Disruption of the RAS–to–mitogen-activated protein kinase (MAPK/ERK) signaling pathway, either directly through activatingRAS gene mutations or indirectly through other genetic aberrations, plays an important role in the molecular pathogenesis of myeloid leukemias. Constitutive activation of ERK-1/2 and MEK-1/2, which elicit oncogenic transformation in fibroblasts, has recently been observed in acute myeloid leukemias (AML). In this study, the activation of the RAS-to-MAPK cascade in 14 AML and 5 chronic myeloid leukemia (CML) cell lines is examined and correlated with the effects of a panel of 9 RAS signaling inhibitors on cell viability, colony formation, cell-cycle progression, and induction of apoptosis. Activation of MEK, ERK, and the transcription factors CREB-1, ATF-1, and c-Myc is demonstrated in the majority of the cell lines (9 of 14 AML and 2 of 5 CML cell lines). Although activation of the ERK cascade did not always correlate with the presence of activatingRAS mutations or BCR-Abl, it is linked to the G0/G1 and the G2/M phase of the cell cycle. In contrast to most inhibitors (eg, B581, Cys-4-Abs-Met, FPT-2, FTI-276, and FTS), a significant growth inhibition was only observed for FTI-277 (19 of 19), FPT-3 (10 of 19), and the MEK inhibitors U0126 (19 of 19) and PD098059 (8 of 19). Treatment of NB-4 cells with FTI-277 primarily resulted in a G2/M block, whereas treatment with FPT-3 and U0126 led to induction of apoptosis. FTI-277 revealed strong toxicity toward normal purified CD34+ cells. The results suggest differences in the mechanisms of action and support a potential therapeutic usefulness of these inhibitors in the treatment of myeloid leukemias.
Introduction
The deregulation of RAS signal transduction has been implicated in the malignant growth of human cancer cells including myeloid leukemias.1,2RAS proto-oncogenes (H-RAS, N-RAS, and K-RAS) encode 21-kd G-proteins that play key roles in signal transduction, proliferation, differentiation, and malignant transformation.3-5 RAS proteins are produced as cytoplasmatic precursors, which require several posttranslational modifications (eg, prenylation, proteolysis, carboxymethylation, and palmitoylation) for membrane binding and full biologic activity.3-8 RAS functions as a biologic switch that relays signals from ligand-stimulated tyrosine kinase, cytokine, and heterotrimeric G-protein–coupled receptors to cytoplasmatic mitgen-activated protein kinase (MAPK) cascades. In its activated, GTP-bound state, RAS binds to and activates effector molecules such as Rafs, MEKK, PI-3K, and Ral-GEF.3-5,8-14 Raf kinases (A-Raf, B-Raf, c-Raf-1) selectively phosphorylate and activate MAPK kinases (MAPKK) MEK-1/2 in the MAPK/ERK pathway.13-17MEK-1/2 are dual specificity kinases that activate MAPKs (ERK-1/2).18,19 The best-characterized ERK substrates are cytoplasmic phospholipase A2 (cPLA2), ribosomal protein S6 kinases (RSKs), and transcription factors Elk-1 and CREB-1.18-20
The importance of deregulation of ERK signaling in the molecular pathogenesis of myeloid leukemias is underscored by the positioning of several oncogene and tumor suppressor gene products on this pathway.5,21-23RAS mutations are frequent genetic aberrations found in 20% to 30% of all human tumors, although the incidences in tumor type vary greatly.1,2 The most commonly observed RAS mutations arise at sites critical for RAS regulation, namely codons 12, 13, and 61.1,2,5,9Additionally, mutations occur at codons 15, 16, 18, and 31.24,25 These mutations result in abrogation of normal intrinsic or GAP-stimulated GTPase activity of RAS, leading to increased half-lives of mutant RAS-GTP.5,9,26Transformation results, at least in part, from deregulated stimulation of mitogenic signal transduction pathways.1,2,5 The highest incidences of RAS mutations were detected in carcinomas of pancreas (90%), thyroid (50%), colon (50%), and lung (30%). RAS mutations are also frequently observed in myelodysplastic syndromes, acute myeloid leukemias (AML), juvenile myelomonocytic myeloid leukemia (JMML), and multiple myelomas (20%-40%).1,2,5,21-23 N-RAS is mutated in the majority of cases and presence of the mutation is not associated with any particular FAB type, cytogenetic abnormality, or clinical feature including prognosis.22
In addition to activation by mutation, RAS is also deregulated in myeloid leukemias by constitutive activation of proto-oncogenes such as receptor or nonreceptor tyrosine kinases (RTKs and NRTKs) or inactivation of tumor suppressor genes.5,21-23 RTKs are constitutively activated by single point mutations (eg, colony-stimulating factor-1 [CSF-1] receptor and c-Kit receptor), duplications of juxtamembrane domain-coding sequences (eg, FLT3 receptor), or deletions of the negative regulatory regions in ligand binding or transmembrane domains.5,21,27-31 Additionally, translocations involving RTKs and NRTKs produce chimeric proteins in which varying N-terminal portions of either the ligand-binding or the transmembrane domain are replaced with novel protein sequences (BCR-Abl in CML, Abl-TEL in AML, and TEL-PDGFRβ in CMML).5,32-37These fusion tyrosine kinases have been shown to activate RAS.32-37 The product of the NF-1 tumor suppressor gene, neurofibromin, encodes an RAS-GTPase–activating protein (GAP) and is mutated in the autosomal dominant type 1 neurofibromatosis, which is associated with an increased tendency to develop myeloid leukemias, especially JMML.5,38-41 Leukemic cells from children with type 1 neurofibromatosis show a moderate elevation in the percentage of GTP-RAS.38-41
Because the frequency of RAS mutations is among the highest for any gene in human cancers, development of inhibitors of the MAPK/ERK pathway as potential anticancer agents is a promising pharmacologic strategy.5,42-44 One strategy to impede oncogenic RAS function in vivo is the inhibition of RAS posttranslational modification (eg, inhibitors of FTase, GGTase, PPMTase, REPase).5 Another strategy is the inhibition of downstream effectors of RAS (eg, geldanamycin, sulindac, and MEK inhibitors).5 Inhibitors of RAS signaling revert RAS-dependent transformation and cause regression of RAS-dependent tumors in animal models. The most promising new class of these potential cancer therapeutics is the farnesyltransferase inhibitors (FTIs).42-44 In contrast to many conventional chemotherapeutics, FTIs are remarkably specific and cause no gross systemic toxicity in animals. Some inhibitors (eg, R115777, SCH66336, and L-778123) are presently being evaluated in phase I and phase II clinical trials.5,45 46
Based on the wealth of data reporting the effectiveness of RAS signaling inhibitors against human carcinomas harboring activated RAS, coupled with the implications of RAS in the pathophysiology of myeloid leukemias, the role of activated RAS signaling and the effects of these inhibitors on leukemia cell growth were investigated. Although we demonstrate an activation of MEKs, ERKs, and CREB-1/ATF-1 in myeloid leukemia cell lines, the presence of RAS mutations and BCR-Abl did not always correlate with an activation of the RAS-to-MAPK pathway. Interestingly, MEK-1/2 activation coincided with the G0/G1 and G2/M phases of the cell cycle. Regardless of the presence of MAPK activation, incubation with various inhibitors (eg, FTI-277, FPT-3, U0126, and PD098059) of the RAS-to-MAPK cascade led to substantial growth inhibition of most myeloid leukemia cell lines tested. Incubation of NB-4 cells with FPT-3 or U0126 induced apoptotic DNA fragmentation and exposure of phosphatidylserine on the outer leaflet of the plasma membrane. In contrast, treatment of NB-4 cells with FTI-277 primarily induced a G2/M block. Treatment with FTI-277 also inhibited colony formation of purified human CD34+ cells. These results suggest differences in the mechanisms of action and a potential role of some of these inhibitors in the treatment of myeloid leukemias.
Materials and methods
Cellsand antibodies
Cell lines were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Antibodies against H-, K-, and N-RAS, ERK-1/2, MEK-1/2, monophospho- and diphospho-ERK-1/2 (PP-ERK-1/2) were from Santa Cruz Biotechnology Inc (Santa Cruz, CA) and Sigma-Aldrich (Deisenhofen, Germany). Diphospho-MEK-1/2 (PP-MEK-1/2), CREB-1, and phospho-CREB-1 antibodies were from New England Biolabs (Frankfurt, Germany). Inhibitors were purchased from Calbiochem-Novabiochem (Bad Soden, Germany).
Trypan blue exclusion assay
Cells (2.5 or 6.25 × 104 cells in 250 μL media) were seeded in 96-well plates and incubated 4 days with either solvent control or the stated concentration of inhibitor. Cell counts were evaluated using a 1:1 dilution of cell suspension in 0.4% trypan blue solution (Sigma-Aldrich). Viable and nonviable cells were counted in a Neubauer cell counting chamber.
