Abstract
Standard therapy in the United States for malignancy-associated hyperuricemia consists of hydration, alkalinization, and allopurinol. Urate oxidase catalyzes the enzymatic oxidation of uric acid to a 5 times increased urine soluble product, allantoin. Rasburicase is a new recombinant form of urate oxidase available for clinical evaluation. This multicenter randomized trial compared allopurinol to rasburicase in pediatric patients with leukemia or lymphoma at high risk for tumor lysis. Patients received the assigned uric acid-lowering agent for 5 to 7 days during induction chemotherapy. The primary efficacy end point was to compare the area under the serial plasma uric acid concentration curves during the first 96 hours of therapy (AUC0-96). Fifty-two patients were randomized at 6 sites. In an intent-to-treat analysis, the mean uric acid AUC0-96 was 128 ± 70 mg/dL.hour for the rasburicase group and 329 ± 129 mg/dL.hour for the allopurinol group (P < .0001). The rasburicase versus allopurinol group experienced a 2.6-fold (95% CI: 2.0-3.4) less exposure to uric acid. Four hours after the first dose, patients randomized to rasburicase compared to allopurinol achieved an 86% versus 12% reduction (P < .0001) of initial plasma uric acid levels. No antirasburicase antibodies were detected at day 14. This randomized study demonstrated more rapid control and lower levels of plasma uric acid in patients at high risk for tumor lysis who received rasburicase compared to allopurinol. For pediatric patients with advanced stage lymphoma or high tumor burden leukemia, rasburicase is a safe and effective alternative to allopurinol during initial chemotherapy.
Introduction
In patients with myeloproliferative diseases or hematopoietic malignancies, nucleic acids are catabolized as a result of increased turnover of malignant cell populations. This results in an increase in purine metabolism, leading to hyperuricemia. Aggressive cancer chemotherapy regimens cause an increase in cell lysis and release of purine metabolites.1 Tumor lysis syndrome is characterized by severe hyperuricemia, hyperphosphatemia, hyperkalemia, hypocalcemia, and acute renal failure.2-6 As a consequence of hyperuricemia, renal insufficiency develops when urine becomes supersaturated with uric acid and crystals of uric acid form in the renal tubules and distal collecting system.2,7 Moreover, despite management of metabolic abnormalities to reduce the risk of acute renal failure, 25% of children with advanced-stage Burkitt lymphoma and B-cell acute lymphoblastic leukemia still experience acute renal failure at onset of cytoreductive chemotherapy.8-10
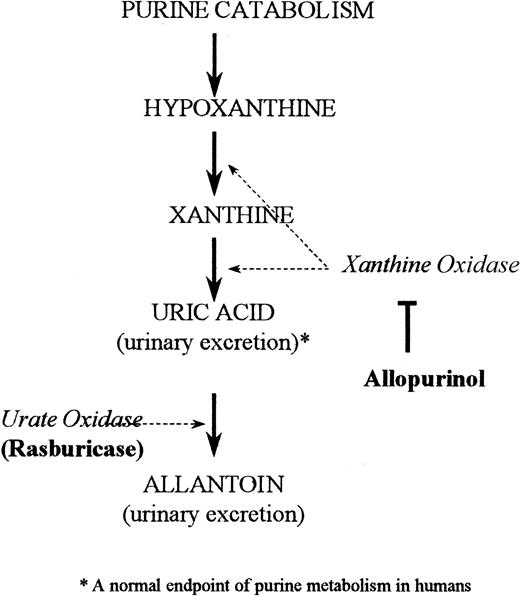
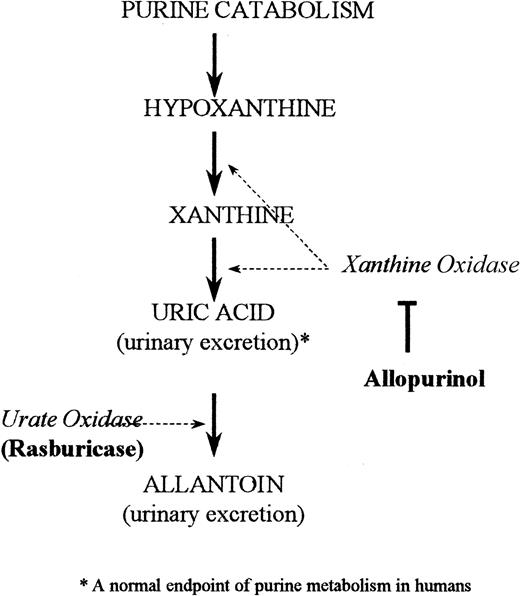
The standard treatment for hyperuricemia in the United States consists of allopurinol, urinary alkalinization, and hydration.11By inhibiting the enzyme xanthine oxidase, allopurinol blocks uric acid formation (Figure 1). This increases the plasma concentrations of the uric acid precursors hypoxanthine and xanthine.11 Patients at high risk for tumor lysis still need to excrete preexisting uric acid, which is not affected by allopurinol treatment. Consequently, a drug that has rapid and highly potent uricolytic properties, reduces metabolic morbidity, and can be conveniently integrated with the initiation of a cancer chemotherapy regimen would be helpful in the supportive management of this patient population.
