Abstract
The mi transcription factor (MITF) is a basic-helix-loop-helix leucine zipper (bHLH-Zip) transcription factor that is important for the development of mast cells. Mast cells ofmi/mi genotype express normal amounts of abnormal MITF (mi-MITF), whereas mast cells of tg/tg genotype do not express any MITFs. The synthesis of heparin is abnormal in the skin mast cells of mi/mi mice. Because N-deacetylase/N-sulfotransferase 2 (NDST-2) is essential for the synthesis of heparin, the amount of NDST-2 messenger RNA (mRNA) was compared among cultured mast cells (CMCs) of +/+,mi/mi, and tg/tg genotypes. The NDST-2 mRNA was detected by in situ hybridization in the skin mast cells of +/+ andtg/tg mice, but not in the skin mast cells ofmi/mi mice. The amount of NDST-2 mRNA decreased significantly in CMCs derived from mi/mi mice when compared to the values of +/+ and tg/tg mice, suggesting that the defective form of MITF inhibited the expression of the NDST-2 transcript. The expression of NDST-2 transcript was mediated by the GGAA motif located in the 5′-untranslated region. GA binding protein (GABP) bound the GGAA motif and increased the amount of NDST-2 transcript. The mi-MITF appeared to inhibit the ability of GABP to express NDST-2 transcript by disturbing its nuclear localization. This is the first study to show that expression of an abnormal form of a bHLH-Zip transcription factor can dramatically alter the intracellular location of another DNA/RNA binding factor, which in turn brings about profound and unexpected consequences on transcript expression.
Introduction
The mi locus of mice encodes a member of the basic-helix-loop-helix leucine zipper (bHLH-Zip) protein family of transcription factors (hereafter called MITF).1,2 Themi/mi mutant mice show depletion of pigment in both hair and eyes, microphthalmia, osteopetrosis, and deficient natural killer activity.3,4 In addition, the development of mast cells is defective in mi/mi mice. The skin mast cells ofmi/mi mice decrease in number and reduce the amount of mouse mast cell protease-4 (MMCP-4), MMCP-6, and c-kit messenger RNAs (mRNAs).5-11 Cultured mast cells (CMCs) derived from the spleen of mi/mi mice are deficient in the expression of various genes, such as MMCP-4,11 MMCP-5,12MMCP-6,13 c-kit,14 p75 nerve growth factor receptor,15 granzyme B (Gr B),16 tryptophan hydroxylase (TPH),16integrin-α4 subunit17 and α-melanocyte-stimulating hormone receptor genes.18
MITF encoded by the mi mutant allele (mi-MITF) deletes 1 of 4 consecutive arginines in the basic domain.1,19,20 The mi-MITF is defective in the DNA binding ability and the nuclear localization potential.21,22 The mi-MITF does not appear to transactivate target genes due to these abnormalities.11-18,22 The tg is another mutant allele of the mi locus.1,23 Thetg/tg mice possess the insertional mutation at the promoter region of mi gene and do not express any MITFs.1,24 The tg/tg and mi/mi mice share several phenotypic features, but the phenotypic abnormality oftg/tg mice is apparently mild when compared to that ofmi/mi mice. The transcription of c-kit, Gr B, and TPH genes as significantly reduced inmi/mi CMCs, but the reduction was moderate intg/tg CMCs.25 The presence ofmi-MITF caused more severe abnormalities than the absence of normal (+) MITF. In addition to the loss of transactivation ability, the mi-MITF possesses an inhibitory effect on the transcription of some particular genes in mast cells.25
Heparin is a highly sulfated proteoglycan that is abundantly contained by mast cells in the skin of mice.26-28 Because berberine sulfate is a dye that binds heparin, most of the skin mast cells of +/+ mice are stained with berberine sulfate.29-31 In the skin of mi/mi mice, the content of heparin decreases, and few mast cells are stained with berberine sulfate.9 In contrast, most of the skin mast cells of tg/tg mice are stained with berberine sulfate as in the case of +/+ skin mast cells,32 indicating that the content of heparin decreases in mi/mi skin mast cells but not in tg/tg skin mast cells. There is a possibility that mi-MITF may possess the inhibitory effect on the synthesis of heparin as in the case of serotonin.25 In fact, serotonin content ofmi/mi CMCs decreases due to the poor transcription ofTPH gene.16,25 N-deacetylase/N-sulfotransferase 2 (NDST-2) is essential for the synthesis of heparin, and the disruption of the NDST-2 gene causes the depletion of heparin in mast cells.33 34 In the present study, we compared the amount of NDST-2 mRNA among mast cells of +/+,mi/mi, and tg/tg genotypes and found the inhibitory effect of mi-MITF on the expression of NDST-2 transcript in mast cells.
Materials and methods
Mice
The original stock of C57BL/6-mi/+ mice was purchased from the Jackson Laboratory (Bar Harbor, ME), and was maintained in our laboratory by consecutive back-cross with our own inbred C57BL/6 colony (> 15 generations at the time of the present experiment). The original stock of VGA-9-tg/tg mice, in which the mouse vasopressin-Escherichia coli β-galactosidase transgene was integrated at the 5′ flanking region of the mi gene, were kindly given by Dr H. Arnheiter (National Institutes of Health, Bethesda, MD).1 The integrated transgene was maintained by repeated back-crosses to our own inbred C57BL/6 colony (> 15 generations at the time of the present experiment). Female and malemi/+ or tg/+ mice were crossed together, and the resulting homozygous mi/mi or tg/tg mice were selected by their white coat color.3 4 C57BL/6-+/+ mice raised in our laboratory were used as a control.