Colony-forming assays
Colony-forming assays were performed essentially as described.47 Briefly, cells were seeded at 1.0 to 2.5 × 105/mL cell suspension in 96-well plates and treated with inhibitors as indicated. After 4 days, aliquots of the cell suspensions were plated in 400 μL methylcult H4230 (Cellsystems Biotech, St Katharinen, Germany) according to the manufacturer's instructions and incubated 7 to 14 days. Cell densities were evaluated and aggregates of more than 25 cells were scored as colonies.
Westernblot analysis
Cells were cultured in RPMI medium containing 10% heat-inactivated fetal calf serum, glutamine (292 μg/mL), and antibiotics (penicillin 60.2 mg/mL, streptomycin 133 μg/mL). Cell extracts were prepared and Western blotting was performed as described.48,49 Cellular protein concentrations were determined using the Coomassie dye-binding assay according to Bradford50 (Bio-Rad Laboratories, Hercules, CA).
MAPK assays
RAS-GTP pulldown assay
The RAS-GTP pulldown assays were accomplished as described.51 52 The pGEX 2T-RBD construct (encoding a GST fusion protein containing amino acids 51-131 of c-Raf-1) was a gift from J. Bos. GST-RBD expression in Escherichia coli was induced with 1 mM isopropyl-β-D-thiogalactopyranoside (IPTG) for 2 to 3 hours. Bacteria were sonicated in 50 mL phosphate-buffered saline (PBS) containing 0.5 mM DTT and 1 μg/mL aprotinin, leupeptin, and pepstatin A. After addition of 1% Triton X-100, clarified lysates were aliquoted and stored at −80°C as glycerol stocks (10%). The fusion protein was purified on glutathione-Sepharose beads (35 μL/sample, Pharmacia, Uppsala, Sweden). For affinity precipitation, the beads were washed 3 times with lysis buffer (1 mL), incubated with fresh cell lysates (1 mg total protein) 30 minutes at 4°C, and collected by centrifugation (12 000g at 4°C). After washing 3 times with 100 to 200 μL lysis buffer and incubation in loading buffer (5 minutes at 95°C), samples were analyzed by 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and subjected to Western blotting using antibodies against H-, K-, and N-RAS to identify GTP-bound RAS.
Cell-cycleanalysis
Cell permeabilization (1-2 × 106 cells) was performed using the GAS-002 kit from Bio Research (Kaumberg, Austria) essentially according to the manufacturer's instructions. After washing in PBS, fixation in 100 μL buffer A (15 minutes), and permeabilization in 100 μL buffer B (10 minutes), 5 μL of the primary antibodies (αMEK-1, αPP-MEK-1/2, αERK-2, αPP-ERK-1/2) or negative control antibodies were added (1 hour at room temperature). Cells were washed with 3 mL 0.1% bovine serum albumin (BSA)–PBS, and 3 μL of the appropriate fluorescein isothiocyanate (FITC)–conjugated secondary immunoglobulins were added (20 minutes in the dark). After washing with 3 mL 0.1% BSA-PBS, cells were incubated in propidium iodide staining buffer (10 mM Tris-HCl pH 7.4, 0.1% Triton-X-100, 15 μg/mL RNase A, 5 mM MgCl2, 50 μg/mL propidium iodide, 15-30 minutes at 4°C in the dark). The cell-cycle profiles (10 000 cells) were analyzed on a FACScan flow cytometer (Becton Dickinson, San Jose, CA) using the Modfit LT 2.0 software (Verity Software House, Topsham, ME).
Immunocytochemicalstaining
Cells were washed twice in PBS and cytospin slides were prepared using standard techniques. Slides were air-dried for 2 to 24 hours and processed for staining or wrapped airtight and stored at −20°C. Immunocytochemical staining of the slides was done using the Dako LSAB+ kit (DAKO, Carpinteria, CA) according to the manufacturer's instructions. After fixation in 3% paraformaldehyde (30 minutes, 4°C), cells were washed 3 times with Tris-buffered saline containing 0.1% Tween-20 (TBS-T buffer; 5 minutes) and once with TBS buffer (2 minutes). Slides were incubated in 100% methanol (10 minutes, −20°C), washed in TBS-T buffer (3 times, each 5 minutes), and blocked with 5% horse serum in TBS-T buffer (1 hour, room temperature). Incubation with primary antibodies (αMEK-1, αPP-MEK-1/2, αERK-2, αPP-ERK-1/2) or negative control antibodies was done overnight at 4°C (1:400 in 5% BSA–TBS-T buffer). After washing in TBS-T (15 minutes) followed by 0.1% BSA–TBS-T (2 minutes) and incubation with appropriate biotinylated secondary immunoglobulins (DAKO) staining and counterstaining with hematoxylin was performed.
Sequencing of RAS mutations
Synthetic oligonucleotides were purchased from MWG-Biotech AG (Ebersberg, Germany) for use as amplification primers to identify mutations in codons 12, 13, and 61 of N-RAS, H-RAS, and K-RAS.53 54 Genomic DNA (gDNA) samples were prepared using a kit from Qiagen (Hilden, Germany). Fragments of 103 and 109 base pairs (bp) containing the codons of interest were obtained by amplification of 200 ng gDNA with AmpliTaq Gold (PE Applied Biosystems, Langen, Germany). The DNA was purified by 3% agarose gel electophoresis, recovered with a Qiaex II gel extraction kit (Qiagen), and cloned using the TOPO-TA Cloning Kit from Invitrogen (Carlsbad, CA). M13 forward and reverse primers were used for sequencing 400- to 600-ng plasmids using the Big Dye Sequencing kit from PE Applied Biosystems. The products of polymerase chain reaction were precipitated, washed with 70% ethanol, air-dried, and redissolved in 20 μL high-performance liquid chromatography-grade H2O and analyzed by an ABI Prism Sequence Detection System (PE Applied Biosystems).
Detectionof apoptosis
For detection and quantification of apoptosis at the single cell level, the in situ cell death detection kit of Roche Diagnostics (Mannheim, Germany) was used. Labeling of DNA strand breaks was done according to the manufacturer's instructions applying the TUNEL method. In addition, the annexin V-PE/7-amino-actinomycin (7-AAD) double-staining method was used to quantitatively determine the percentage of cells that are actively undergoing apoptosis. After washing once with PBS and twice with annexin V-binding buffer (10 mM Hepes, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2), cells were resuspended in annexin V-binding buffer (1 mL) and 5 μL annexin V-PE (BD Pharmingen/Biosciences, Heidelberg, Germany) was added. After 15 minutes at room temperature in the dark, washing twice with annexin V-binding buffer (1 mL) and resuspension in 200 μL, 40 μL 7-AAD solution (0.2 mg/mL PBS) was added (20 minutes at 4°C in the dark). Finally, 300 μL annexin V- binding buffer was added to each tube and cells were analyzed by flow cytometry.
Results
Activation of the RAS-to-MAP kinase cascade
RAS mutations.
The gDNA of several myeloid cell lines was analyzed for activating mutations in codons 12, 13, 15, 16, 18, and 61 of H-, K- and N-RAS to correlate the frequency of RAS mutations with the presence of constitutive activation of the ERK pathway. Four of 14 AML cell lines (28.6%) contained activating RASmutations (Table 1). As previously reported, a mutation of codon 12 of N-RAS was detected in THP-1 (GGT to GAT) and a N-RAS codon 61 mutation in HL-60 (CAA to CTA) resulting in G12D and Q61L.55 Two AML cell lines (Kasumi-1 and MV4-11) were found to harbor a K-RAScodon 12 mutation (GGT to GAT), which also exchanges Gly12 with Asp. Interestingly, an additional K-RAS codon 18 mutation was found in the second allele of MV4-11 (GCC to GAC), which leads to replacement of A18D. No activating mutations of codons 15 and 16 or of the H-RAS gene were detected. Three cell lines (MV4-11, Kasumi-1, and NB-4) harbored a silent point mutation in codon 59 (GCC to GCT).
RAS activation assays.