Mechanism of action: rasburicase and allopurinol.
Depicted is the pathway of purine catabolism. Rasburicase is a recombinant form of urate oxidase, an enzyme that converts uric acid to allantoin. Allopurinol in comparison acts by inhibiting the endogenous enzyme xanthine oxidase, thereby inhibiting formation of uric acid.
Mechanism of action: rasburicase and allopurinol.
Depicted is the pathway of purine catabolism. Rasburicase is a recombinant form of urate oxidase, an enzyme that converts uric acid to allantoin. Allopurinol in comparison acts by inhibiting the endogenous enzyme xanthine oxidase, thereby inhibiting formation of uric acid.
Urate oxidase catalyzes the enzymatic oxidation of uric acid into allantoin (Figure 1), a readily excretable substance about 5 times more soluble than uric acid.12 Urate oxidase is an endogenous enzyme found in most mammals, but not in humans because of a nonsense mutation in the coding region in the gene during hominoid evolution.13 Rasburicase is a recombinant form of the urate oxidase enzyme.14 A study in healthy adult male volunteers established that single daily intravenous doses of rasburicase were well tolerated and led to dramatic decreases in plasma uric acid levels.15 A recent multicenter phase II trial of rasburicase in pediatric patients with leukemia and lymphoma (n = 131) demonstrated that 100% of patients achieved uric acid control at a dose of rasburicase of 0.2 mg/kg despite concomitant intensive chemotherapy.16 The present study reports the efficacy of rasburicase compared to allopurinol in reducing exposure to plasma uric acid in children with leukemia or lymphoma at high risk for developing tumor lysis syndrome.
Materials and methods
Study design
From November 1996 through December 1997, 52 pediatric patients with leukemia or lymphoma were enrolled on this open-labeled, randomized, multicenter, comparative trial of rasburicase versus allopurinol. The study investigational new drug (IND) was approved by the Food and Drug Administration. The Institutional Review Boards of all participating institutions approved the study. Patients or families gave written informed consent before entry into the study and randomization.
Eligibility criteria
The study enrolled pediatric oncology patients with leukemia or lymphoma deemed at high risk for tumor lysis syndrome. Eligibility criteria included: (1) Murphy stage III or IV non-Hodgkin lymphomas (NHL),17 (2) acute lymphocytic leukemia (ALL) with a peripheral white blood cell (WBC) count of 25 000/μL or higher at presentation, or (3) any childhood lymphoma or leukemia with a uric acid level of 8 mg/dL or higher at the time of study entry. Patients must have been scheduled to receive chemotherapeutic agents that were not investigational in nature. A minimum life expectancy of 4 weeks and a Eastern Cooperative Oncology Group (ECOG) performance scale of 3 or less, or a Karnofsky scale of 30% or more were additional eligibility criteria at patient entry.
Exclusion criteria included patients with previous treatment with rasburicase or Uricozyme, treatment with allopurinol within 7 days, and a significant history of documented asthma, atopy, or deficiency of glucose-6-phosphate dehydrogenase (G6PD).
Randomization and treatment
Via telephone entry, treatment (rasburicase or allopurinol) was randomly allocated to patients according to a computer-generated randomization code schema. Randomization was stratified according to presentation uric acid (< 8.0 mg/dL or ≥ 8.0 mg/dL) and disease (leukemia or lymphoma).
Patients were scheduled to receive 5 to 7 days of either uric acid-lowering agent (rasburicase or allopurinol). The dose and schedule of rasburicase was 0.20 mg/kg intravenously given over 30 minutes daily.16 Oral allopurinol therapy was dosed by the individual investigators according to the package insert and standard clinical practice. Standard pediatric allopurinol dosing was 300 mg/m2 (or 10 mg/kg) divided every 8 hours. Intravenous sodium bicarbonate (generally with 20-40 mEq/L fluid) to increase urine alkalinization was permitted at the investigators' discretion. All patients, regardless of treatment group, received hydration (∼ 3 L/m2 daily). The study duration was to be 14 days for each patient, including 5 to 7 days of treatment and a final safety assessment on day 14.