In situ hybridization
Skin pieces were removed from the backs of 20-day-old mice, fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (PB; pH 7.4), and embedded in paraffin. The technique of in situ hybridization has been described in detail.35 To obtain the NDST-2 probe, single-stranded complementary DNA (cDNA) was generated from total RNA extracted from CMCs of +/+ mouse origin by lithium chloride-urea method.36 The specific cDNA of NDST-2 was then amplified by polymerase chain reaction (PCR). The primers for PCR were 5′-CGAGCACATGGAGACCCCATTGCTC (corresponding to the region between nt 2661 and nt 2685, the number represents the nucleotide numbers of the NDST-2 cDNA reported by Orellana and colleagues37) and 5′-TGCTGGTAAAGAAAGACTGGGCCA (corresponding to the region between nt 3188 and nt 3165). The NDST-2 cDNA was subcloned into the EcoRV site of Bluescript KS (-) plasmid (pBS; Stratagene, La Jolla, CA) that contains T3 and T7 promoters to generate probes. Skin mast cells were identified with alcian blue staining, and the presence of NDST-2 mRNA was examined in the serial section by hybridization of antisense probe. The sense probe for NDST-2 cDNA was used as a negative control.
Cells
Pokeweed mitogen-stimulated spleen cell-conditioned medium (PWM-SCM) was prepared according to the method described by Nakahata and coworkers.38 Mice of mi/mi,tg/tg, and control +/+ genotype were used at 2 to 3 weeks of age to obtain CMCs. Mice were killed by decapitation after ether anesthesia and spleens were removed. Spleen cells were cultured in α-minimal essential medium (α-MEM; ICN Biomedicals, Costa Mesa, CA) supplemented with 10% PWM-SCM and 10% fetal calf serum (FCS; Nippon Bio-supp Center, Tokyo, Japan). Half of the medium was replaced every 5 days. Cells derived from the spleen of each mutant genotype reached to 1 × 107 in number within 4 weeks. More than 95% of cells contained alcian blue–positive granules and were considered to be CMCs 4 weeks after initiation of the culture. The MST cells, a heparin-producing cell line derived from the Furth murine mastocytoma, were maintained in α-MEM supplemented with 10% FCS.39The NIH/3T3 cells and COS-7 cells were maintained in Dulbecco modified Eagle medium (DMEM, Flow Laboratories, Irvine, United Kingdom) supplemented with 10% FCS.
Northern blot analysis
Each RNA sample was prepared from 1.0 × 107 of CMCs or MST cells by the lithium chloride-urea method.36Northern blot analysis was performed using NDST-237 and GAPDH cDNAs40 labeled with α-[32P]-dCTP (DuPont/NEN Research Products, Boston, MA; 10 mCi/mL) by random oligonucleotide priming. The NDST-2 cDNA used was same as that used for in situ hybridization. After hybridization at 42°C, blots were washed to a final stringency of 0.2 × standard sodium citrate (SSC; 1 × SSC is 150 mM NaCl and 15 mM trisodium citrate, pH 7.4), and subjected to autoradiography.
Determination of 5′-end of cDNA
The 5′-rapid amplification of cDNA end (5′-RACE) was performed with the SMART RACE cDNA Amplification Kit (Clontech Laboratories, Palo Alto, CA) according to the manufacturer's instructions. The primer for the first PCR was 5′-AGATTCCCATCCTGTGTCTGCCAATGAG (corresponding to the region between nt 66 and nt 38)37 and the primer for the nested PCR was 5′-CTGGTGGGCTCTCGATAACAAGTGGATG (corresponding to the region between nt 30 and nt 3).37 The total RNA obtained from MST cells was used as the template.
Promoter region of the NDST-2 gene
The DNA fragment containing the promoter region of theNDST-2 gene was isolated with the Mouse Genome Walker Kit (Clontech Laboratories) according to the manufacturer's instructions. The primers used were same as those used in 5′-RACE. The isolated fragment was cloned into pBS and sequenced. The isolated fragment contained the most upstream point of the 5′-RACE product, and the remaining upstream sequence was considered to be the promoter region.
Construction of reporter plasmids
The luciferase gene subcloned into pSP72 (pSPLuc) was generously provided by Dr K. Nakajima (Osaka City University Medical School, Osaka, Japan).41 To construct reporter plasmids, a DNA fragment containing a promoter and 5′-untranslated region (5′-UTR) of the NDST-2 gene (−1300 to +668, +1 shows the transcription initiation site determined by 5′-RACE) was cloned into the upstream of luciferase gene in pSPLuc. The deletion of the promoter or 5′-UTR of the NDST-2 gene was produced by PCR. The mutation was also introduced by PCR. All of the PCR products were verified by sequencing.
Construction of expression plasmids
The pBS containing the whole coding region of +-MITF ormi-MITF was constructed in our laboratory (hereafter called pBS-+-MITF and pBS-mi-MITF, respectively). TheSmaI-HincII fragment of pBS-+-MITF, or pBS-mi-MITF was introduced into the blunted Xba I site of pEF-BOS expression vector kindly provided by Dr S. Nagata (Osaka University, Osaka, Japan).42 To generate the Myc-tagged MITF construct, we subcloned the SmaI-HincII fragment of pBS-+-MITF or pBS-mi-MITF into theStuI site of the CS2+MT expression vector that provides 6 copies of the Myc epitope tag at the N-terminal end of the protein (a gift from Dr I. Matsumura, Osaka University, Osaka, Japan).43 The resultant chimeric gene was subcloned into pEF-BOS expression vector. The dominant negative form of GABP cloned in the pET3d expression vector (Novagen, Madison, WI) was digested with the BamHI and BglII. TheBamHI-BglII fragment of the pET3d expression plasmid was introduced into the BglII site of pCAGGS expression vector.
Transfection and luciferase assay
The transfection to MST cells was performed by electroporation. The transfection to NIH/3T3 cells or COS-7 cells was performed with the TransFast Transfection Reagent (Promega, Madison, WI) according to the manufacturer's instructions. In luciferase assays, 10 μg of a reporter and 3 μg of an expression vector containingβ-galactosidase gene were cotransfected. The expression vector containing β-galactosidase gene was used as an internal control. In some experiments, 10 μg of an expression plasmid containing effector cDNA was cotransfected with 10 μg of reporter plasmids. The cells were harvested 48 hours after the transfection and lysed with 0.1 M potassium phosphate buffer (pH 7.4) containing 1% Triton X-100. Soluble extracts were then assayed for luciferase activity with a luminometer LB96P (Berthold, Wildbad, Germany) and for β-galactosidase activity. The luciferase activity was normalized by the β-galactosidase activity and total protein concentration according to the method described by Yasumoto and coworkers.44 The normalized value was expressed as the relative luciferase activity.