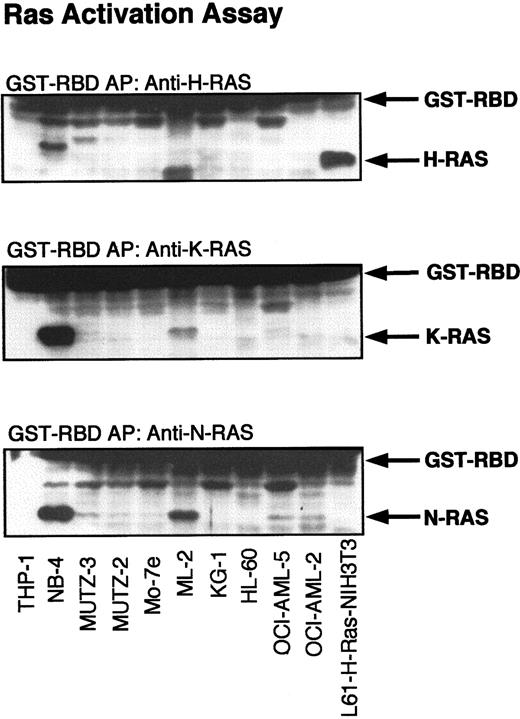
The expression of all 3 RAS isoforms (H-, K- and N-RAS) was detected by Western blotting of the cellular lysates of all myeloid cell lines tested (not shown). To identify the presence of GTP-RAS, cell lysates were incubated with the minimal RAS-binding domain (RBD) of C-Raf-1. Western blotting with antibodies against H-, K- and N-RAS was performed to detect the binding of GTP-RAS with GST-RBD. Cellular lysates of H-RAS (L-61)–transformed NIH-3T3 fibroblasts were used as a positive control for activated H-RAS. As shown in Figure1, only oncogenic H-RAS (L61) could be precipitated with GST-RBD from NIH-3T3 lysates, but not endogenous K- or N-RAS. THP-1 and HL-60, which harbor N-RAS mutations in codon 12 and codon 61, showed only a slight activation of N-RAS in some experiments but no activation of H-RAS or K-RAS. Surprisingly, lysates of Kasumi-1 and MV4-11, which harbor a K-RAS codon 12 mutation, did not contain substantial amounts of activated K-RAS. Although most lysates of cell lines with wild-type RAS did not contain significant amounts of GTP-RAS, high levels of activated H-, K- and N-RAS were found in NB-4 and ML-2 cell lysates. No apparent activation of RAS was detected in the CML cell lines, although all express the activated BCR-Abl fusion tyrosine kinase, which has been reported to induce RAS activation.5,39 40 Of the 5 growth factor–dependent myeloid cell lines (AML-OCI-5, Mo-7e, JK-1, Mutz-2, and Mutz-3), activation of H-, K-, and N-RAS was observed only in AML-OCI-5 on stimulation by interleukin 3 or conditioned medium of 5637 kidney cells (not shown).
Activation of RAS in myeloid leukemia cell lines.
Lysates of myeloid leukemia cell lines were subjected to affinity precipitation (AP) with GST-RBD as described in “Materials and methods.” RAS proteins were detected by immunoblotting with H-, K-, and N-RAS antibodies. H-RAS (L61)–transformed NIH-3T3 fibroblasts were used as a positive control for activated H-RAS.
Activation of RAS in myeloid leukemia cell lines.
Lysates of myeloid leukemia cell lines were subjected to affinity precipitation (AP) with GST-RBD as described in “Materials and methods.” RAS proteins were detected by immunoblotting with H-, K-, and N-RAS antibodies. H-RAS (L61)–transformed NIH-3T3 fibroblasts were used as a positive control for activated H-RAS.
Activation of the MAPK cascade.
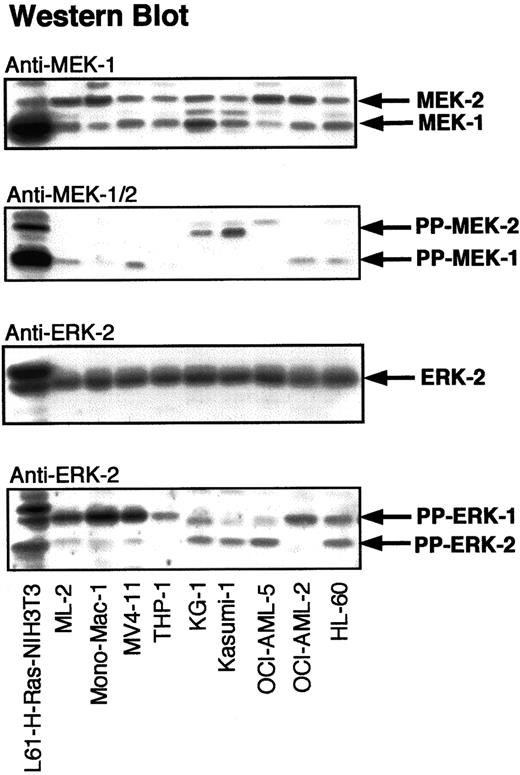
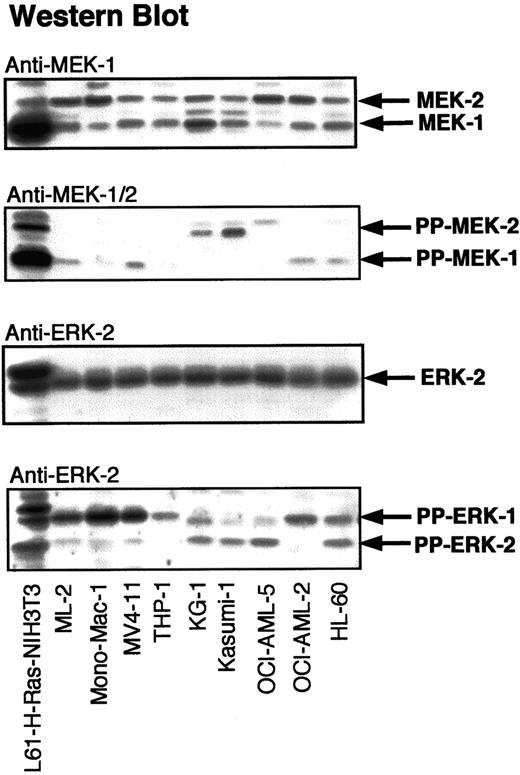
To determine the frequency and the level of activation of signaling proteins downstream of RAS, lysates of myeloid cells were analyzed by Western blotting with antibodies against PP-MEK-1/2 and PP-ERK-1/2 (Figure 2). Cellular lysates of H-RAS (L-61)–transformed NIH-3T3 fibroblasts were used as a positive control for activated MEK-1/2 and ERK-1/2. Significant activation of MEK-1/2 was detected in 8 of 14 AML cell lines and 2 of 5 CML cell lines. ERK-1/2 activation was observed in 9 of 14 AML cell lines and 2 of 5 CML cell lines. MEK-1/2 activation correlated with ERK-1/2 activation in 7 of 14 AML cell lines and 2 of 5 CML cell lines. Western blots for diphospho-ERK-1/2 were confirmed by immunocomplex MAPK assays using an ERK-2 antibody, which cross-reacts with ERK-1 (Figure3). The presence of PP-ERK-1/2 in Western blots of cellular lysates correlated closely with MAPK/ERK activity in immunocomplex kinase assays. Compared to leukemia cell lines, H-RAS (L-61)–transformed NIH-3T3 fibroblasts harbored at least 50% higher amounts of PP-ERK-1/2. The presence of RAS mutations or BCR-Abl did not always correlate with significant ERK-1/2 activation (Table 1).
Activation of ERK-1/2 and MEK-1/2 in myeloid leukemia cell lines.
Myeloid leukemia cell lysates were adjusted for protein concentration and equal amounts of total cell protein were subjected to SDS-PAGE. Western analysis was performed with antibodies against MEK-1, ERK-2, PP-MEK-1/2, and PP-ERK-1/2. L61-H-RAS–transformed NIH-3T3 cell lysates were used as positive controls for the RAS-induced activation of ERKs and MEKs.
Activation of ERK-1/2 and MEK-1/2 in myeloid leukemia cell lines.
Myeloid leukemia cell lysates were adjusted for protein concentration and equal amounts of total cell protein were subjected to SDS-PAGE. Western analysis was performed with antibodies against MEK-1, ERK-2, PP-MEK-1/2, and PP-ERK-1/2. L61-H-RAS–transformed NIH-3T3 cell lysates were used as positive controls for the RAS-induced activation of ERKs and MEKs.
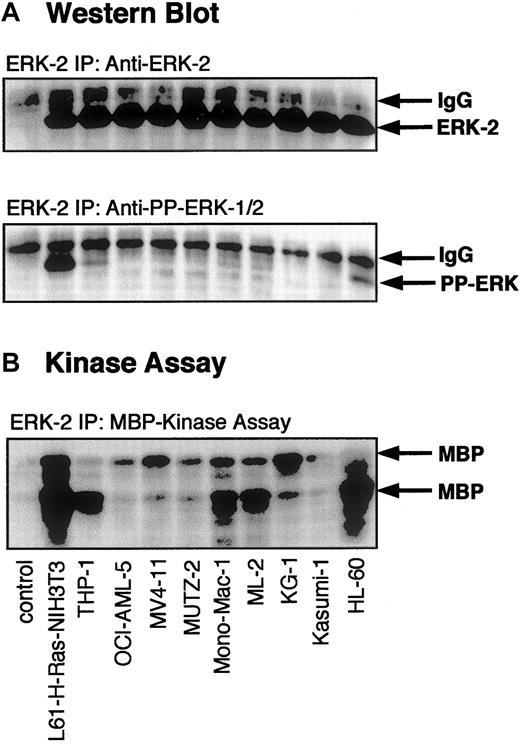
Activation of ERK-2 in myeloid leukemia cell lines.
