-specific CD8+T cells become either deleted or dysfunctional in patients who have developed AIDS-NHL. In addition, using a real-time quantitative polymerase chain reaction (PCR) assay, EBV DNA in infected cells was quantitated. Presence and function of CD8+ T lymphocytes specific for peptides from both latent and lytic EBV antigens were studied, and EBV load was measured in the course of HIV-1 infection in AIDS-NHL patients. For comparison, HIV-1–infected individuals who progressed to AIDS with opportunistic infections (AIDS-OI) or were long-term asymptomatic (LTA) were studied.
Patients, materials, and methods
Study population
This study was performed on participants of the Amsterdam Cohort Studies on AIDS and HIV-1 infection. From these individuals at risk of HIV-1 infection, we selected HIV+ homosexual males according to duration of follow-up, availability of samples, and HLA type. Blood samples from these individuals were collected every 3 months for HIV-1 serology and immunologic studies. In addition, at all time points peripheral blood mononuclear cells (PBMCs) were cryopreserved.
We analyzed longitudinal PBMC samples from 5 HIV-1 infected individuals progressing to AIDS-related diffuse large cell NHL (NHL6006, NHL0118, NHL6118, NHL0139, NHL0308), starting at or soon after HIV-1 seroconversion. For comparison, we studied PBMC samples from 3 HIV-1–infected individuals progressing to AIDS (classification of the Centers for Disease Control, 1993) with opportunistic infections (PROG0232, PROG0341, PROG0642) and 3 HIV-infected LTA (LTA0036, LTA1160, LTA0057) individuals with CD4+ T-cell counts above 500/μL during more than 8 years of asymptomatic follow-up. Characteristics of the HIV-1–infected individuals are summarized in Table 1. In addition, PBMC samples from 6 HIV− individuals who were either HLA-B8+ or HLA-A2+B8+ were analyzed. Approval for these studies was obtained from the Academic Medical Center Institutional Review Board. Informed consent was provided according to the Declaration of Helsinki.
Flow cytometry and tetramer staining
MHC class I tetramers complexed with EBV peptides were produced as previously described.13 The peptides used (synthesized by solid-phase methods using an automated multiple-peptide synthesizer and Fmoc chemistry) were 2 immunodominant epitopes from EBV lytic cycle proteins, the HLA-A2–restricted epitope GLCTLVAML (A2-GLC) from BMLF-115 and the HLA-B8–restricted epitope RAKFKQLL (B8-RAK) from BZLF-1,16 and 1 immunodominant epitope from the latent antigen EBNA-3A, the HLA-B8–restricted epitope FLRGRAYGL (B8-FLR).17 Biotinylated class I peptide complexes were tetramerized by addition of allophycocyanin or phycoerythrin-conjugated streptavidin.
Two-color fluorescence analysis was performed as previously described.18 Briefly, PBMCs were thawed, and 1.5 × 106 cells were stained in phosphate-buffered saline (PBS) supplemented with 0.5% (vol/vol) bovine serum albumin (BSA) with MHC class I tetramers and peridinin chlorophyll protein (PerCP)-conjugated monoclonal antibody (mAb) CD8 (Becton Dickinson, San Jose, CA). After staining, cells were washed with PBS/BSA and fixed in PBS/1% paraformaldehyde, and at least 250 000 events were acquired using a FACSCalibur flow cytometer (Becton Dickinson). The tetramer staining was very reproducible because multiple stainings on PBMCs from the same donor gave similar results.
To determine the percentage of dead cells in each sample, a propidium iodide staining was performed. Lymphocytes were gated by forward and side scatter. Data were analyzed using the software program Cell Quest (Becton Dickinson).
T-lymphocyte immunophenotyping for CD4 and CD8 membrane markers was performed in real time by flow cytometry.
Elispot assay for single-cell IFN-γ release
IFN-γ–producing antigen-specific T cells were enumerated using IFN-γ–specific Elispot assays as previously described.14 A total of 96-well nylon-backed plates (Nunc, Roskilde, Denmark) were coated overnight with 50 μL of 15 μg/mL anti–IFN-γ mAb, 1-DIK (Mabtech, Stockholm, Sweden), in 0.1 M carbonate/bicarbonate buffer, pH 9.6. After 6 wash steps with culture medium (RPMI 1640, Gibco, Life Technologies, Breda, The Netherlands) to remove unbound antibody, plates were blocked for 1 hour with RPMI 1640 supplemented with 10% fetal calf serum. Subsequently, PBMCs were added in triplicate wells at 1 × 105 cells per well in case of HLA-B8–restricted responses or 2 × 105 cells per well in case of HLA-A2–restricted responses in the absence or presence of 2 μM peptide. As a positive control to test the capacity of PBMCs to produce IFN-γ in general, phytohemagglutinin (Murex Diagnostics, Dartford, United Kingdom) was added. Cultures were incubated overnight at 37°C in 5% CO2. The next day, cells were removed by washing with PBS/0.05% Tween 20, and the second biotinylated anti–IFN-γ mAb, 7-B6-1 biotin (Mabtech), was added at 1 μg/mL in PBS and left for 3 hours at room temperature, followed by streptavidin-conjugated alkaline phosphatase (Mabtech) for an additional 2 hours. Individual cytokine-producing cells were detected as dark purple spots after a 10-minute reaction with 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium (BCIP/NBT, Sigma, St Louis, MO). Reactions were stopped by extensive washing in water. Nylon membranes were dried, and spots were counted after computerized visualization by a scanner (Hewlett-Packard, Boise, ID). The number of specific T-cell responders per 106 PBMCs were calculated after subtracting negative control values. Because the percentage of dead cells and the percentage of CD8+ T cells were assessed in the same samples, the number of specific T-cell responders per 106 living CD8+ T cells could be calculated. This assay was very reproducible when performed on multiple samples from EBV+ donors, detecting as low as 1 positive cell per 1 × 105 PBMCs (0.001%).
Intracellular IFN-γ staining after antigen-specific stimulation
A total of 2 ×106 PBMCs per milliliter were either stimulated with 1 μg EBV (RAK) peptide, used in the tetrameric complexes, or PMA/ionomycin (positive control) or not stimulated (medium alone as negative control) at 37°C for 4 hours in the presence of 3 μM monensin.19 This stimulation was sufficient to induce IFN-γ production in most potential cells. Stronger stimulation protocols (10 μg peptide per milliliter for 6 hours) did not substantially increase the number of IFN-γ–producing cells. After incubation, cells were washed and stained in PBS supplemented with 0.5% (vol/vol) BSA for 15 minutes with HLA-B8–RAK tetramers (APC) and PerCP-conjugated mAb CD8 (Becton Dickinson). After membrane staining, cells were washed with PBS/BSA and fixed with 4% paraformaldehyde, permeabilized (permeabilization kit, Becton Dickinson), and stained intracellularly with IFN-γ–PE (Becton Dickinson) for 30 minutes at 4°C. At least 200 000 events in the lymphogate were acquired using a FACSCalibur flow cytometer (Becton Dickinson).