Electrophoretic gel mobility shift assay
Nuclear extract of MST cells was obtained as described before.22 The sequence of oligonucleotide used as a probe was 5′-GGAGAAGCGGAAGGGGAAGGGA (the GGAA motifs are underlined). The oligonucleotide was labeled with α-[32P]-dCTP by filling 5′-overhangs and used as probes of EGMSA. DNA-binding assays were performed in a 20 μL reaction mixture containing 10 mM Tris-HCl (pH 8.0), 1 mM EDTA, 75 mM KCl, 1 mM dithiothreitol (DTT), 4% Ficoll type 400, 50 ng poly (dI-dC), and 25 ng labeled DNA probe. After the incubation at 4°C for 15 minutes, the reaction mixture was subjected to electrophoresis at 14 V/cm on a 5% polyacrylamide gel in 0.25 × TBE buffer (1 × TBE is 90 mM Tris-HCl, 64.6 mM boric acid, and 2.5 mM EDTA, pH 8.3). In the competition experiment, 10-fold molar excess amount of unlabeled oligonucleotide was added. In the supershift experiment, the antibody (Ab) against GABPα, GABPβ, or Ets-1 (Santa Cruz Biotechnology, Santa Cruz, CA) was added. The polyacrylamide gels were dried on Whatman 3MM chromatography paper and subjected to autoradiography.
In vitro binding assay
The 35S-labeled +-MITF or mi-MITF protein was synthesized using the reticulocyte lysate system (TNT system, Promega). The +-MITF or mi-MITF cDNA in pBluescript was transcribed with T7 RNA polymerase and translated in the presence of35S-methionine. For the binding assays, the35S-labeled +-MITF or mi-MITF protein was incubated for 1 hour at room temperature with GST-GABPα, GST-GABPβ, or GST alone immobilized on glutathione-agarose beads. The beads were washed 4 times. Proteins retained on the beads were subsequently analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiography.
Immunoprecipitation
The Myc-tagged +-MITF or Myc-tagged mi-MITF was coexpressed with GABPα or GABPβ in COS-7 cells. The whole cell extract was obtained by the method as described before.22The whole cell extract was incubated with LIP buffer (10 mM HEPES, 250 mM NaCl, 0.1% Nonidet P-40, 5 mM EDTA, 1 mM phenylmethylsulfonyl fluoride) and protein G Sepharose (Amersham-Pharmacia Boitec, Bucks, United Kingdom) for 1 hour with gentle rocking and centrifuged at 3000 rpm for 3 minutes. The supernatants were transferred into new tubes and incubated with protein G Sepharose and anti-GABPα or anti-GABPβ Ab for 1 hour in LIP buffer. Immunecomplexes were washed 4 times with LIP buffer, resuspended in loading buffer, boiled, and analyzed by immunoblot with anti-Myc monoclonal Ab (9E10, Pharmingen, San Diego, CA).
Immunocytochemistry
The GABPα cDNA or GABPβ cDNA was transfected alone, or both GABPα and GABPβ cDNAs were cotransfected to NIH/3T3 cells. The cells were fixed with 100% methanol and permialized by treatment with 0.2% Triton X-100 in PBS. The cells transfected with GABPα cDNA were incubated with mouse anti-GABPα Ab, and the cells transfected with GABPβ cDNA were incubated with mouse anti-GABPβ Ab, respectively. The cells transfected with both GABPα and GABPβ cDNAs were incubated with mouse anti-GABPα Ab, or with mouse anti-GABPβ Ab, independently. Immunoreacted cells were detected with antimouse IgG Ab conjugated with fluorescein isothiocyanate (FITC) (MBL, Nagoya, Japan). In a separate experiment, GABPα cDNA was cotransfected to NIH/3T3 cells with +-MITF cDNA. Because the anti-GABPα Ab was the mouse Ab47 and the anti-MITF Ab was the rabbit Ab,22we detected the cells expressing both GABPα and +-MITF using the double-staining method. The transfected cells were incubated with the mixture of anti-GABPα Ab and anti-MITF Ab, and then with the mixture of the above-mentioned antimouse IgG Ab conjugated with FITC and the antirabbit IgG Ab conjugated with rhodamine (MBL). The cells expressing GABPα were detected with the green filter and the cells expressing +-MITF were detected with the red filter of the fluorescence microscope (Olympus BX-50, Tokyo, Japan). We observed the cells possessing both the green and red signals. The GABPα cDNA was cotransfected withmi-MITF cDNA. The GABPβ cDNA was also cotransfected with either +-MITF cDNA or mi-MITF cDNA. The cells expressing both GABPα and mi-MITF, the cells expressing both GABPβ and +-MITF, or the cells expressing both GABPβ and mi-MITF were detected with the double-staining method, as described above.
Results
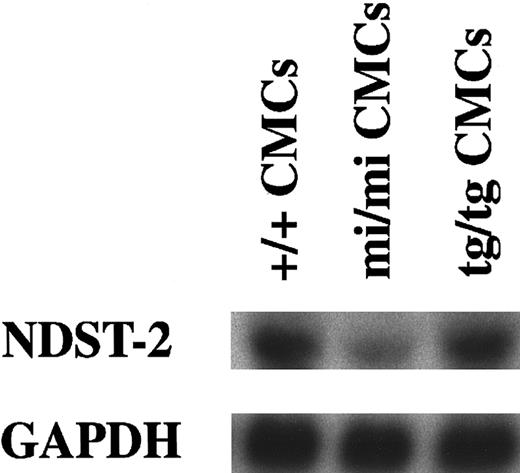
The expression of the NDST-2 gene was analyzed in skin mast cells of +/+, mi/mi, and tg/tg mice by in situ hybridization. Signals for NDST-2 mRNA were detected in skin mast cells of +/+ and tg/tg mice but not in skin mast cells ofmi/mi mice (Figure 1). We compared the amount of NDST-2 mRNA among CMCs derived from +/+,mi/mi, or tg/tg mice using Northern blot. The amount of NDST-2 mRNA reduced significantly in mi/mi CMCs when compared to that of +/+ or tg/tg CMCs (Figure2).