Lysates of myeloid leukemia cell lines were subjected to immunoprecipitation with an antibody against ERK-2 (which also cross-reacts with ERK-1). After washing the immunoprecipitates, immunocomplex kinase assays were performed as described in “Materials and methods.” (A) Western blot of the immunoprecipitates with an antibody specific for ERK-2 and an antibody specific for activated PP-ERK-1/2. (B) Autoradiogram of kinase assay demonstrating MBP phosphorylation by immunoprecipitated ERK-1/2. N-RAS (L61)–transformed NIH 3T3 fibroblasts were used as a positive control for the RAS-induced activation of ERK-1/2. A nonspecific antibody was used as a negative control.
Activation of ERK-2 in myeloid leukemia cell lines.
Lysates of myeloid leukemia cell lines were subjected to immunoprecipitation with an antibody against ERK-2 (which also cross-reacts with ERK-1). After washing the immunoprecipitates, immunocomplex kinase assays were performed as described in “Materials and methods.” (A) Western blot of the immunoprecipitates with an antibody specific for ERK-2 and an antibody specific for activated PP-ERK-1/2. (B) Autoradiogram of kinase assay demonstrating MBP phosphorylation by immunoprecipitated ERK-1/2. N-RAS (L61)–transformed NIH 3T3 fibroblasts were used as a positive control for the RAS-induced activation of ERK-1/2. A nonspecific antibody was used as a negative control.
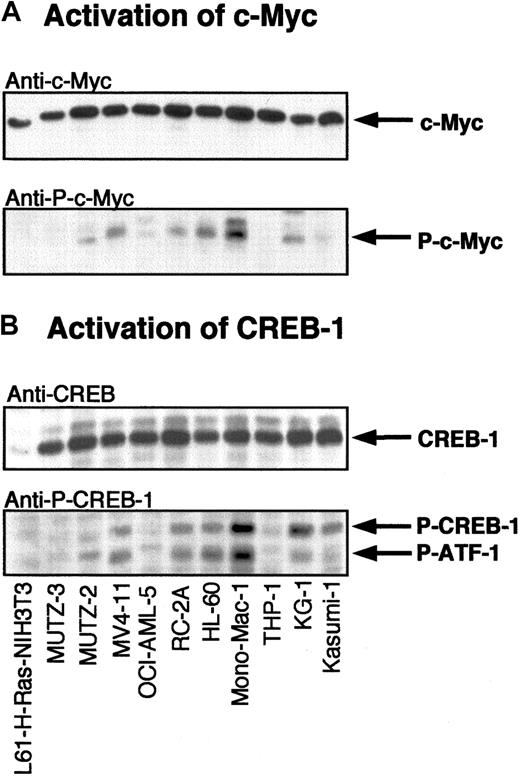
Activation of transcription factors.
Following activation, ERK-1/2 translocates to the nucleus resulting in phosphorylation and activation of transcription factors such as ELK-1, CREB-1, and Myc. To determine whether activation of ERK-1/2 induces activation of these transcription factors, myeloid leukemia cell lysates were subjected to Western blotting with specific antibodies against phospho-ELK, phospho-CREB, and phospho-Myc. Activated CREB-1/ATF-1 was found in 11 of 14 AML cell lines and 2 of 5 CML cell lines and did not always coincide with the presence of phospho-ERK-1/2 (Figure 4 and Table 1).
Activation of MAPK-dependent transcription factors in myeloid leukemia cell lines.
Lysates of myeloid leukemia cell lines were subjected to SDS-PAGE followed by Western blotting with anti–c-Myc (A) and anti–CREB-1 (B) antibodies. Activated c-Myc and CREB-1/ATF-1 were detected with phosphospecific antibodies against activated c-Myc and CREB-1.
Activation of MAPK-dependent transcription factors in myeloid leukemia cell lines.
Lysates of myeloid leukemia cell lines were subjected to SDS-PAGE followed by Western blotting with anti–c-Myc (A) and anti–CREB-1 (B) antibodies. Activated c-Myc and CREB-1/ATF-1 were detected with phosphospecific antibodies against activated c-Myc and CREB-1.
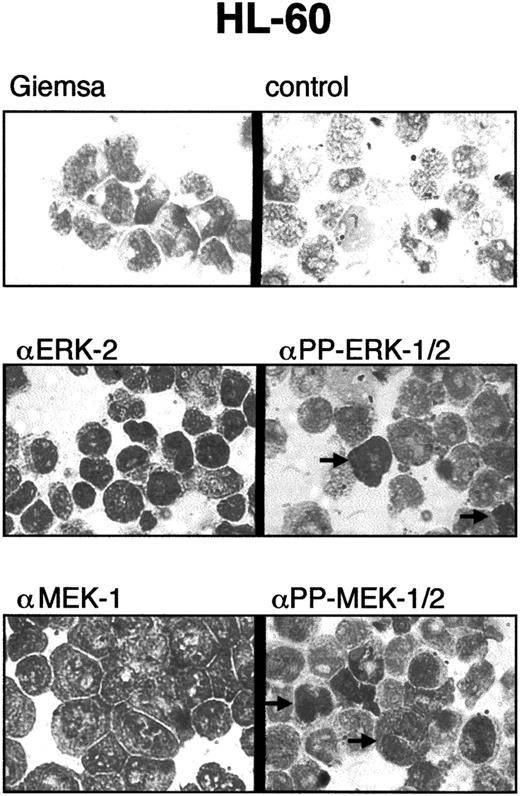
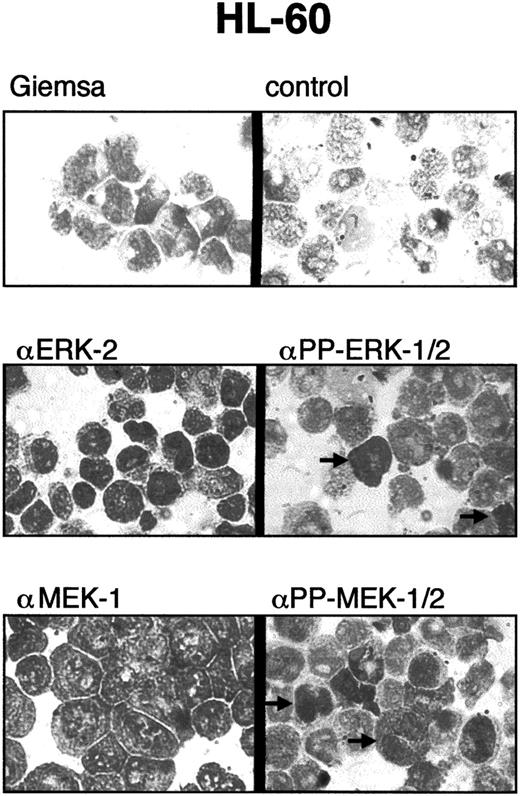
Intracellular localization of PP-ERK-1/2 and PP-MEK-1/2.
To determine whether activation of ERK-1/2 and MEK-1/2 was limited to subpopulations of myeloid leukemia cells, cytospins were prepared and stained with specific antibodies against ERK-2, MEK-1, PP-ERK-1/2, and PP-MEK-1/2 (Figure 5). All cells were stained with anti–ERK-2 and anti–MEK-1 antibodies. However, only 1% to 5% of cells showed strong staining with anti-PP-ERK-1/2 and anti-PP-MEK-1/2 antibodies. Strong nuclear staining was found in approximately 50% of PP-ERK-1/2+ and PP-MEK-1/2+ cells, whereas the other portion showed mainlycytoplasmic staining. These results were obtained for all samples tested and suggest activation of ERKs and MEKs in subpopulations (eg, cells in certain phases of the cell cycle).
Immunocytostaining of myeloid leukemia cells with PP-ERK-1/2 and PP-MEK-1/2 antibodies.
Cytospins of myeloid leukemia cells were stained with antibodies specific for ERK-2, PP-ERK-1/2, MEK-1, and PP-MEK-1/2 as described in “Materials and methods.” Approximately 1% to 5% of the cells were strongly stained with anti–PP-ERK-1/2 and anti–PP-MEK-1/2 antibodies. Note the nuclear staining of some cells, whereas others are stained mainly in the cytoplasm.
Immunocytostaining of myeloid leukemia cells with PP-ERK-1/2 and PP-MEK-1/2 antibodies.
Cytospins of myeloid leukemia cells were stained with antibodies specific for ERK-2, PP-ERK-1/2, MEK-1, and PP-MEK-1/2 as described in “Materials and methods.” Approximately 1% to 5% of the cells were strongly stained with anti–PP-ERK-1/2 and anti–PP-MEK-1/2 antibodies. Note the nuclear staining of some cells, whereas others are stained mainly in the cytoplasm.