DNA extraction and real-time quantitative TaqMan assay
PBMCs (1 × 106) were lysed by addition of L6-lysis buffer.20 Genomic DNA was extracted by precipitation with isopropanol, and DNA from 2 × 105cells was amplified using PCR primers selective for the EBV DNA genome encoding the nonglycosylated membrane protein BNRF1 p143.21,22 PCR amplification was performed as previously described23 using EBV/p143 forward and reverse primers resulting in a 74–base pair DNA product. In the PCR reaction, a fluorigenic EBV/p143-specific probe was added with a FAM reported molecule attached to the 5′ end and a TAMRA quencher linked at the 3′ end to detect amplified DNA. Amplification and detection was performed with an ABI Prism 7700 Sequence Detection System (PE Applied Biosystems, Foster City, CA). Real-time measurements were taken, and a threshold cycle value was calculated for each sample by determining the point at which the fluorescence exceeded a threshold limit of 0.04. Each run contained several negative controls (no template or EBV− DNA), a positive control (a known amount of EBV copies), and a standard dilution of plasmid DNA containing the PCR product as insert, which was calibrated with an EBV-quantified standard (Advanced Biotechnologies, Epsom, United Kingdom). The analyzed sensitivity of the assay was between 50 and 5 × 106 copies per milliliter. All reactions were performed in duplicate and only considered positive when both replications were above the threshold limit. The variation between duplicates was as low as 7.5%.
Statistical analysis
To compare EBV-specific tetramer+ T cells, IFN-γ–producing CD8+ T cells, and EBV load early and late in HIV infection, Wilcoxon tests were performed. To compare all variables early or late between groups, Mann-Whitney tests were performed using the software program SPSS 7.5 (SPSS, Chicago, IL). To test the relation between CD4+ T-cell numbers and EBV-specific functional CD8+ T cells, EBV-tetramer+ T cells, or total CD8+ T cells, regression analyses (mixed linear model) were performed. Regression analysis and multivariate analysis to search for predictors of the number of functional T cells were performed after cube root transformation of all variables. To correct for a possible correlation between multiple observations from one person, compound symmetry was used as correlation structure using the Proc Mixed procedure of the software program SAS.
Results
Direct visualization and functional analysis of EBV-specific CD8+ T lymphocytes
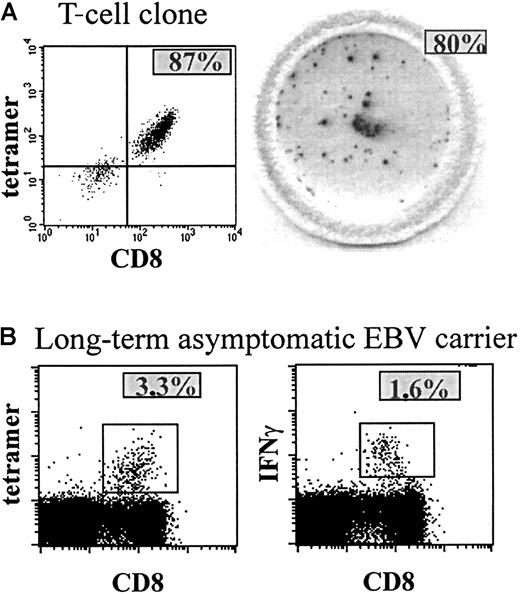
To investigate the presence and function of EBV-specific CD8+ T cells, we studied CD8+ T cells specific for 2 epitopes derived from lytic antigens, the HLA-A2–restricted epitope GLCTLVAML (A2-GLC) and the HLA-B8–restricted epitope RAKFKQLL (B8-RAK), and for 1 epitope derived from a latent antigen, the HLA-B8–restricted epitope FLRGRAYGL (B8-FLR). To detect the presence of antigen-specific CD8+ T cells, we stained antigen-specific T cells using tetrameric HLA-peptide complexes. To assess the function of antigen-specific CD8+ T cells, we used the IFN-γ Elispot assay, which shows the number of IFN-γ–producing T cells after peptide stimulation. To determine the sensitivity of both assays, we applied them both to an HIV-specific T-cell clone that was selected in vitro to respond to one specific (HIV RT) peptide.24 In Figure 1(left panel), tetramer staining of this T-cell clone is shown, revealing that 87% of the T-cell clone was specific for the tetramer containing the specific peptide. IFN-γ Elispot revealed that virtually all tetramer+ T cells from the T-cell clone produced IFN-γ upon stimulation with the specific peptide (Figure 1A, right panel), because almost every (93%) tetramer+ T cell could be accounted for in the Elispot assay. In addition, IFN-γ production was similar in both Elispot assay and intracellular FACS staining (manuscript in preparation). Tetramer staining of PBMCs from a healthy EBV-carrying individual showed that approximately 3% of the CD8+ T cells were specific for an EBV-peptide (Figure 1B, left panel), half of which produced IFN-γ after peptide stimulation in a direct ex vivo assay using intracellular staining (Figure 1B, right panel) or Elispot assay (data not shown). Furthermore, both assays were reproducible and sensitive on both fresh and frozen material (data not shown).
Tetramer staining and IFN-γ production of a T-cell clone and PBMCs.
(A) The left panel shows tetramer staining of a T-cell clone of which most of the T cells are specific for one peptide, revealing the presence of ∼ 87% tetramer+ CD8+ T cells (upper right quadrant). In the right panel, a well of a 96-well nitrocellulose-backed plate containing spots is shown, revealing ∼100 IFN-γ–producing T cells (∼80%) after peptide stimulation of ∼125 cells of the T-cell clone, which comes down to ∼93% IFN-γ–producing tetramer+ T cells. (B) Percentage tetramer staining (3.3%, left panel) and percentage IFN-γ–producing CD8+ T cells after peptide (RAK) stimulation and intracellular IFN-γ staining (1.6%, right panel) of PBMCs of a healthy EBV carrier.
Tetramer staining and IFN-γ production of a T-cell clone and PBMCs.
(A) The left panel shows tetramer staining of a T-cell clone of which most of the T cells are specific for one peptide, revealing the presence of ∼ 87% tetramer+ CD8+ T cells (upper right quadrant). In the right panel, a well of a 96-well nitrocellulose-backed plate containing spots is shown, revealing ∼100 IFN-γ–producing T cells (∼80%) after peptide stimulation of ∼125 cells of the T-cell clone, which comes down to ∼93% IFN-γ–producing tetramer+ T cells. (B) Percentage tetramer staining (3.3%, left panel) and percentage IFN-γ–producing CD8+ T cells after peptide (RAK) stimulation and intracellular IFN-γ staining (1.6%, right panel) of PBMCs of a healthy EBV carrier.