Expression of NDST-2 mRNA by +/+ and
tg/tg skin mast cells but not bymi/mi skin mast cells. A section of +/+,mi/mi, or tg/tg skin was stained with alcian blue and nuclear fast red, and the adjacent section was hybridized with the antisense probe for the NDST-2 gene. Another section of +/+,mi/mi, or tg/tg skin was stained with alcian blue and nuclear fast red, and the adjacent section was hybridized with the sense probe for the NDST-2 gene. Identical mast cells in the pairing sections are shown by arrows (original magnification × 400).
Expression of NDST-2 mRNA by +/+ and
tg/tg skin mast cells but not bymi/mi skin mast cells. A section of +/+,mi/mi, or tg/tg skin was stained with alcian blue and nuclear fast red, and the adjacent section was hybridized with the antisense probe for the NDST-2 gene. Another section of +/+,mi/mi, or tg/tg skin was stained with alcian blue and nuclear fast red, and the adjacent section was hybridized with the sense probe for the NDST-2 gene. Identical mast cells in the pairing sections are shown by arrows (original magnification × 400).
Expression of the
NDST-2 gene in CMCs derived from +/+,mi/mi, or tg/tg mice.The blot was hybridized with 32P-labeled cDNA probe of NDST-2 or of GAPDH. Three independent experiments were done, and comparable results were obtained. A representative experiment is shown.
Expression of the
NDST-2 gene in CMCs derived from +/+,mi/mi, or tg/tg mice.The blot was hybridized with 32P-labeled cDNA probe of NDST-2 or of GAPDH. Three independent experiments were done, and comparable results were obtained. A representative experiment is shown.
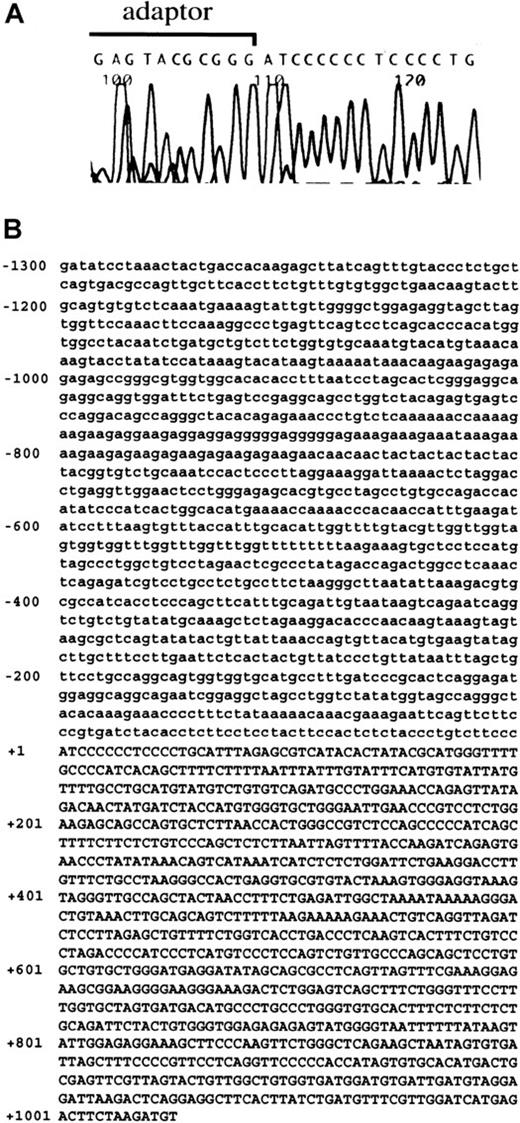
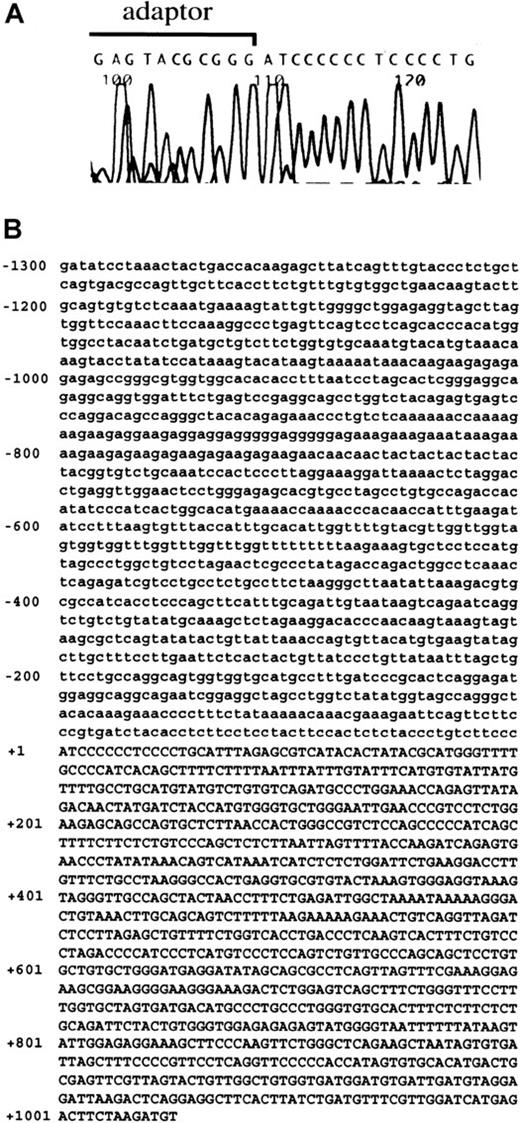
We attempted to isolate the promoter region and 5′-UTR of theNDST-2 gene. The sequence of 512 base pair (bp) upstream of the translation initiation site has been reported by Orellana and colleagues, but further upstream regions have not been cloned.37 First, we carried out 5′-RACE to determine the transcription initiation site of the NDST-2 gene. MST cells containing abundant NDST-2 transcript were used as the source of mRNA. The 5′-RACE product of the NDST-2 cDNA started from an adenine, which was located 1013 bp upstream of the 5′-end reported by Orellana and coworkers (Figure 3A).37 The 5′-UTR of the NDST-2 gene was composed of 1525 bp (512 bp for the previously reported part + 1013 bp for the isolated part). Then, we isolated the DNA fragment containing the promoter region. The isolated fragment contained the above-mentioned 5′-RACE product (1013 bp) and the remaining upstream sequence (1300 bp) (Figure 3B, the transcription initiation site was indicated as +1).