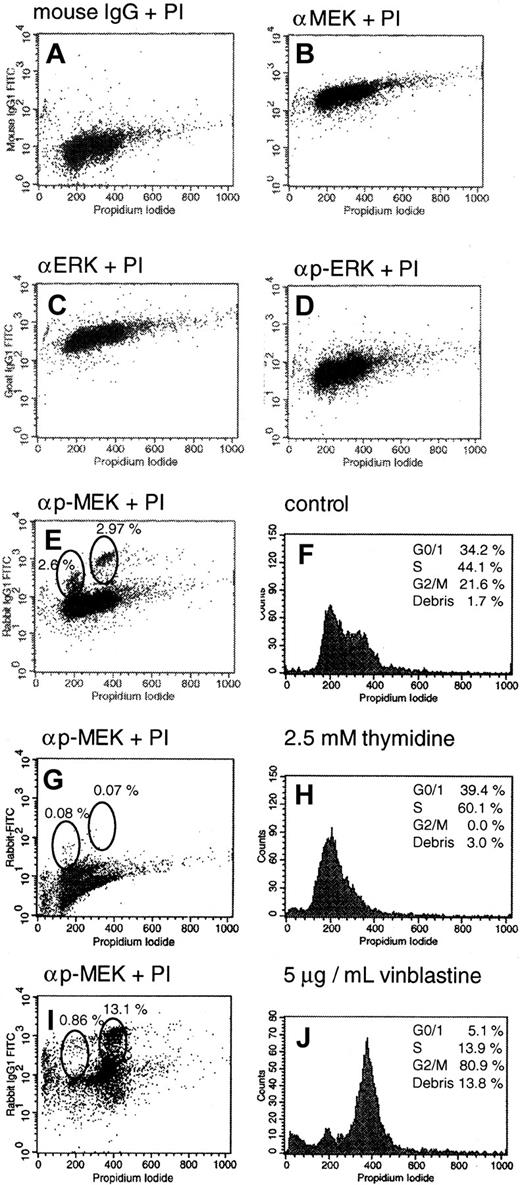
MEK activation during cell-cycle progression.
To address the possibility of ERK-1/2 and MEK-1/2 activation during cell-cycle progression, a novel FACS method for cytoplasmic staining of activated kinases of the RAS pathway was developed. Cells were double stained with propidium iodide and antibodies specific for different members of the ERK signaling pathway (eg, ERK-2, PP-ERK-1/2, MEK-1, and PP-MEK-1/2). As shown in Figure 6, panels B and C, all cells were strongly positive for ERK-2 and MEK-1. Using anti-PP-ERK-1/2 and anti-PP-MEK-1/2 antibodies, most cells were only slightly positive (Figure 6D,E). Interestingly, PP-MEK-1/2 staining revealed 2 additional, strongly positive cell populations (Figure 6E) that corresponded to the G0/G1 and G2/M phases of the cell cycle. The difference in PP-ERK-1/2 and PP-MEK-1/2 staining might be due to rapid nuclear translocation of PP-ERK-1/2. Incubation with excess thymidine (2.5 mM) resulted in a G1/S-phase block with an increase of S-phase cells to 60.1% and a concomitant reduction of PP-MEK-1/2+ cells in G0/G1 and G2/M (< 0.1% of the total cell population; Figure 6G,H). Cell viability was not significantly affected by this treatment. Treatment of cells with vinblastine (5 μg/mL, 10 hours), which induces depolymerization of mitotic interpolar microtubules and a cell metaphase block, increased the mitotic population of cells in G2/M to 80.9% and the amount of strongly positive PP-MEK-1/2 cells in G2/M to 13.1% (Figure 6I,J). These results demonstrate MEK-1/2 activation in the G0/G1 and G2/M phases of the cell cycle of myeloid leukemia cells.
Activation of MEK-1/2 during cell-cycle progression.
HL-60 cells were prepared for FACS analysis as described in “Materials and methods.” Graph A shows a representative control with unspecific mouse IgG antibodies. Similar results were obtained with rabbit and goat controls (data not shown). All cells were clearly positive when probed with ERK-2 or MEK-1 antibodies (graphs B,C), and only slightly positive when stained with phosphospecific antibodies against activated PP-ERK-1/2 and PP-MEK-1/2 (graphs D,E). Graph F shows the cell-cycle profile of untreated HL-60 cells stained with propidium iodide. Additionally, PP-MEK-1/2 staining revealed 2 strongly positive populations, which correlated with the G0/G1and G2/M phases of the cell cycle (graphs E,F). Treatment of HL-60 cells with excess thymidine (2.5 mM) for 10 hours: G, staining with anti–PP-MEK-1/2 antibodies; H, cell-cycle profile. Treatment of HL-60 cells with 5 μg/mL vinblastine for 10 hours: I, staining with anti–PP-MEK-1/2 antibodies; J, cell-cycle profile.
Activation of MEK-1/2 during cell-cycle progression.
HL-60 cells were prepared for FACS analysis as described in “Materials and methods.” Graph A shows a representative control with unspecific mouse IgG antibodies. Similar results were obtained with rabbit and goat controls (data not shown). All cells were clearly positive when probed with ERK-2 or MEK-1 antibodies (graphs B,C), and only slightly positive when stained with phosphospecific antibodies against activated PP-ERK-1/2 and PP-MEK-1/2 (graphs D,E). Graph F shows the cell-cycle profile of untreated HL-60 cells stained with propidium iodide. Additionally, PP-MEK-1/2 staining revealed 2 strongly positive populations, which correlated with the G0/G1and G2/M phases of the cell cycle (graphs E,F). Treatment of HL-60 cells with excess thymidine (2.5 mM) for 10 hours: G, staining with anti–PP-MEK-1/2 antibodies; H, cell-cycle profile. Treatment of HL-60 cells with 5 μg/mL vinblastine for 10 hours: I, staining with anti–PP-MEK-1/2 antibodies; J, cell-cycle profile.
Effect of inhibitors of RAS-to-MAPK signaling on myeloid leukemia cell growth
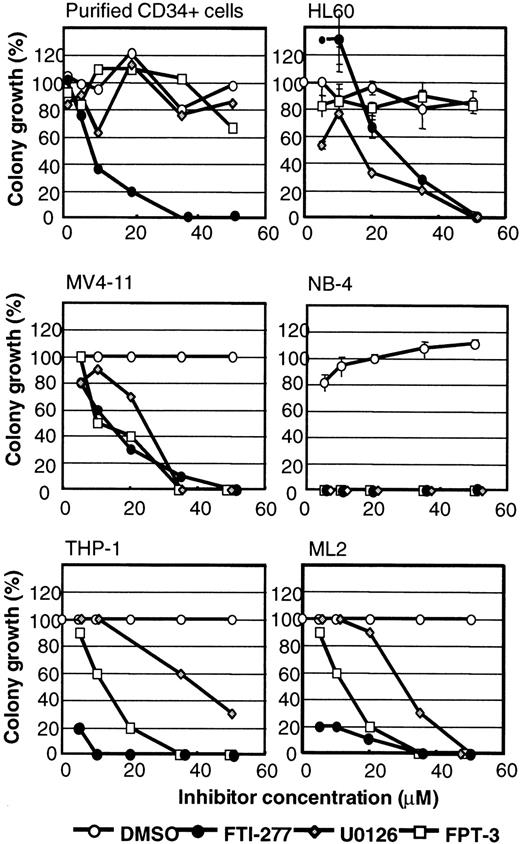
Because activation of the ERK pathway has been implicated in the pathogenesis of myeloid leukemias, the effectiveness of several different types of inhibitors of RAS signaling was tested on viability and colony formation of myeloid leukemia cell lines. The cell lines tested are shown in Table 1 and included some that express mutationally activated N-RAS, K-RAS, or BCR-Abl. Purified CD34+ cells were used as a control for inhibitor specificity. Inhibitors used in this study included (1) MEK inhibitors U0126 and PD098059, (2) farnesyl pyrophosphate (FPP)-based farnesyltransferase (FTase) inhibitors FPT-II and FPT-III, (3) CAAX-based FTIs FTI-276, FTI-277, B581, and Cys-4-Abz-Met, and the (4) prenylated protein methyltransferase (PPMTase) inhibitor FTS. To screen the compounds for growth inhibition of myeloid leukemia cells, liquid suspension cultures were incubated with 50 μM inhibitor or an equal volume of solvent. After 24 to 96 hours of incubation, a decrease in cell viability was observed in trypan blue exclusion assays in some inhibitor-treated samples. Therefore, all myeloid cell lines were preincubated in liquid suspension cultures for 96 hours followed by incubation in methylcellulose to assay colony formation in the presence or absence of inhibitor. Limited growth inhibition was obtained by treatment with 50 μM FTI-276, B-581, and Cys-4-Abz-Met (Tables2 and 3). Growth inhibition greater than 70% was observed with 50 μM FPP-competitive FTIs FPT-3 (10 of 19), FPT-2 (4 of 19), the CAAX-based FTase inhibitor FTI-277 (19 of 19), and the PPMTase inhibitor FTS (3 of 19; Tables 2 and 3). Growth inhibition greater than 70% was also observed with MEK inhibitors PD098059 (8 of 19) and U0126 (19 of 19) (Table 3). In contrast to other inhibitors, FTI-277 elicited substantial toxicity toward purified CD34+ human stem cells. Because it has previously been reported that this compound causes nonspecific toxicity at concentrations greater than 20 μM, we studied the concentration dependency of these inhibitors on cell viability and colony formation. Only minor growth inhibitory effects on purified CD34+ cells were observed in the presence of DMSO, or U0126 and FPT-3 at concentrations up to 50 μM (Figure7). FTI-277 caused inhibition of stem cell colony formation with an inhibitory concentration of 50% (IC50) value of 10 μM. The NB-4, ML-2, and THP-1 cells showed significant leukemia selective inhibition of colony formation in the presence of FPT-3, U0126, and FTI-277. The IC50 values for FTI-277 were less than 5 μM for these cell lines, suggesting that FTI-277 may elicit leukemia-specific toxicity in some cell lines. However, HL-60 and MV4-11 cells revealed IC50 values for FTI-277 similar to the IC50 values determined for purified CD34+ cells. HL-60 and MV4-11 colony formation was significantly inhibited in the presence of U0126, but HL-60 cells were resistant to FPT-3 (Figure 7).