In preliminary studies using IFN-γ Elispot assay, the selected EBV epitopes were shown to be immunodominant in that they were recognized by a high frequency of CD8+ T cells (100-400 peptide-specific T cells per 106 PBMCs) in all HIV-infected individuals studied. HLA-A2–restricted latent antigen-specific T cells were either not present or present in a very low range (data not shown). Therefore, HLA-A2–restricted T cells against latent epitopes were not studied.
Lower numbers of functional EBV-specific CD8+ T cells in HIV+ versus HIV− individuals
After immunodominance was established, the number and function of EBV peptide–specific CD8+ T lymphocytes was measured in HIV− and HIV+ individuals in the course of HIV-1 infection. For comparisons, both the total number of circulating (tetramer+ as percentage of CD8+) and the total number of functional (IFN-γ–producing as number per 106CD8+) EBV-specific CD8+ T cells was calculated from the individual peptide-specific CD8+ T cells. Combining these 2 parameters, the proportion of IFN-γ+ tetramer+ T cells could be determined.
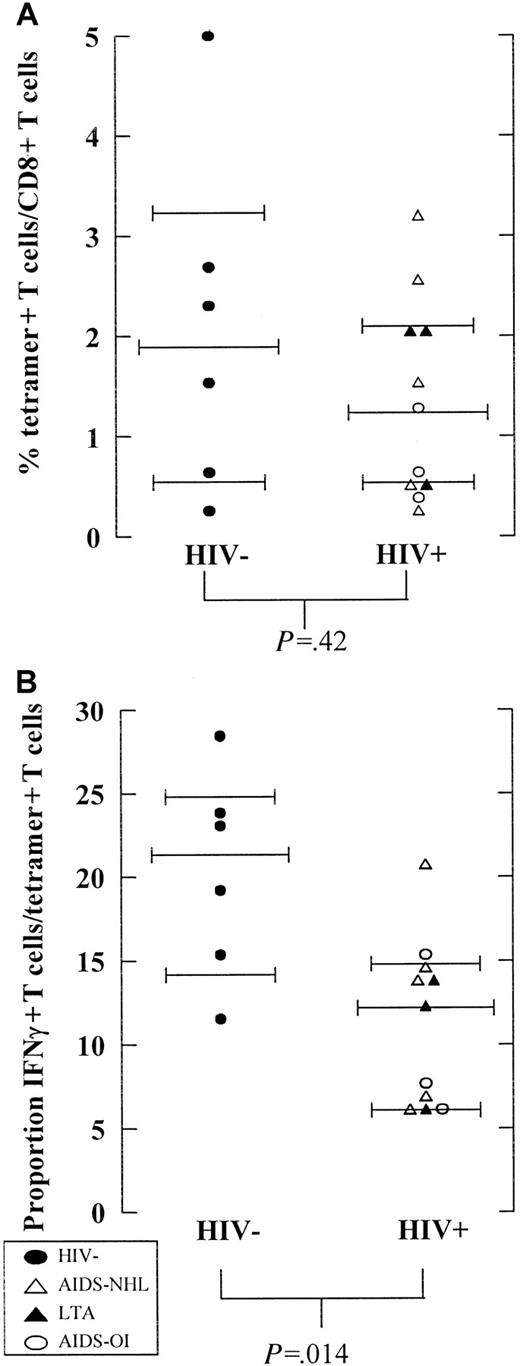
In this study population, 0.2% to 5% of the CD8+ T cells were EBV-specific. (Figure 2A). No difference in the percentage of tetramer+ EBV-specific CD8+ T cells was observed between HIV− and HIV+ individuals early in HIV infection (P = .42, Mann-Whitney test) (Figure 2A). Interestingly, in HIV− individuals a higher proportion of these EBV-specific T cells produced IFN-γ in response to the selected dominant EBV peptides (median 22%, range 12%-28%) than in HIV+ individuals early in HIV infection (median 13%, range 6%-15%) (P = .014, Mann-Whitney test) (Figure 2B).
Percentage tetramer+ EBV-specific T cells and functionality of tetramer+ T cells in HIV−and HIV+ individuals.
(A) The number of tetramer+ (percentage of CD8+T cells as assessed by tetramer staining) and (B) proportion of IFN-γ–producing T cells of tetramer+ T cells (percentage of tetramer+ T cells determined after peptide stimulation) are shown for HIV− (n = 6, filled circles) and HIV+ individuals (n = 11) early in HIV infection (average of time points studied until CD4+ T cells drop below 200/μL or halfway through follow-up). HIV+ individuals include individuals progressing to AIDS-NHL (n = 5, open triangles) or AIDS-OI (n = 3, open circles) and LTA HIV-infected individuals (n = 3, filled triangles).
Percentage tetramer+ EBV-specific T cells and functionality of tetramer+ T cells in HIV−and HIV+ individuals.
(A) The number of tetramer+ (percentage of CD8+T cells as assessed by tetramer staining) and (B) proportion of IFN-γ–producing T cells of tetramer+ T cells (percentage of tetramer+ T cells determined after peptide stimulation) are shown for HIV− (n = 6, filled circles) and HIV+ individuals (n = 11) early in HIV infection (average of time points studied until CD4+ T cells drop below 200/μL or halfway through follow-up). HIV+ individuals include individuals progressing to AIDS-NHL (n = 5, open triangles) or AIDS-OI (n = 3, open circles) and LTA HIV-infected individuals (n = 3, filled triangles).
Progressive loss of function of EBV-specific CD8+ T lymphocytes in AIDS-NHL patients
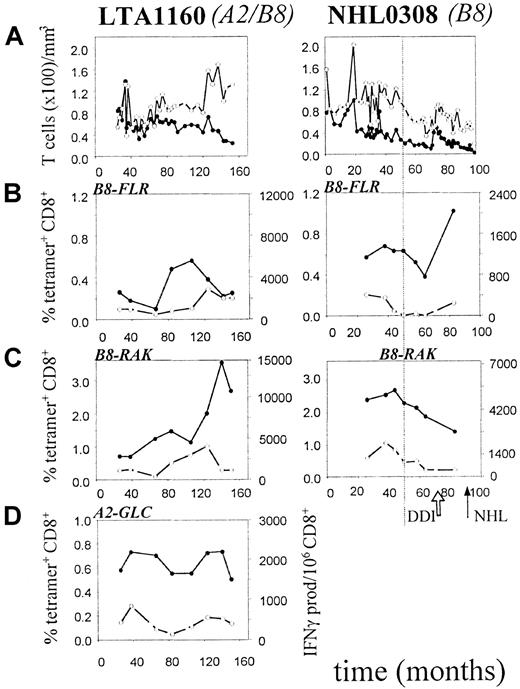
To study the cause of the defective EBV-immune surveillance in AIDS-NHL patients, we investigated both the number and function of EBV-specific CD8+ T cells in the course of HIV-1 infection in AIDS-NHL patients. For comparison, progressors to AIDS-OI and LTA individuals were studied. Detailed results from 2 study participants (1 LTA, 1 AIDS-NHL) are shown in Figure 3. Most EBV-specific CD8+ T cells were directed against the lytic epitope B8-RAK (0.7%-3.6% in Figure 3C), whereas fewer T cells were directed against the latent epitope B8-FLR (0.1%-1.1% in Figure 3B). In HLA-A2+ individuals, 0.2% to 1.2% of the T cells were directed against the lytic epitope A2-GLC (0.6%-0.8% in Figure 3D).