The result of 5′-RACE and the sequence of promoter region and 5′-UTR of
NDST-2 gene. (A) 5′-RACE using mRNA derived from MST cells. A part of adaptor used in 5′-RACE and 5′-end of NDST-2 cDNA are shown. (B) The sequence of promoter region and 5′-UTR of theNDST-2 gene. The transcription initiation site was indicated as +1. The sequence in the promoter region is shown by lower case, and that of the 5′-UTR is shown by capitals.
The result of 5′-RACE and the sequence of promoter region and 5′-UTR of
NDST-2 gene. (A) 5′-RACE using mRNA derived from MST cells. A part of adaptor used in 5′-RACE and 5′-end of NDST-2 cDNA are shown. (B) The sequence of promoter region and 5′-UTR of theNDST-2 gene. The transcription initiation site was indicated as +1. The sequence in the promoter region is shown by lower case, and that of the 5′-UTR is shown by capitals.
To determine the motif(s) that mediate the transactivation, we constructed the reporter plasmid containing the promoter region and 5′-UTR of the NDST-2 gene (−1300 to +668). The reporter plasmid, of which promoter region was deleted, was also constructed (−196 to +668). Each reporter plasmid was transfected to MST cells, and the luciferase activity was measured. The expression of reporter plasmid starting from −1300 and that of reporter plasmid starting from −196 showed comparable luciferase activity (Figure4A). Then, we constructed the reporter plasmid, of which 5′-UTR was deleted (−196 to +649). The luciferase activity of reporter plasmid ending at +649 reduced to one fifth that of the reporter plasmids ending at +668 (Figure 4A).
Luciferase assay in MST cells that are spontaneously expressing the
NDST-2 gene. (A) Luciferase activity under the control of promoter region and 5′-UTR of the NDST-2 gene in MST cells. (B) A part of 5′-UTR of the NDST-2 gene between +649 and +668. Two GGAA motifs are underlined. (C) Luciferase activity of the reporter plasmid containing the intact GGAA motifs or the motifs mutated at either 5′-GGAA or 3′-GGAA sequence. The data represent the mean ± SE of 3 experiments. In some cases, the SE was too small to be shown by bars. *P < .01 by t test when compared with the luciferase activity obtained by the transfection of the reporter plasmid containing −196 to +668 region.
Luciferase assay in MST cells that are spontaneously expressing the
NDST-2 gene. (A) Luciferase activity under the control of promoter region and 5′-UTR of the NDST-2 gene in MST cells. (B) A part of 5′-UTR of the NDST-2 gene between +649 and +668. Two GGAA motifs are underlined. (C) Luciferase activity of the reporter plasmid containing the intact GGAA motifs or the motifs mutated at either 5′-GGAA or 3′-GGAA sequence. The data represent the mean ± SE of 3 experiments. In some cases, the SE was too small to be shown by bars. *P < .01 by t test when compared with the luciferase activity obtained by the transfection of the reporter plasmid containing −196 to +668 region.
We examined the region between +649 and +668 in detail. Between +649 and +668, no CANNTG motif that is recognized and bound by MITF was present. On the other hand, the region contained 2 GGAA motifs, which are the consensus sequences recognized by GA binding protein (GABP) (Figure 4B, named 5′-GGAA and 3′-GGAA, respectively). GABP is a member of ets-family transcription factors, and is composed of 2 subunits, GABPα and GABPβ.45 46 The mutation at the 5′-GGAA motif (GGAA to CCAA) reduced the luciferase activity to one fifth, but the mutation at the 3′-GGAA motif did not change the luciferase activity (Figure 4C).
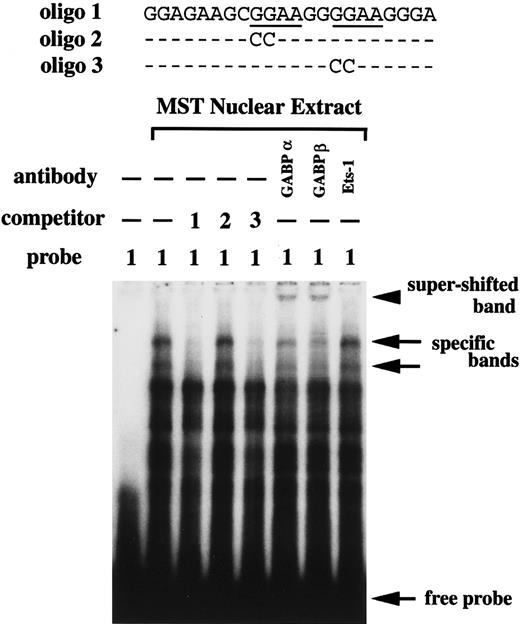
The EGMSA was done using the nuclear extract of MST cells. The oligonucleotide containing the 2 GGAA motifs was used as a probe. Multiple bands were detected by mixing the probe and nuclear extract, but the addition of excess amount of nonlabeled oligonucleotide with the same sequence as the probe reduced the intensity of 2 bands (Figure5). The intensity of these 2 bands was not reduced by the addition of the nonlabeled oligonucleotide mutated at the 5′-GGAA motif, but were reduced by the addition of the nonlabeled oligonucleotide mutated at the 3′-GGAA motif (Figure 5). This indicated that the protein in the nuclear extract of MST cells specifically bound the 5′-GGAA motif but not the 3′-GGAA motif. To characterize the protein that specifically bound the 5′-GGAA motif, we performed super-shift assay with the Ab against the GABPα, GABPβ, or Ets-1. The addition of the Ab against GABPα or GABPβ showed the super-shifted band, but the addition of the Ab against Ets-1 did not (Figure 5).