Inhibition of colony growth by the addition of U0126, FPT-3, and FTI-277.
Purified human CD34+ cells and myeloid leukemia cell lines were incubated in liquid suspension cultures with increasing concentrations of the MEK inhibitor U0126, the FPP-based FTI FPT-3, and the CAAX-based FTase inhibitor FTI-277. After 4 days, viability of the cells was determined by trypan blue dye assays. Aliquots of the samples were incubated in methylcellulose for an additional 7 to 14 days in the presence of freshly added inhibitors. Representative experiments are shown. Results are expressed in percentage inhibition of maximal spontaneous colony growth or cell density. Error bars indicate the SE of margin.
Inhibition of colony growth by the addition of U0126, FPT-3, and FTI-277.
Purified human CD34+ cells and myeloid leukemia cell lines were incubated in liquid suspension cultures with increasing concentrations of the MEK inhibitor U0126, the FPP-based FTI FPT-3, and the CAAX-based FTase inhibitor FTI-277. After 4 days, viability of the cells was determined by trypan blue dye assays. Aliquots of the samples were incubated in methylcellulose for an additional 7 to 14 days in the presence of freshly added inhibitors. Representative experiments are shown. Results are expressed in percentage inhibition of maximal spontaneous colony growth or cell density. Error bars indicate the SE of margin.
Effect of inhibitors of RAS-to-MAPK signaling on cell-cycle progression
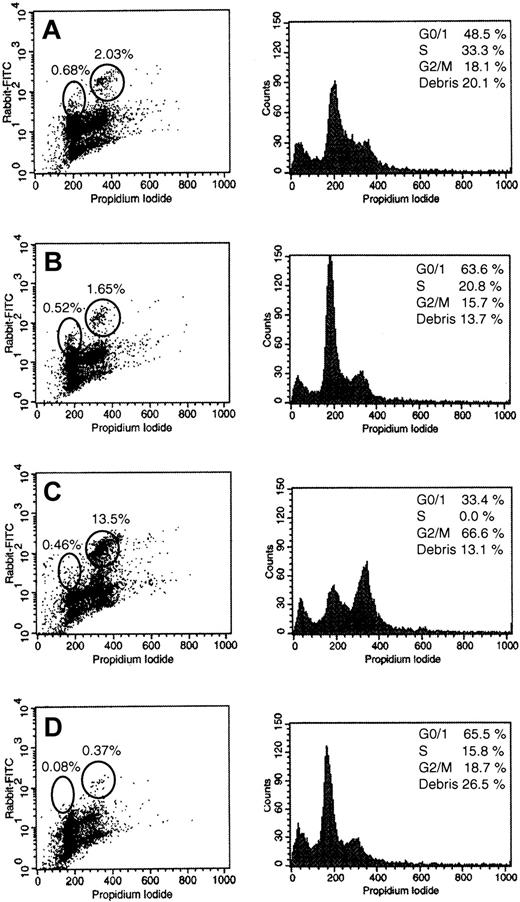
To elucidate the effects of the most potent inhibitors on cell-cycle progression of leukemia cells, NB-4 cells were incubated 18 and 36 hours in the presence of 50 μM FPT-3, 50 μM U0126, and 20 μM FTI-277 (Figures 8 and9). NB-4 cells were studied because they showed strong activation of the RAS pathway and revealed high sensitivity toward inhibitors of RAS signaling. After incubation with FPT-3, U0126, and FTI-277, double staining with propidium iodide and anti–P-MEK-1/2 antibodies was performed and cells were analyzed by flow cytometry. Incubation with DMSO for 18 hours elicited no substantial changes in NB-4 cell-cycle progression (Figure 8). However, incubation with DMSO for 36 hours resulted in an increase of the G0/G1 fraction to 63.5% compared to 48.5% in untreated controls (Figure 9A,B). In DMSO-treated control cells, 0.83% showed strong activation of MEK-1/2 in the G0/G1 phase and 2.4% in the G2/M phase of the cell cycle. These subpopulations were slightly reduced after 36 hours (Figure 9A,B).
Effects of U0126, FPT-3, or FTI-277 treatment for 18 hours on the cell-cycle–dependent activation of MEK-1/2.
NB-4 cells were incubated in the absence or the presence of 50 μM FPT-3, 50 μM U0126, or 20 μM FTI-277. After 18 hours, double staining with propidium iodide and an antibody specific for activated PP-MEK-1/2 was performed as described in “Materials and methods.” Representative cell-cycle profiles (left) and FACS profiles of the double staining (right) are shown. (A) Untreated NB-4 cells; (B) solvent-treated (DMSO) NB-4 cells; (C) NB-4 cells treated with 50 μM FPT-3; (D) NB-4 cells treated with 20 μM FTI-277; (E) NB-4 cells treated with 50 μM U0126.
Effects of U0126, FPT-3, or FTI-277 treatment for 18 hours on the cell-cycle–dependent activation of MEK-1/2.
NB-4 cells were incubated in the absence or the presence of 50 μM FPT-3, 50 μM U0126, or 20 μM FTI-277. After 18 hours, double staining with propidium iodide and an antibody specific for activated PP-MEK-1/2 was performed as described in “Materials and methods.” Representative cell-cycle profiles (left) and FACS profiles of the double staining (right) are shown. (A) Untreated NB-4 cells; (B) solvent-treated (DMSO) NB-4 cells; (C) NB-4 cells treated with 50 μM FPT-3; (D) NB-4 cells treated with 20 μM FTI-277; (E) NB-4 cells treated with 50 μM U0126.
Effects of U0126 or FTI-277 treatment for 36 hours on the cell-cycle–dependent activation of MEK-1/2.
NB-4 cells were incubated in the absence or the presence of 50 μM U0126 or 20 μM FTI-277. After 36 hours, double staining with propidium iodide and an antibody specific for activated PP-MEK-1/2 was performed as described in “Materials and methods.” Representative cell-cycle profiles (left) and FACS profiles of the double staining (right) are shown. (A) Untreated NB-4 cells; (B) solvent-treated (DMSO) NB-4 cells; (C) NB-4 cells treated with 20 μM FTI-277; (D) NB-4 cells treated with 50 μM U0126. NB-4 cells treated with FPT-3 are not shown because of nearly complete apoptosis at this time point.
Effects of U0126 or FTI-277 treatment for 36 hours on the cell-cycle–dependent activation of MEK-1/2.
NB-4 cells were incubated in the absence or the presence of 50 μM U0126 or 20 μM FTI-277. After 36 hours, double staining with propidium iodide and an antibody specific for activated PP-MEK-1/2 was performed as described in “Materials and methods.” Representative cell-cycle profiles (left) and FACS profiles of the double staining (right) are shown. (A) Untreated NB-4 cells; (B) solvent-treated (DMSO) NB-4 cells; (C) NB-4 cells treated with 20 μM FTI-277; (D) NB-4 cells treated with 50 μM U0126. NB-4 cells treated with FPT-3 are not shown because of nearly complete apoptosis at this time point.