Presence and function of lytic and latent EBV antigen-specific CD8+ T lymphocytes in an AIDS-NHL patient (left) and an LTA individual (right).
On the x-axis, follow-up is indicated in months after HIV-1+ entry in the study. The arrows indicate the time of NHL-diagnosis (NHL) and start of therapy (DDI). The vertical dotted line indicates the time point at which CD4+ T-cell counts drop below 200/μL. (A) Longitudinal analysis of CD4+ and CD8+ T-lymphocyte numbers. (●) indicates CD4; (○), CD8. (B) Longitudinal analysis of B8-FLR–specific CD8+ T lymphocytes as assessed by tetramer staining (percentage of CD8+ T cells, solid line) and IFN-γ Elispot assay (per 106 CD8+ T cells, dashed line). (●) indicates tetramer; (○), IFN-γ. (C) Longitudinal analysis of B8-RAK–specific CD8+ T lymphocytes as assessed by tetramer staining (solid line) and IFN-γ Elispot assay (dashed line). (●) indicates tetramer; (○), IFN-γ. (D) Longitudinal analysis of A2-GLC–specific CD8+ T lymphocytes as assessed by tetramer staining (solid line) and IFN-γ Elispot assay (dashed line), as described in “Patients, materials, and methods.” (●) indicates tetramer; (○), IFN-γ.
Presence and function of lytic and latent EBV antigen-specific CD8+ T lymphocytes in an AIDS-NHL patient (left) and an LTA individual (right).
On the x-axis, follow-up is indicated in months after HIV-1+ entry in the study. The arrows indicate the time of NHL-diagnosis (NHL) and start of therapy (DDI). The vertical dotted line indicates the time point at which CD4+ T-cell counts drop below 200/μL. (A) Longitudinal analysis of CD4+ and CD8+ T-lymphocyte numbers. (●) indicates CD4; (○), CD8. (B) Longitudinal analysis of B8-FLR–specific CD8+ T lymphocytes as assessed by tetramer staining (percentage of CD8+ T cells, solid line) and IFN-γ Elispot assay (per 106 CD8+ T cells, dashed line). (●) indicates tetramer; (○), IFN-γ. (C) Longitudinal analysis of B8-RAK–specific CD8+ T lymphocytes as assessed by tetramer staining (solid line) and IFN-γ Elispot assay (dashed line). (●) indicates tetramer; (○), IFN-γ. (D) Longitudinal analysis of A2-GLC–specific CD8+ T lymphocytes as assessed by tetramer staining (solid line) and IFN-γ Elispot assay (dashed line), as described in “Patients, materials, and methods.” (●) indicates tetramer; (○), IFN-γ.
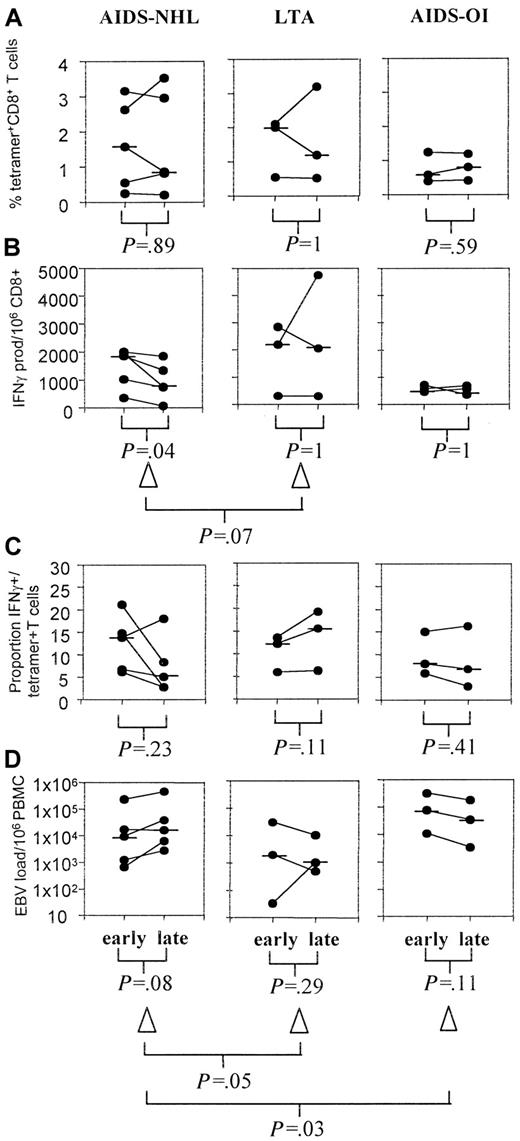
For all individuals, the total percentage of tetramer+ and number of IFN-γ–producing (functional) EBV-specific CD8+T cells was calculated from the individual peptide-specific CD8+ T cells. In Figure 4, average numbers of EBV-specific CD8+ T cells early and late in HIV-1 infection are shown for the 3 groups studied. Figures 5 and6 give detailed longitudinal information for several of the investigated participants.
EBV-specific T cells and EBV load early and late in HIV-1 infection.
For 3 groups of HIV-infected individuals, from the longitudinal data the average of early (period studied until CD4+ T cells drop below 200/μL or halfway through follow-up) and late (average of time points from the drop in CD4+ T cells below 200/μL or halfway through follow-up until AIDS diagnosis or last time point studied) time points in HIV-infection were calculated excluding time periods on therapy. For AIDS-NHL patients (left), LTA individuals (middle), and AIDS-OI patients (right), the figure depicts (A) the percentage of EBV tetramer+ CD8+ T cells, (B) the number of IFN-γ–producing EBV-specific T cells per 106 CD8+ T cells, (C) the proportion of IFN-γ+ tetramer+ T cells, and (D) EBV load per 106 PBMCs.
EBV-specific T cells and EBV load early and late in HIV-1 infection.
For 3 groups of HIV-infected individuals, from the longitudinal data the average of early (period studied until CD4+ T cells drop below 200/μL or halfway through follow-up) and late (average of time points from the drop in CD4+ T cells below 200/μL or halfway through follow-up until AIDS diagnosis or last time point studied) time points in HIV-infection were calculated excluding time periods on therapy. For AIDS-NHL patients (left), LTA individuals (middle), and AIDS-OI patients (right), the figure depicts (A) the percentage of EBV tetramer+ CD8+ T cells, (B) the number of IFN-γ–producing EBV-specific T cells per 106 CD8+ T cells, (C) the proportion of IFN-γ+ tetramer+ T cells, and (D) EBV load per 106 PBMCs.