EGMSA using nuclear extract of MST cells.
Oligo 1 containing the intact GGAA motifs was used as a probe. As a competitor, nonlabeled oligo 1, oligo 2 mutated at the 5′-GGAA motif, or oligo 3 mutated at the 3′-GGAA motif was added. Arrows indicate the 2 specific DNA/protein complexes and the free probe. The super-shift assay was performed using the anti-GABPα, GABPβ, or Ets-1 Ab. The arrowhead indicates the super-shifted band.
EGMSA using nuclear extract of MST cells.
Oligo 1 containing the intact GGAA motifs was used as a probe. As a competitor, nonlabeled oligo 1, oligo 2 mutated at the 5′-GGAA motif, or oligo 3 mutated at the 3′-GGAA motif was added. Arrows indicate the 2 specific DNA/protein complexes and the free probe. The super-shift assay was performed using the anti-GABPα, GABPβ, or Ets-1 Ab. The arrowhead indicates the super-shifted band.
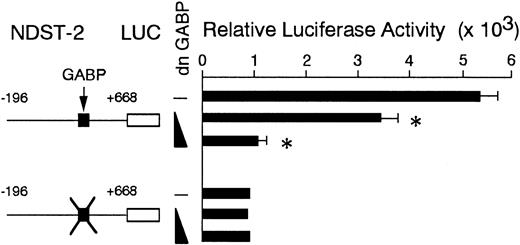
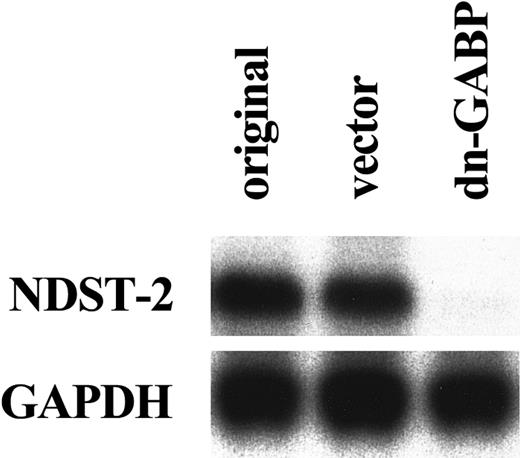
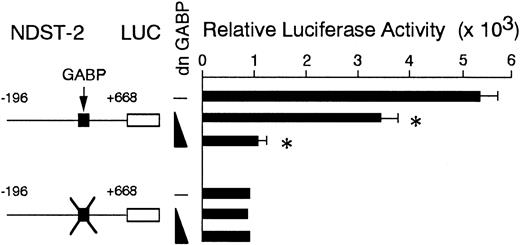
To examine whether the endogenous GABP increased the amount of NDST-2 transcript through the 5′-GGAA motif, we used a dominant negative form of GABP. The dominant negative form of GABP is a mutant of α subunit that lacks the DNA binding ability.47 We overexpressed the dominant negative GABP in MST cells. After 6 days of culture, the expression of the NDST-2 gene was examined by Northern blotting. The overexpression of dominant negative GABP significantly reduced the amount of NDST-2 mRNA (Figure6). Next, we cotransfected the dominant negative GABP to MST cells with the reporter plasmid. When various amounts of dominant negative GABP were cotransfected, the luciferase activity of the reporter plasmid containing the intact 5′-GGAA motif deceased in a dose-dependent manner (Figure7).
Reduced expression of NDST-2 transcript by the overexpression of a dominant negative GABP cDNA.
Total RNA was extracted from original MST cells (indicated as original), MST cells overexpressing empty expression vector alone (indicated as vector), or MST cells overexpressing the dominant negative GABP (indicated as dn-GABP). The expression of theNDST-2 gene was examined with Northern blot.
Reduced expression of NDST-2 transcript by the overexpression of a dominant negative GABP cDNA.
Total RNA was extracted from original MST cells (indicated as original), MST cells overexpressing empty expression vector alone (indicated as vector), or MST cells overexpressing the dominant negative GABP (indicated as dn-GABP). The expression of theNDST-2 gene was examined with Northern blot.
The effect of coexpression of dominant negative GABP cDNA on the luciferase activity.
The reporter plasmids were transfected to MST cells with the expression plasmid containing the dominant negative GABP cDNA (5 or 10 μg). The transfected DNA was always kept in equal amount using the backbone expression vector in each transfection assay. The data represent the mean ± SE of 3 experiments. In some cases, the SE was too small to be shown by bars. P < .01 by t test when compared with the luciferase activity obtained by the cotransfection of the reporter plasmid with the expression vector alone.
The effect of coexpression of dominant negative GABP cDNA on the luciferase activity.
The reporter plasmids were transfected to MST cells with the expression plasmid containing the dominant negative GABP cDNA (5 or 10 μg). The transfected DNA was always kept in equal amount using the backbone expression vector in each transfection assay. The data represent the mean ± SE of 3 experiments. In some cases, the SE was too small to be shown by bars. P < .01 by t test when compared with the luciferase activity obtained by the cotransfection of the reporter plasmid with the expression vector alone.
We examined the effect of MITF on the expression of NDST-2 transcript by GABP. We cotransfected the reporter plasmid to MST cells with the effector plasmid containing +-MITF or mi-MITF cDNA. The coexpression of +-MITF did not affect the luciferase activity, but the coexpression of mi-MITF reduced the luciferase activity in a dose-dependent manner (Figure 8).
The effect of coexpression of +-MITF or
mi-MITF cDNA on the luciferase activity. The reporter plasmids were transfected to MST cells with the expression plasmid containing +-MITF or mi-MITF cDNA (5 or 10 μg). The transfected DNA was always kept in equal amount using the backbone expression vector in each transfection assay. The data represent the mean ± SE of 3 experiments. In some cases, the SE was too small to be shown by bars. P < .01 by t test when compared with the luciferase activity obtained by the cotransfection of the reporter plasmid with the expression vector alone.