After 18 hours of incubation with 50 μM FPT-3, a strong increase of the sub-G0/G1 fraction (debris) was observed, suggesting rapid induction of apoptotic DNA fragmentation (increase of debris from 13.5% to 35.8%; Figure 8C). Furthermore, the G0/G1 fraction increased to 46.8%. PP-MEK-1/2+ cells were reduced to 1.3% in G2/M, but remained unchanged in G0/G1 (1.09% versus 0.83% and 1.34% for DMSO and negative controls). A 36-hour treatment of NB-4 cells with FPT-3 resulted in nearly complete apoptotic DNA fragmentation (not shown).
Incubation with FTI-277 for 18 hours led to an increase of cellular debris to 16.1%, an increase of PP-MEK-1/2+ cells in G2/M to 5.5% but a decrease in G0/G1 to 0.34% (Figure 8D). FTI-277 treatment for 36 hours induced a G2/M block with 66.6% of cells in G2/M as well as an increase of PP-MEK-1/2+ cells in G2/M to 13.5%, but a decrease in G0/G1 to 0.46% (Figure9C).
Treatment with U0126 for 18 hours induced an increase of cellular debris to 22.4%, an increase in the G0/G1 fraction to 46.6%, and a slight decrease in PP-MEK-1/2+ cells in G0/G1 (0.56% versus 0.83%; Figure8B,D). After 36 hours of incubation with U0126 the sub-G0/G1 debris increased to 26.5% and the PP-MEK-1/2+ cells were further reduced to 0.08% in G0/G1 and 0.37% in G2/M (Figure 9D).
Apoptosis induction by treatment with FPT-3, U0126, and FTI-277
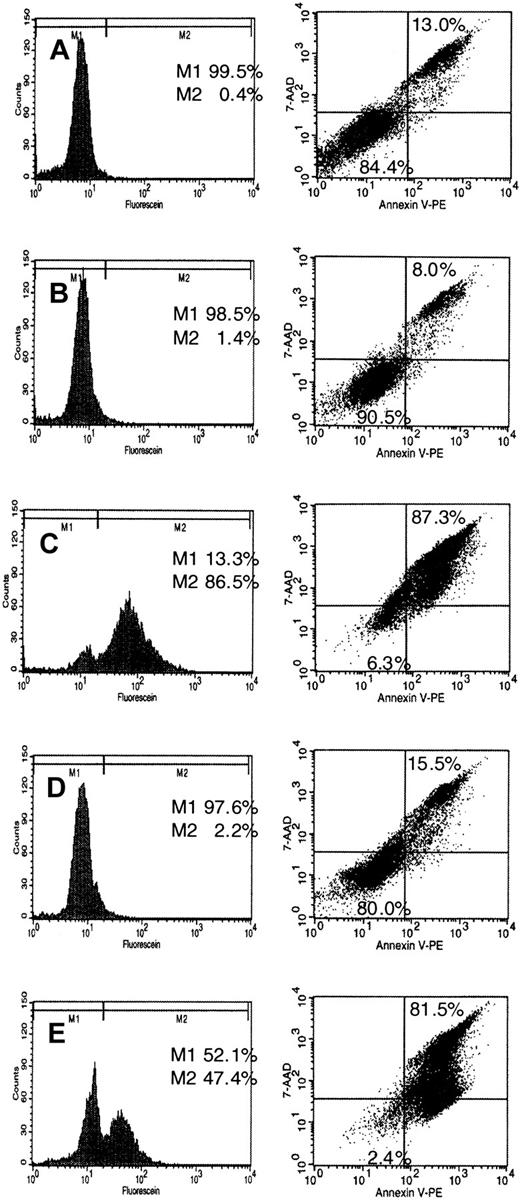
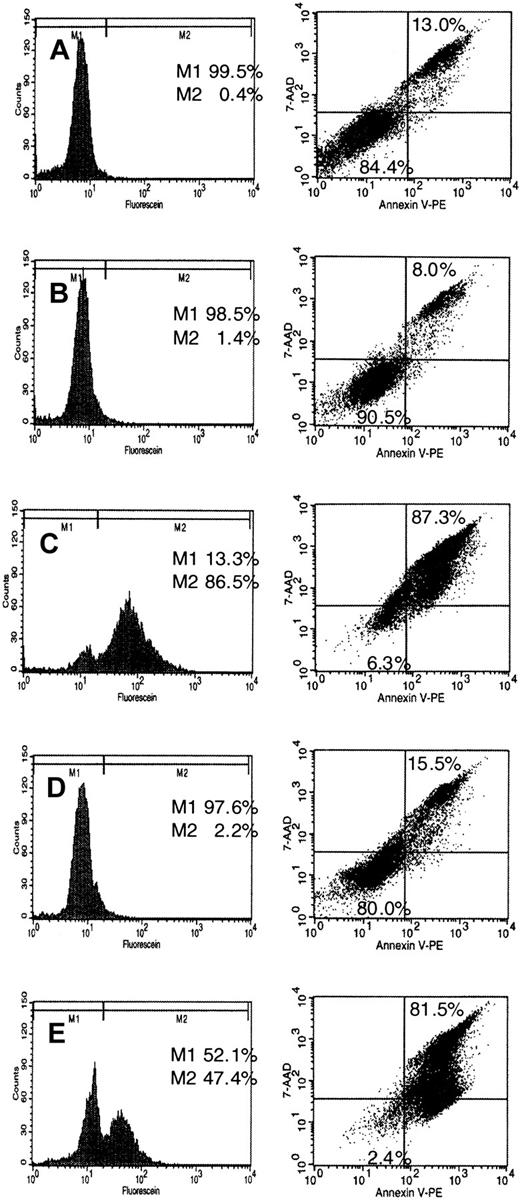
Staining with propidium iodide demonstrated an increase of the sub-G0/G1 fraction (debris) after treatment of NB-4 cells with U0126 and FPT-3 (Figures 8 and 9) suggesting apoptotic DNA fragmentation. To confirm induction of apoptosis by these inhibitors, NB-4 cells were incubated for 18 and 36 hours with FPT-3, U0126, and FTI-277. DNA strand breaks were detected by labeling with flourescein-dUTP using the TUNEL method. Externalization of membrane phosphatidylserine during early stages of apoptosis was monitored by a 7-AAD assay. Annexin V binds to negatively charged phospholipid surfaces with a higher specificity for phosphatidylserine than most other phospholipids. 7-AAD was used for the exclusion of nonviable cells. Samples were analyzed by flow cytometry for detection and quantification of apoptosis at the single cell level. The presence of DMSO had no effect on DNA degradation or exposure of phosphatidylserine (Figure10B). Treatment with FPT-3 resulted in apoptotic DNA fragmentation (86.5% after 18 hours) and exposure of phosphatidylserine on the outer leaflet of the plasma membrane (87.3% after 36 hours of incubation; Figure 10C). Similarly, treatment with U0126 induced DNA cleavage (47.4%) and externalization of phosphatidylserine (81.5%; Figure 10E). In contrast, FTI-277 treatment for 18 and 36 hours, which caused a G2/M block, did not induce apoptosis (Figure 10D). These differences toward FPT-3, U0126, and FTI-277 in cellular response suggest different mechanisms of action.
Induction of apoptosis by treatment with FPT-3, FTI-277, or U0126.
NB-4 cells were incubated in the presence or absence of 50 μM FPT3, 50 μM U0126, or 20 μM FTI-277. After 18 hours, cells were harvested and labeling of DNA strand breaks was performed applying the TUNEL method as described in “Materials and methods” (left). M1 indicates no DNA fragmentation; M2, DNA fragmentation. Exposure of phosphatidylserine on the outer leaflet of the plasma membrane was detected using an Annexin V-PE/7AAD double staining method as described in “Materials and methods” (right). Apoptotic exposure of phosphatidylserine is shown in the upper right square. Results are given in percentage of total cell population. (A) Untreated NB-4 cells; (B) solvent-treated (DMSO) NB-4 cells; (C) FPT-3–treated NB-4 cells; (D) FTI-277–treated NB-4 cells; (E) U0126-treated NB-4 cells.
Induction of apoptosis by treatment with FPT-3, FTI-277, or U0126.
NB-4 cells were incubated in the presence or absence of 50 μM FPT3, 50 μM U0126, or 20 μM FTI-277. After 18 hours, cells were harvested and labeling of DNA strand breaks was performed applying the TUNEL method as described in “Materials and methods” (left). M1 indicates no DNA fragmentation; M2, DNA fragmentation. Exposure of phosphatidylserine on the outer leaflet of the plasma membrane was detected using an Annexin V-PE/7AAD double staining method as described in “Materials and methods” (right). Apoptotic exposure of phosphatidylserine is shown in the upper right square. Results are given in percentage of total cell population. (A) Untreated NB-4 cells; (B) solvent-treated (DMSO) NB-4 cells; (C) FPT-3–treated NB-4 cells; (D) FTI-277–treated NB-4 cells; (E) U0126-treated NB-4 cells.