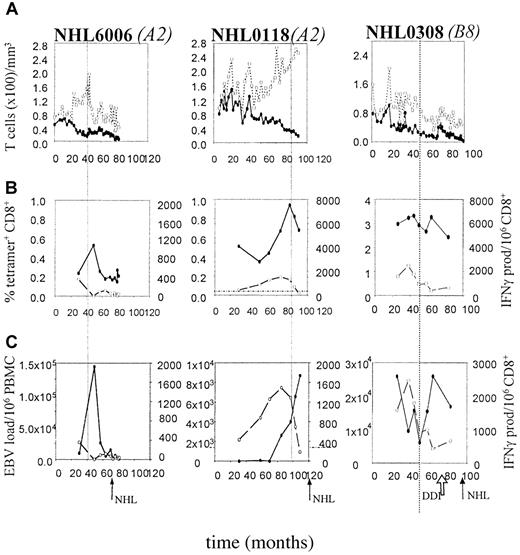
Presence and function of EBV-specific CD8+ T cells and EBV load in 3 HIV-1 infected individuals with AIDS-NHL.
On the x-axis follow-up is indicated in months after HIV-1+entry in the study. The arrows indicate the time of AIDS-NHL diagnosis (NHL) and start of antiretroviral therapy (DDI). The vertical dotted line indicates the time point at which CD4+ T cell counts drop below 200/μL. (A) Longitudinal analysis of CD4+ and CD8+ T-lymphocyte numbers. (●) indicates CD4; (○), CD8. (B) Longitudinal analysis of EBV-specific CD8+ T lymphocytes as assessed by tetramer staining as a composite of all tested tetramers (solid line) and IFN-γ Elispot assay (dashed line), as described in “Patients, materials, and methods.” (●) indicates tetramer; (○), IFN-γ. (C). Longitudinal analysis of EBV load, expressed as the number of virus copies per 106 PBMCs (solid line), in comparison with the number of functional EBV-specific CD8+ T lymphocytes per 106 CD8+ T cells (dashed line). (●) indicates EBV load; (○), IFN-γ.
Presence and function of EBV-specific CD8+ T cells and EBV load in 3 HIV-1 infected individuals with AIDS-NHL.
On the x-axis follow-up is indicated in months after HIV-1+entry in the study. The arrows indicate the time of AIDS-NHL diagnosis (NHL) and start of antiretroviral therapy (DDI). The vertical dotted line indicates the time point at which CD4+ T cell counts drop below 200/μL. (A) Longitudinal analysis of CD4+ and CD8+ T-lymphocyte numbers. (●) indicates CD4; (○), CD8. (B) Longitudinal analysis of EBV-specific CD8+ T lymphocytes as assessed by tetramer staining as a composite of all tested tetramers (solid line) and IFN-γ Elispot assay (dashed line), as described in “Patients, materials, and methods.” (●) indicates tetramer; (○), IFN-γ. (C). Longitudinal analysis of EBV load, expressed as the number of virus copies per 106 PBMCs (solid line), in comparison with the number of functional EBV-specific CD8+ T lymphocytes per 106 CD8+ T cells (dashed line). (●) indicates EBV load; (○), IFN-γ.
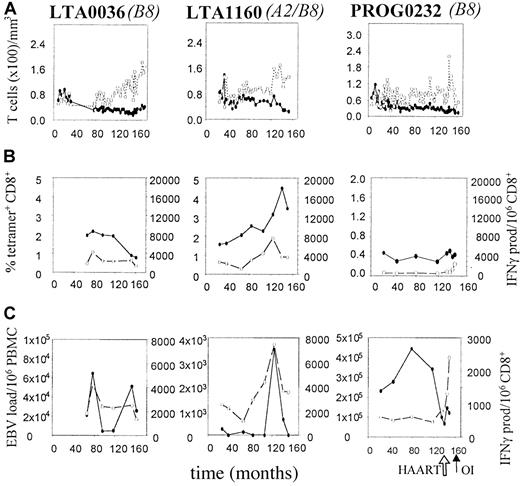
Presence and function of EBV-specific CD8+ T cells and EBV load in 2 HIV-1 infected LTA individuals and 1 HIV-1 infected individual progressing to AIDS-OI.
On the x-axis, follow-up is indicated in months after HIV-1+ entry in the study. The arrows indicate the time of AIDS diagnosis (OI) and start of HAART. (A) Longitudinal analysis of CD4+ and CD8+ T-lymphocyte numbers. (●) indicates CD4; (○), CD8. (B) Longitudinal analysis of EBV-specific CD8+ T lymphocytes as assessed by tetramer staining as a composite of all tested tetramers (solid line) and IFN-γ Elispot assay (dashed line), as described in “Patients, materials, and methods.” (●) indicates tetramer; (○), IFN-γ. (C) Longitudinal analysis of EBV load, expressed as the number of virus copies per 106 PBMCs (solid line), in comparison with the number of functional EBV-specific CD8+ T lymphocytes per 106 CD8+ T cells (dashed line). (●) indicates EBV load; (○), IFN-γ.
Presence and function of EBV-specific CD8+ T cells and EBV load in 2 HIV-1 infected LTA individuals and 1 HIV-1 infected individual progressing to AIDS-OI.
On the x-axis, follow-up is indicated in months after HIV-1+ entry in the study. The arrows indicate the time of AIDS diagnosis (OI) and start of HAART. (A) Longitudinal analysis of CD4+ and CD8+ T-lymphocyte numbers. (●) indicates CD4; (○), CD8. (B) Longitudinal analysis of EBV-specific CD8+ T lymphocytes as assessed by tetramer staining as a composite of all tested tetramers (solid line) and IFN-γ Elispot assay (dashed line), as described in “Patients, materials, and methods.” (●) indicates tetramer; (○), IFN-γ. (C) Longitudinal analysis of EBV load, expressed as the number of virus copies per 106 PBMCs (solid line), in comparison with the number of functional EBV-specific CD8+ T lymphocytes per 106 CD8+ T cells (dashed line). (●) indicates EBV load; (○), IFN-γ.
In most individuals no change in the percentage of tetramer+ T cells was observed in the course of HIV-1 infection (Figure 4A). In AIDS-NHL patients, the function of these EBV-specific T cells significantly decreased in the course of HIV-1 infection (P = .04, Wilcoxon test) (Figure 4B). The decrease in IFN-γ–producing cells (δ IFN-γ) tended to be stronger than the decrease in tetramer+ cells (δ tetramer+) corrected for the first time point (−29% and −6%, respectively, P = .07, Mann-Whitney test), indicating a discrepancy in the kinetics of the number of EBV-specific T cells present in comparison with their functionality.
The decrease in IFN-γ–producing T cells was most pronounced in patients NHL6006, NHL0308, and NHL0118, in whom almost no IFN-γ–producing CD8+ T cells were left after a few years of HIV-1 infection (NHL6006, left panel in Figure 5B,C; and B8-FLR–specific T cells in NHL0308, Figure 2B) or near lymphoma diagnosis (NHL0118, middle panel in Figure 5B). This loss of function was observed for both lytic and latent antigen-specific responses (patient NHL0308, Figure 2B,C).