The effect of coexpression of +-MITF or
mi-MITF cDNA on the luciferase activity. The reporter plasmids were transfected to MST cells with the expression plasmid containing +-MITF or mi-MITF cDNA (5 or 10 μg). The transfected DNA was always kept in equal amount using the backbone expression vector in each transfection assay. The data represent the mean ± SE of 3 experiments. In some cases, the SE was too small to be shown by bars. P < .01 by t test when compared with the luciferase activity obtained by the cotransfection of the reporter plasmid with the expression vector alone.
The physical interaction between MITF and GABP was examined by an in vitro binding experiment. The 35S-labeled +-MITF or35S-labeled mi-MITF was subjected to coprecipitation with GST, GST-GABPα, or GST-GABPβ fusion protein that was immobilized on glutathione-agarose beads. Protein complexes were analyzed by SDS-PAGE. The complex of +-MITF and GST-GABPα and that of +-MITF and GST-GABPβ were detected, but that of +-MITF and GST was not (Figure 9). We also examined the complex formation of mi-MITF. The complex ofmi-MITF and GST-GABPα and that of mi-MITF and GST-GABPβ were detected, but that of mi-MITF and GST was not (Figure 9).
In vitro binding of MITF to GABP.
35S-labeled +-MITF or 35S-labeledmi-MITF was subjected to coprecipitation with GST-GABPα, GST-GABPβ, or GST, which had been attached to beads, and the protein complex was analyzed by SDS-PAGE.
In vitro binding of MITF to GABP.
35S-labeled +-MITF or 35S-labeledmi-MITF was subjected to coprecipitation with GST-GABPα, GST-GABPβ, or GST, which had been attached to beads, and the protein complex was analyzed by SDS-PAGE.
We carried out coimmunoprecipitation studies to confirm the interaction between MITF and GABP. The Myc-tagged +-MITF or Myc-taggedmi-MITF was coexpressed with GABP in COS-7 cells, and their whole cell lysate was analyzed. The immnoprecipitated product with anti-GABPα Ab or with anti-GABPβ Ab was immunoblotted by anti-Myc Ab. The immunoprecipitated product with anti-GABPα Ab contained either +-MITF or mi-MITF (Figure10A). The immunoprecipitated product with anti-GABPβ Ab also contained either +-MITF or mi-MITF (Figure 10B).
Coimmunoprecipitation of MITF and GABP.
Myc-tagged +-MITF or Myc-tagged mi-MITF was cotransfected to COS-7 cells with GABPα (A) or GABPβ (B). The whole cell extract was subjected to immunoprecipitation with anti- GABPα or GABPβ Ab. Precipitates were separated by SDS-PAGE and immunoblotted with anti-Myc Ab.
Coimmunoprecipitation of MITF and GABP.
Myc-tagged +-MITF or Myc-tagged mi-MITF was cotransfected to COS-7 cells with GABPα (A) or GABPβ (B). The whole cell extract was subjected to immunoprecipitation with anti- GABPα or GABPβ Ab. Precipitates were separated by SDS-PAGE and immunoblotted with anti-Myc Ab.
The effect of MITF on nuclear translocation of GABP was examined by immunocytochemistry. First, we examined the subcellular localization of GABPα or GABPβ without any MITFs. When expressed alone, GABPα diffused both in the nucleus and cytoplasm, whereas GABPβ was localized in the nucleus (Figure 11A). GABPα became localized in the nucleus when coexpressed with GABPβ (Figure 11A, left). Next, we coexpressed +-MITF with GABPα or GABPβ. By coexpressing +-MITF, GABPα was localized in the nucleus (Figure 11B, left). GABPβ was also localized in the nucleus when coexpressed with +-MITF (Figure 11B, right). Then, we coexpressedmi-MITF with GABPα or GABPβ. GABPα diffused in both nucleus and cytoplasm when coexpressed with mi-MITF (Figure11C, left). GABPβ also diffused in both nucleus and cytoplasm when coexpressed with mi-MITF (Figure 11C, right). When expressed alone, the +-MITF was localized in the nucleus, whereas themi-MITF diffused both in the nucleus and cytoplasm, as reported previously (data not shown).22
Subcellular localization of GABPα, GABPβ, +-MITF, or
mi-MITF examined by immunocytochemistry. (A) GABPα or GABPβ was expressed alone, or GABPα and GABPβ were coexpressed. Cells were stained with either anti-GABPα Ab or anti-GABPβ Ab. (B) GABPα and +-MITF, or GABPβ and +-MITF were coexpressed. Cells were stained with the mixture of anti-GABPα Ab and anti-MITF Ab, or the mixture of anti-GABPβ Ab and anti-MITF Ab. (C) GABPα and mi-MITF, or GABPβ and mi-MITF were coexpressed. Cells were stained with the mixture of anti-GABPα Ab and anti-MITF Ab, or the mixture of anti-GABPβ Ab and anti-MITF Ab (original magnification × 2000).
Subcellular localization of GABPα, GABPβ, +-MITF, or
mi-MITF examined by immunocytochemistry. (A) GABPα or GABPβ was expressed alone, or GABPα and GABPβ were coexpressed. Cells were stained with either anti-GABPα Ab or anti-GABPβ Ab. (B) GABPα and +-MITF, or GABPβ and +-MITF were coexpressed. Cells were stained with the mixture of anti-GABPα Ab and anti-MITF Ab, or the mixture of anti-GABPβ Ab and anti-MITF Ab. (C) GABPα and mi-MITF, or GABPβ and mi-MITF were coexpressed. Cells were stained with the mixture of anti-GABPα Ab and anti-MITF Ab, or the mixture of anti-GABPβ Ab and anti-MITF Ab (original magnification × 2000).
Discussion
NDST-2 catalyzes the initial sulfation step of heparin synthesis.37,48 Recently, 2 groups reported the phenotype of the mice whose NDST-2 genes were disrupted (NDST-2−/−).33,34 In NDST-2−/− mice, skin mast cells show depletion of heparin and CMCs do not form normal granules. We previously reported that the synthesis of heparin is abnormal in the skin mast cells ofmi/mi mice.9 In the present study, we compared the expression of the NDST-2 gene among mast cells of +/+,mi/mi, and tg/tg genotypes. The NDST-2 mRNA was detected by in situ hybridization in the skin mast cells of +/+ andtg/tg mice, but not in the skin mast cells ofmi/mi mice. The amount of NDST-2 mRNA in CMCs ofmi/mi genotype decreased significantly when compared to the value of +/+ or tg/tg genotype. This indicated that themi-MITF possessed the inhibitory effect on the expression of NDST-2 transcript.