Discussion
Accumulating evidence supports a role of the deregulation of RAS function in the molecular pathogenesis of myeloid leukemias.5,21-23 The importance of the RAS-to-MAPK pathway is underscored by the positioning of several oncogene and tumor suppressor gene products on this pathway. Furthermore, mutant N-RAS induces myeloproliferative disorders resembling human CML, AMLs, and apoptotic syndromes similar to human myelodysplastic syndromes in bone marrow–repopulated mice.56 Possible effects of deregulated signaling through the RAS-to-MAPK cascade include an increase in proliferation and cell-cycle progression or inhibition of differentiation and apoptosis.
Constitutive activation of ERKs has previously been reported in 37 of 73 cases of AML.57-59 Consistently, our present study demonstrates ERK activation in 9 of 14 AML and 2 of 5 CML cell lines. Furthermore, 4 of 14 AML cell lines (28.6%) harbored activating mutations of N-RAS and K-RAS. Interestingly, in MV4-11 2 allelic K-RAS mutations were detected (codons 12 and 18). Mutations of “non–hot spot” codons 15, 16, 18, and 31 in K-RAS frequently occur in addition to the classical codon 12, 13, and 61 mutations.24,25 The identification of K-RAS and “non–hot spot” mutations in our study demonstrates that the frequency of RAS mutations in myeloid leukemia may be underestimated in the published literature because most series did not analyze all 3 RAS genes and “non–hot spot” codons such as 15, 16, 18, and 31. In agreement with recent reports, we demonstrate that ERK-1/2 activation in myeloid leukemia cell lines does not always correlate with the presence of activatingRAS mutations or BCR-Abl.57-59 Others have suggested that the constitutive activation of ERK-1/2 in AML cells is a result of ERK-1/2 hyperexpression and down-regulation of PAC1, a member of the MAPK phosphatase family.59 Our study reveals that approximately 5% of all cells showed strong ERK-1/2 and MEK-1/2 activation on immunocytochemical staining with specific antibodies against PP-ERK-1/2 and PP-MEK-1/2. Two patterns of staining were observed: cytoplasmic and nuclear staining. These observations are in agreement with reports that ERKs and MEKs are phosphorylated and activated after growth factor stimulation in the cytoplasm and then translocated into the nucleus.60-68Nuclear translocation of ERK-1/2 is a key signaling event for activation of nuclear processes such as transcription.69In contrast to stable nuclear presence of PP-ERK for several hours, a nuclear export signal (NES) between residues 32 and 42 of MEK-1/2 leads to rapid removal of MEKs from the nucleus.63 67
Additionally, our study shows that activation of the ERK cascade in myeloid leukemia cell lines is linked to the G0/G1 and G2/M phases of the cell cycle. Requirement for ERK activation in the G1 phase and the M phase of the cell cycle of fibroblasts has been reported in several studies.69-76 ERK-1/2 expression increases in mid-G1 phase and ERK phosphorylation and activation occurs during G1 phase.72,73 After phosphorylation and activation in the cytoplasm, PP-ERK is translocated to the nucleus during late/mid-G1 phase.72,73 Nuclear ERK-regulated events required for progression into S phase include expression of immediate early genes such as Fos andEgr-1, and transcriptional up-regulation of cyclin D1. ERKs and MEKs are also activated early in prophase of mitosis before nuclear envelope breakdown.75-78 In prophase, activated ERKs associate at kinetochores and within the chromosomal periphery of condensed chromosomes.77,78 Activated ERKs and MEKs localize to spindle poles between prophase and anaphase and to the midbody during cytokinesis.78 Candidate mitotic substrates include the kinetochore motor protein CENP-E and topoisomerase IIa suggesting potential targets for the ERK pathway in the modulation of chromatin reorganization events during mitosis and in other phases of the cell cycle.77 78
The role of RAS in the molecular pathophysiology of myeloid leukemias has considerable potential implications for a therapeutic approach, which targets the RAS-to-MAPK signaling pathway.5,42-45Although leukemogenesis is a multistep process, the positioning of several oncogenes and tumor suppressor genes on this pathway strongly suggests that inhibition of oncogenic RAS function and signaling is a promising pharmacologic strategy.5,42-45 Therefore, we evaluated a panel of 9 inhibitors of RAS-to-MAPK signaling for growth inhibition of myeloid leukemia cell lines. This panel included inhibitors of the posttranslational modification of RAS and specific MEK inhibitors.5 42-45 Most FTIs were less effective in the inhibition of leukemia cell growth (eg B581, FPT-2, Cys-4-Abs-Met).
Treatment with the CAAX-based FTI-277 resulted in a significant reduction in cell viability and colony formation in all myeloid leukemia cell lines tested. Unfortunately, at concentrations greater than 20 μM this inhibitor also displayed a strong toxicity toward purified human CD34+ cells suggesting that myelotoxicity may be a side effect of treatment with some FTIs. Similar toxicities have not been reported in recent animal studies with this inhibitor.79-81 However, mild myelosuppression, neutropenia, and anemia have been observed in phase I clinical trials of another FTI, R115777.45,82 Treatment of myeloid cell lines with FTI-277 resulted in a G2/M block and a subsequent increase in the number of PP-MEK-1/2+ cells. Similarly, FTI-277 treatment of lung adenocarcinoma A-549 cells caused enrichment in the G2/M phase of the cell cycle.83
Additionally, L-739749 and L-744832, both CAAX-based FTIs, have recently been shown to inhibit spontaneous JMML granulocyte-macrophage colony growth (at 1-10 μM).84,85 Unfortunately, these inhibitors were not tested for toxicity toward normal purified stem cells. L-744832 inhibited H-RAS prenylation and colony growth of NF-1–deficient hematopoietic cells in response to granulocyte-macrophage colony-stimulating factor but did not reduce constitutively activated MAPK activity in these cells. Furthermore, a myeloproliferative disorder in NF-1 deficient (NF−/−) mice did not respond to L-744832 treatment.85 It was speculated that the lack of efficacy in this model was due to the resistance of N-RAS and K-RAS processing to inhibition by this FTI.85
The FPP-based FTI, FPT-3, caused inhibition of colony formation of 10 of 19 myeloid cell lines at concentrations that did not significantly affect colony growth of purified human CD34+ cells. FPT-3 incubation of NB-4 cells resulted in rapid induction of apoptotic DNA fragmentation and exposure of phosphatidylserine. FPT-3 also induces apoptosis in ovarian cancer cells by up-regulation of Bax and Bcl-xs expression and activation of caspase family proteases.86 87
Treatment with MEK inhibitors PD098059 and U0126 significantly inhibited viability, growth, and colony formation of most cell lines tested (10 of 19 and 19 of 19, respectively). MEK inhibitors, which display broad inhibitory effects, caused only minor toxicity in purified human CD34+ cells. The stronger effect of U0126 is most likely due to a higher affinity of U0126 to all forms of MEK (44- to 357-fold) as compared to PD098059.5 In contrast to PD098059, U0126 reverses Ki-RAS–mediated transformation by blocking MAPK and p70 S6 kinase pathways.88
In agreement with previous observations,82 a correlation between susceptibility toward these inhibitors and the RAS status (eg, mutation or activation) was not always observed in our study. For example, NB4 cells displayed strong activation of the RAS-to-MAPK cascade and profound inhibitory effects of most inhibitors, whereas Mono-Mac-1 cells were resistant to most inhibitors despite a strong MAPK activation.
The different cellular responses invoked by FPT-3, U0126, and FTI-277 suggest the possibility of different mechanisms of action. The differences between FPT-3 and FTI-277 are particularly interesting because these compounds target RAS farnesylation. It has been suggested that RAS may not be the therapeutically relevant loci.82Determining whether leukemic growth inhibition occurs because of inhibition of RAS-specific cellular events such as MAPK-induced cell-cycle progression or because of alteration of other cellular targets such as RhoB, an endosomal Rho protein that functions in receptor trafficking, is currently under investigation. Regardless of the locus of action, our results demonstrate profound in vitro inhibitory effects of FTase, PPMTase, and MEK inhibitors on the growth of several myeloid leukemia cell lines irrespective of the presence ofRAS mutations, expression of BCR-Abl, or MAPK activation. Our findings support a potential role of inhibitors of RAS signaling in the future treatment of myeloid leukemias.
We thank Natalja Möbius and Dieter Kirchner for excellent technical assistance and Dr Kristine A. Henningfeld for valuable discussions. Purified human CD34+ stem cells were a gift from Dr Wiesneth, German Red Cross (DRK), Blutspendezentrale Baden-Württemberg, Ulm, Germany.
Supported by a grant to C.W.M.R. from the Deutsche Forschungsgemeinschaft (Re 864/4-1) and a grant from the University of Ulm (P.541).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Christoph W. M. Reuter, Department of Hematology & Oncology, Center for Internal Medicine, Hannover Medical School, Carl-Neuberg-Str 1, D-30625 Hannover, Germany; e-mail: reuter.christoph@mh-hannover.de.