Furthermore, the decline in IFN-γ–producing T cells, while the number of tetramer+ T cells remained stable, leads to a loss of the proportion of tetramer+ T cells that produced IFN-γ in individuals progressing to AIDS-NHL (Figure 4C). In contrast, in LTAs the proportion of tetramer+ T cells that produced IFN-γ even increased in the course of HIV-1 infection. As a result, the decrease in IFN-γ (Δ IFN-γ) in AIDS-NHL patients tended to be stronger than the delta IFN-γ in LTAs corrected for the first time point (−29% and +3%, respectively, P = .07, Mann-Whitney test) (Figure 4C).
Progressors to AIDS-OI showed lower but stable percentages of tetramer+ T cells (Figure 4A) and IFN-γ–producing T cells specific for EBV (Figure 4B), resulting in a stable proportion of IFN-γ+/tetramer+ T cells (Figure 4C). Interestingly, PROG0232, a slow progressor to AIDS, showed an increase in the total number of IFN-γ–producing T cells just after the start of highly active antiretroviral therapy (HAART) (Figure 6B).
Comparison of the number of IFN-γ–producing T cells determined by Elispot assay and intracellular staining
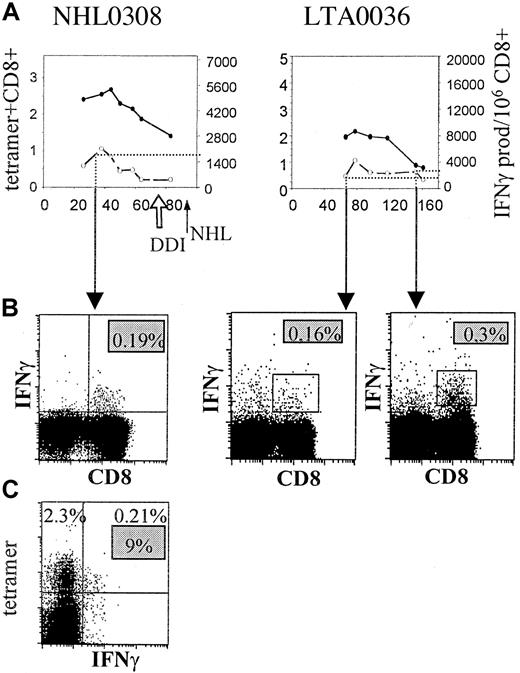
To confirm our findings with the Elispot assay, we also performed intracellular IFN-γ staining after peptide stimulation (B8-RAK) of PBMCs from an AIDS-NHL patient at an early time point (Figure7, left panel) and PBMCs from an LTA individual at 2 time points (Figure 7, right panel). At an early time point in NHL0308, the percentage of RAK-specific IFN-γ–producing T cells as assessed by intracellular staining (0.19%, or 1900 per 106 CD8) (Figure 7B) is of the same magnitude as the number of IFN-γ producers found by Elispot assay (∼1900, Figure 7A, dotted line). Similar to the Elispot results, the number of CD8+ T cells with intracellular IFN-γ staining increased in the course of infection for the LTA individual (from 0.16% to 0.3%) after stimulation, which correlated well with IFN-γ production in Elispot (Figure 7A). Moreover, the proportion of IFN-γ–producing tetramer+ T cells for the AIDS-NHL patient early in infection was 9% (Figure 7C), which corresponded well with the percentages observed using tetramer staining in combination with IFN-γ Elispot assay (around 8% early in HIV-1 infection) (Figure 4C).
Correlation of IFN-γ–producing T cells by Elispot and intracellular FACS-staining.
(A) Number of tetramer+ (percentage of CD8+ T cells, solid line) and IFN-γ–producing T cells (per 106CD8+ T cells, dashed line), as assessed by Elispot assay, are shown in the course of HIV-1 infection for RAK-specific T cells of one AIDS-NHL patient (NHL0308, left panel) and one LTA individual (LTA0036, right panel). (●) indicates tetramer; (○), IFN-γ. (B) Intracellular IFN-γ staining, as described in “Patients, materials, and methods,” after stimulation with RAK peptide is shown at an early time point in HIV-1 infection for the AIDS-NHL patient and an early and late time point for the LTA individual, revealing the percentage of IFN-γ–producing CD8+ T cells (upper right quadrant). (C) Combination of tetramer and intracellular IFN-γ staining after peptide stimulation is shown at an early time point for the AIDS-NHL patient, revealing the percentage tetramer+ (2.3%, upper left quadrant) T cells, IFN-γ+ (0.21%, upper and lower right quadrant) T cells, and proportion of IFN-γ+tetramer+ CD8+ T cells (9%, gray box).
Correlation of IFN-γ–producing T cells by Elispot and intracellular FACS-staining.
(A) Number of tetramer+ (percentage of CD8+ T cells, solid line) and IFN-γ–producing T cells (per 106CD8+ T cells, dashed line), as assessed by Elispot assay, are shown in the course of HIV-1 infection for RAK-specific T cells of one AIDS-NHL patient (NHL0308, left panel) and one LTA individual (LTA0036, right panel). (●) indicates tetramer; (○), IFN-γ. (B) Intracellular IFN-γ staining, as described in “Patients, materials, and methods,” after stimulation with RAK peptide is shown at an early time point in HIV-1 infection for the AIDS-NHL patient and an early and late time point for the LTA individual, revealing the percentage of IFN-γ–producing CD8+ T cells (upper right quadrant). (C) Combination of tetramer and intracellular IFN-γ staining after peptide stimulation is shown at an early time point for the AIDS-NHL patient, revealing the percentage tetramer+ (2.3%, upper left quadrant) T cells, IFN-γ+ (0.21%, upper and lower right quadrant) T cells, and proportion of IFN-γ+tetramer+ CD8+ T cells (9%, gray box).
EBV-specific CD8+ T-lymphocyte function is dependent on CD4+ T cells
Next, we investigated whether there was a possible relation between CD4+ T-cell numbers and the number of EBV-specific CD8+ T cells. In AIDS-NHL patients, CD4+ T-cell numbers decreased significantly in the course of HIV-infection (P = .04, Wilcoxon test). In 4 of 5 AIDS-NHL patients, loss of functional EBV-specific CD8+ T cells (Figure 5B,C) was related in time to a drop in CD4+ T-cell counts below 200/μL (Figure 5A). Furthermore, when CD4+ T-cell counts increased again after antiretroviral treatment (dideoxyinosine, or DDI) in patient NHL0308, a parallel increase in functional EBV-specific CD8+ T cells was observed (Figures 3 and 5B).
In LTAs and progressors to AIDS-OI, who did not show a functional loss of EBV-specific CD8+ T cells (Figure 6B), CD4+T-cell numbers were stable during most time points and no significant decrease in CD4+ T-cell numbers was observed (P = .11, Wilcoxon test). In LTAs and progressor P0232, CD4+ T-cell numbers never dropped below 200 CD4+ T cells per microliter (Figure 6A).