In mast cells of NDST-2−/− mice, the unsulfated heparin precursors are found instead of heparin.33,34 We previously reported that mast cells of mi/mi mice not only reduced the content of heparin but contained unidentified glycosaminoglycans.9 The unidentified glycosaminoglycans in mi/mi skin mast cells might be unsulfated heparin precursors, which were found in mast cells of NDST-2−/−mice.
To examine the inhibitory effect of mi-MITF, we cloned the promoter region and 5′-UTR of the NDST-2 gene and found that the 5′-UTR between +649 and +668 was important for the transactivation of the NDST-2 gene. Unexpectedly, no CANNTG motifs, which were recognized and bound by +-MITF, were present between +649 and +668. Instead, a GGAA motif, which was recognized and bound by GABP, was observed. The expression of dominant negative GABP reduced the luciferase activity of the reporter plasmid containing the GGAA motif, indicating that GABP increased the amount of NDST-2 transcript through the GGAA motif. The mi-MITF reduced the luciferase activity of the reporter plasmid containing the GGAA motif in a dose-dependent manner. This suggested that the mi-MITF possessed the inhibitory effect on the ability of GABP to express NDST-2 transcript.
The GGAA motif was located in the 5′-UTR of the NDST-2gene. The protein that binds to the motif in 5′-UTR regulates the expression of genes in the following 2 ways.49-51 One is that the protein binds to the transcribed RNA and stabilizes it, in which the protein regulates the expression of genes in a post-transcription level.49,50 Another is that the protein binds to the DNA and regulates the expression in a transcription level, as in the case of usual transcription factors.51-55Further studies including nuclear run-on analysis will clarify whether the GABP regulated the expression of NDST-2 transcript as an RNA-binding factor or a DNA-binding factor.
GABP is a heterodimer and is composed of GABPα and GABPβ.45,46 GABPα itself had no nuclear localization ability, but was localized in the nucleus when coexpressed with GABPβ, as reported previously.47 GABPα was also localized in the nucleus when coexpressed with +-MITF. The +-MITF and GABPβ showed the same effect on the nuclear localization of GABPα. Because +/+ CMCs expressed both +-MITF and GABPβ, GABPα may enter into the nucleus of +/+ CMCs.
When coexpressed with mi-MITF, either GABPα or GABPβ was not localized in the nucleus. This was reminiscent of the previous finding that the mi-MITF disturbed the nuclear accumulation of other transcription factors, that is, Pu.1 and c-Fos.56The mi-MITF may inhibit the ability of GABP to express NDST-2 transcript by disturbing the nuclear localization.
The +-MITF did not affect the luciferase activity of the reporter plasmid under the control of the 5′-UTR of the NDST-2 gene containing the GGAA motif. The +-MITF might not be necessary for the expression of NDST-2 transcript, because the NDST-2 gene was normally expressed in tg/tg mast cells that lacked any MITFs. Besides the NDST-2 transcript, the expression of c-kit, Gr B, and TPH transcripts were negatively affected by the presence of mi-MITF.25 The level of expression of c-kit, Gr B, and TPH transcripts did decrease in tg/tg CMCs25 but that of NDST-2 transcript did not decrease in tg/tg CMCs. In other words, the +-MITF may enhance the expression of c-kit, Gr B, and TPH transcripts but not the expression of NDST-2 transcript.NDST-2 is the first gene whose expression was affected negatively by mi-MITF but whose expression was not affected positively by +-MITF.
In vitro binding assay and immunoprecipitation assay showed that GABP and MITF physically interacted with each other. The physical interactions between the ets family transcription factors and the bHLH-Zip family transcription factors have been reported.57 The present result may be another example of such interactions. GABPβ contains a Zip-like motif.47 de la Brousse and coworkers58 reported that the Zip-like motif of GABPβ can be replaced by a Zip domain. Because Zip domains are known to mediate the protein-protein interactions,59GABPβ and MITF might interact with each other through the Zip-like domain of GABPβ and the Zip domain of MITF. Further biochemical studies are necessary to reveal the detailed mechanism of the interaction.
Heparin plays an important role for the storage of mediators in mast cell granules, because amounts of various mediators, such as MMCP-4, MMCP-5, and MMCP-6, decrease in NDST-2−/− mast cells.33,34 We have demonstrated that MITF regulates the transcription of MMCP-4, MMCP-5, and MMCP-6genes.11-13 Here we showed that MITF was involved in the expression of NDST-2 transcript. MITF appeared to regulate both transcription and storage of MMCP-4, MMCP-5, and MMCP-6.
Heparin is synthesized with multiple steps. Besides NDST-2, various enzymes, such as copolymerase EXT-1 and EXT-2, are involved in heparin synthesis in mast cells.60 Further studies will reveal the importance of such enzymes.
Taken together, the mi-MITF possessed the inhibitory effect on the expression of NDST-2 transcript by disturbing the nuclear localization of GABP. The decreased heparin content in mi/miskin mast cells may be attributable to the deficient expression of the NDST-2 transcript.
The authors thank Dr H. Arnheiter of National Institutes of Health for VGA9-tg/tg mice, Dr K. Nakajima of Osaka City University for pSP-Luc, Dr S. Nagata of Osaka University for pEF-BOS, and Dr I. Matsumura of Osaka University for CS2+MT.
Supported by grants from the Ministry of Education, Science and Culture, the Ministry of Health and Welfare, the Organization of Pharmaceutical Safety and Research, the Welfide Medicinal Research Foundation, and the Uehara Memorial Foundation. GenBank accession number AF293452.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Eiichi Morii, Dept of Pathology, Osaka University Medical School, Yamada-oka 2-2, Suita 565-0871, Japan; e-mail:morii@patho.med.osaka-u.ac.jp.