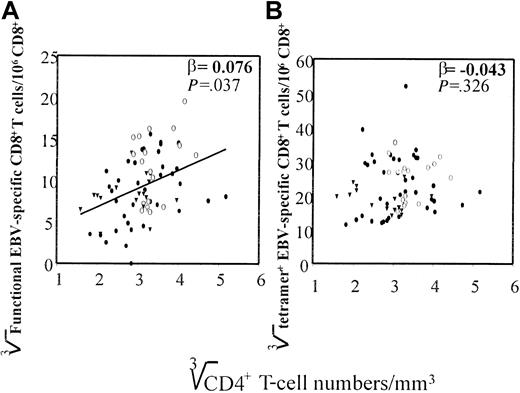
Indeed, when CD4+ T-cell numbers measured at all time points were plotted against the number of EBV-specific CD8+T cells that produced IFN-γ, a correlation was found (β = 0.076), which was highly significant (P < .037), whereas no correlation was found between CD4+ T-cell numbers and the number of tetramer+ T cells (β = −0.043) (Figure8). In addition, no correlation was found between CD4+ T-cell numbers and CD8+ T-cell numbers, indicating that the correlation between CD4+ T cells and the number of functional EBV-specific T cells was not due to an increase in total CD8+ T cells (data not shown).
Correlation of CD4+ T-cell numbers with functional EBV-specific CD8+ T cells.
CD4+ T-cell numbers (cube root transformed) of all time points of the 11 HIV-1–infected individuals are shown, determined as described in “Patients, materials, and methods,” against (A) total number of functional EBV-specific CD8+ T cells per 106 T cells (cube root transformed), determined by IFN-γ Elispot assay as described in “Patients, materials, and methods,” and (B) the cumulative percentage EBV-tetramer+CD8+ T cells (cube root transformed), assessed by staining PBMCs with HLA–EBV-peptide tetrameric complexes as described in “Patients, materials, and methods.” (●) indicates AIDS-NHL; (○), LTA; and (▾), AIDS-OI.
Correlation of CD4+ T-cell numbers with functional EBV-specific CD8+ T cells.
CD4+ T-cell numbers (cube root transformed) of all time points of the 11 HIV-1–infected individuals are shown, determined as described in “Patients, materials, and methods,” against (A) total number of functional EBV-specific CD8+ T cells per 106 T cells (cube root transformed), determined by IFN-γ Elispot assay as described in “Patients, materials, and methods,” and (B) the cumulative percentage EBV-tetramer+CD8+ T cells (cube root transformed), assessed by staining PBMCs with HLA–EBV-peptide tetrameric complexes as described in “Patients, materials, and methods.” (●) indicates AIDS-NHL; (○), LTA; and (▾), AIDS-OI.
Functional loss of EBV-specific CD8+ T lymphocytes is paralleled by an increase in EBV load
To investigate whether loss of functional T cells is associated with an increase in EBV load and whether this precedes the development of AIDS-NHL, EBV load was determined using a sensitive and specific real-time quantitative PCR. As shown in Figure 4, loss of EBV-specific CD8+ T-cell function (Figure 4B) was associated with an increase in EBV load in AIDS-NHL patients (P = 0.08, Wilcoxon test) (Figure 4D), although the absolute number of EBV copies was not different from absolute EBV load observed in LTAs and other progressors to AIDS (D.v.B. et al, manuscript submitted). Interestingly, in patient NHL0308 after the start of DDI treatment, EBV-specific CD8+ T cells slightly increased and a small reduction in EBV load was observed (Figure 5C).
In contrast, in LTAs no increase and, in progressors to AIDS-OI, even a decrease in EBV load was observed (Figure 4D, middle and right panels). The increase in load (Δ load) in AIDS-NHL patients was significantly different from the decrease in load (Δ load) in LTAs (P = .05) and progressors to AIDS-OI (P = .03, Mann-Whitney test) (Figure 4D). Interestingly, in LTAs occasional EBV peak loads (Figure 6C) were paralleled by an increase in the number of functional EBV-specific CD8+ T cells (Figure 6B) after which EBV load decreased, suggestive of EBV control. HAART, which led to an increase in the number of IFN-γ–producing CD8+ T cells (Figure 6B), resulted in a decrease of EBV load in progressor PROG0232 (Figure 6C).
To study whether EBV load is able to drive the number of IFN-γ–producing T cells, we performed multivariate analysis including the number of CD4+ T cells, EBV load, and the number of IFN-γ–producing T cells. Although multivariate analysis of all HIV-infected individuals (n = 11) showed that only the number of CD4+ T cells could predict the number of functional cells (β = 0.92, P = .0188), analysis of only AIDS-OI patients and LTAs (n = 6) showed that both EBV load (β = 0.16,P = .001) and the number of CD4+ T cells (β = 0.89, P = .0022) could predict the number of functional T cells. This suggests that EBV load is indeed able to drive EBV-specific T cells when sufficient CD4+ T cells are present.
Discussion
To investigate the cause of decreasing numbers of CTLp in AIDS-NHL patients, which is believed to lead to defective EBV-immune control, we studied number (using MHC class I tetramers) and function (using IFN-γ Elispot assay) of EBV-specific CD8+ T lymphocytes in the course of HIV infection in relation to EBV load. This is, to our knowledge, the first longitudinal study demonstrating a discrepancy between direct visualization using tetramer staining and functional assays enumerating IFN-γ–producing antigen-specific T cells. The major conclusions from our study are that (1) HIV+individuals have lower numbers of functional EBV-specific CD8+ T cells than HIV− individuals; (2) in AIDS-NHL patients, EBV-specific CD8+ T cells are lost preferentially at the functional level and are not physically lost; (3) loss of EBV-specific CD8+ T-cell function is correlated with lower CD4+ T-cell numbers; (4) increasing EBV load correlates with loss of EBV-specific immunity; and (5) the number of T cells directed against lytic antigens is higher than against latent antigens.
The observed correlation between loss of function of EBV-specific CD8+ T cells and lower CD4+ T-cell numbers indicates an important role for CD4+ T cells in maintaining the functional capacity of CD8+ T cells. Our data are in good agreement with studies on T-helper dependence of chronic lymphocytic choriomeningitis virus (LCMV)-specific CTL in mouse models.25-27 A critical role for CD4+ T cells has also been shown during immunization,28 and progressive loss of CTL in the absence of adequate helper cell function has been demonstrated for several murine viral infections.29-31Furthermore, CD4+ T cells also appear to be essential for long-term persistence of adoptively transferred virus-specific CTL in humans.27,32 33
In the natural course of HIV infection, it has been shown that progressors to AIDS lose CD8+ CTLs when functional HIV-specific CD4+ T cells disappear. In contrast, in nonprogressors, who have stable CD4+ T-cell numbers, HIV-specific CTL responses can be sustained for long periods of time,34-37 indicating that sustained HIV-specific helper activity is required for maintenance of functional CD8+T-cell responses.34,38 39
The fact that most EBV-specific CD8+ T cells were directed against lytic epitopes suggests that these lytic antigen-specific T cells play a role not only during acute infection40 but also in controlling EBV reactivation by eliminating virus-producing cells at an early stage. Loss of functional lytic antigen-specific CD8+ T cells could therefore lead to an increase in EBV DNA, as we indeed observed. As the pool of EBV-infected B cells grows, there is an increased risk of subsequent genetic hits resulting in malignant outgrowth of EBV-infected B cells. Because functional CD8+ T cells specific for latent antigens appear to be lost as well, newly developed tumor cells will not be destroyed. Our data suggest that loss of both lytic and latent antigen-specific CD8+ T cells may contribute to the risk for development of AIDS-NHL.
In HIV infection, 0.2% to 5% of the CD8+ T cells were found to be EBV-specific, of which 13% were shown to produce IFN-γ in response to EBV peptides. This is lower than in healthy HIV− EBV carriers, where the percentage of IFN-γ–producing T cells was approximately 22% (our data), consistent with data from literature.41 In AIDS-NHL patients, the number of IFN-γ–producing T cells was found to decrease progressively in the course of HIV infection, resulting in a much lower percentage of IFN-γ–producing T cells in the tetramer+ T-cell population. Therefore, our data indicate that, next to tetramer staining, functional analysis of antigen-specific T cells may be required to fully appreciate the role of CD8+ T cells in various clinical conditions. The observed discrepancy between number and function of EBV-specific CD8+ T cells is underscored by the finding that CD4+ T-cell numbers correlated with the number of IFN-γ–producing EBV-specific CD8+ T cells but not with the number of tetramer+ CD8+ T cells.
The observed low percentage of IFN-γ–producing T cells is not likely to be a consequence of susceptibility to rapid activation-induced cell death in vitro, caused by preactivation in vivo. Because tetramer staining results before and after stimulation are virtually the same (data not shown), this indicates that antigen-specific cells are not lost after stimulation. In addition, when dead cells are included in the analysis no increase in the percentage of tetramer+ T cells was observed, suggesting that even if cells are lost, this is not occurring selectively in the EBV-specific CD8+ T cells. Furthermore, we studied expression of CD69, which has been shown to be highly expressed on nonresponsive T cells in LCMV-infected CD4 knockout mice.27 However, expression of CD69 on EBV-specific CD8+ T cells was low, reaching no higher levels than 5% of the tetramer+ T cells (data not shown). Indeed, it has been shown that, compared with healthy individuals, a higher percentage of PBMCs from HIV-infected individuals undergo apoptosis after overnight stimulation.42 However, death of T cells was not correlated with CD4+ T-cell numbers or T-cell function and was not confined to expression of T-cell activation markers.43 Moreover, it has been shown that virus-specific CD8+ T cells are able to rapidly reinitiate cytokine production after recent stimulation.44 Thus, even if the EBV-specific T cells are recently activated or preactivated in vivo, this should result in a rapid production of IFN-γ. Overall, these observations make considerable in vitro death of EBV-specific T cells unlikely.
The low percentage of IFN-γ–producing T cells was also not due to a suboptimal assay condition, because the Elispot assay was shown to detect virtually all antigen-specific T cells when a T-cell clone was analyzed. Furthermore, the percentage IFN-γ–producing T cells as assessed by intracellular staining was similar to the percentage of IFN-γ producers found by Elispot assay in both healthy controls (data not shown) and patients (Figure 7).
The phenomenon of antigen-specific CD8+ T cell dysfunction has also recently been shown for hepatitis C virus45during a period of acute infection, for tumor-specific T cells in melanoma patients,46 and for HIV-specific T cells.47 This state of dysfunction has been shown to occur both at the level of IFN-γ production and cytolytic activity.45,46 In addition, in healthy individuals there is a correlation between IFN-γ–producing T cells and CTLp frequencies.41 Furthermore, our own observations indicate that the number of CTLp8 and IFN-γ–producing T cells correlate, and both decreased in the course of HIV-infection in AIDS-NHL patients.
In AIDS-NHL patients the observed loss of function of EBV-specific CD8+ T cells was accompanied by an increase in EBV load, although absolute EBV load did not differ between groups (data not shown). This increase in EBV load cannot be attributed to technical variation, because we have low variation in duplicate measurements (7.5%) and correction for the quantity of input DNA did not change the observed patterns. In addition, increases in EBV load are not due to increases in the number of total B cells, because correction for the number of B cells in PBMC samples did not lead to altered patterns (data not shown).
Thus, finally, in AIDS-NHL patients immune control over EBV seemed to be lost. Surprisingly, in LTAs enormous transient bursts of EBV load were observed. These peaks in viral load seemed to be paralleled by expansions of functional CD8+ T cells specific for EBV. Because EBV load subsequently decreased, these cells apparently were able to control EBV viremia. Indeed, multivariate analysis of AIDS-OI patients and LTAs showed that EBV load, besides the number of CD4+ T cells, could predict the number of functional T cells, indicating that EBV load is indeed able to drive EBV-specific T cells when sufficient CD4+ T cells are present.
In progressors to AIDS-OI, EBV-specific CD8+ T cells on total were lower, suggestive of physical loss or lack of expansion of these T cells. In these patients, the relatively low number of EBV-specific T cells did, however, not lead to an increase in EBV load, suggesting that there is adequate control. Alternatively, it could be that these individuals eventually would have developed AIDS-NHL had they not developed AIDS-OI.
In conclusion, our data suggest that the development of AIDS-NHL is a multifactorial process involving at least virologic and immunologic parameters. Thus, both determinants are required to obtain a complete picture of the virus-host balance. We show that not so much the total number of circulating EBV-specific CD8+ T cells but, mainly, the number of functional EBV-specific CD8+ T cells is important in keeping EBV infection under control. When EBV-specific CD8+ T cells start to lose function, in most cases as a consequence of a decrease in CD4+ T cells, this is paralleled by an increase in EBV load. To be able to predict the occurrence of an AIDS-NHL, all these factors should be taken into account.
This study was part of the Amsterdam Cohort Studies on AIDS and HIV-1 infection, a collaboration of the Municipal Health Service, the Academic Medical Center, and Central Laboratory of the Blood Transfusion Service (CLB). We thank Dr M. Roos and collaborators for T-lymphocyte immunophenotyping, and N. Dukers for statistical analysis.
Supported by the Dutch Cancer Society (grant 96-1168), the Dutch AIDS Fund (grant 1007), and the Dutch Organization for Scientific Research (NWO).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Debbie van Baarle, Dept of Clinical Viro-Immunology, CLB, Plesmanlaan 125, 1066 CX Amsterdam, The Netherlands; e-mail: d_van_baarle@clb.nl.