Outcome measurements and follow-up
All study patients had detailed analysis of plasma uric acid values during the first 96 hours of uric acid-lowering agent therapy. Plasma uric acid was measured at the clinical laboratories of the participating institutions. Special handling procedures and strict temperature conditions (0°C-4°C) were observed during the blood collection procedures to block ex vivo enzymatic activity of rasburicase. These temperature conditions were previously shown to result in less than 3.4% ex vivo degradation for up to 4 hours after blood withdrawal (data not shown). Plasma uric acid levels were measured immediately before administration of uric acid-lowering agents (T0h) and at hours 4 and 12, and then every 12 hours through 96 hours. Complete physical examinations and symptom and toxicity assessments were performed at least daily while the children were hospitalized. Complete blood counts with differential and serum chemistries were performed as required by the patients' chemotherapy protocols or as clinically appropriate. In addition, antibody to urate oxidase protein was determined in patients receiving rasburicase on day 0 and day 14 of the study. After treatment, detailed physical examination, plasma uric acid, complete blood counts, serum chemistries, and urinalysis were required on day 8 of study or 24 to 48 hours after completion of uric acid- lowering therapy (whichever occurred first). On day 14 a symptom/toxicity assessment was performed.
The main objective of the study was to compare the decrease in plasma uric acid levels by the 2 uric acid-lowering agents during the first 5 days of cytoreductive chemotherapy. The primary efficacy end point was the area under the serial plasma uric acid concentration curves from the start of study drug until 96 hours (AUC0-96). The AUC0-96 was constructed for all patients and across treatment groups as well as subgroups of interest. Last value carried forward method was used to estimate the missing data for patients who discontinued the study prematurely and linear interpolation method was used to impute the missing data between visits. Hyperuricemic patients were defined as patients with a plasma uric acid value of 8 mg/dL or above either at presentation or hour 0 (ie, immediately before first dose of study drug administration). A secondary end point was the percent reduction of uric acid at 4 hours after first dose of uric acid-lowering agent therapy (ie, prior to induction chemotherapy).
Safety and toxicity
Safety analyses were performed in all patients and compared between treatment groups. All adverse events were coded according to the World Health Organization Adverse Reactions Terminology (WHO-ART) and assigned a system-organ class. Antirasburicase antibody determination was performed under the responsibility of Sanofi Research Division, Great Valley, PA via an enzyme-linked immunosorbent assay (ELISA) technique as previously reported.18
Statistical analysis
The primary efficacy end point was the plasma uric acid AUC0-96. The end point was computed for each patient using the trapezoidal rule. Linear interpolation was used to compute a missing value. Values collected below the limit of quantification (LOQ) were replaced by the LOQ value. Descriptive summary statistics (N, mean, median, SD) were computed for each treatment group. A one-way analysis of variance (ANOVA) model was used to evaluate the treatment effect on plasma uric acid AUC0-96 and percent change from baseline plasma uric acid concentrations at 4 hours. Subgroup analyses were similarly performed by disease (lymphoma or leukemia) or presenting uric acid values (< 8 mg/dL or ≥ 8 mg/dL). Descriptive statistics and statistical analyses of the safety and efficacy data were calculated using SAS software, Version 6.12 on a Sanofi Alphaserver.
Results
Patients
Fifty-two patients were randomized at 6 sites within the United States between November 1996 and December 1997. Patient baseline demographics and disease type are shown in Table1. There were no statistical differences in the age, race, male/female ratios, or performance status for patients randomized to rasburicase versus allopurinol. The mean age for patients assigned rasburicase versus allopurinol was 7.1 years versus 7.8 years, respectively. Eighty-nine percent of patients assigned to rasburicase had ECOG scores of one or less compared to 84% assigned to allopurinol. Twenty patients with leukemia received rasburicase and 19 patients received allopurinol. Seven patients with lymphoma received rasburicase versus 6 receiving allopurinol. A similar number of patients with advanced Burkitt leukemia/lymphoma (mature B-cell leukemia or ≥ stage III Burkitt small noncleaved-cell lymphoma) were in each treatment group (5 rasburicase, 4 allopurinol). In addition, there was no significant differences in initial tumor burden markers between treatment arms (ie, WBC count in the leukemia patients and lactic dehydrogenase [LDH] in the lymphoma patients). Patient randomizations were also stratified according to presenting uric acid values. Despite this, mean presenting uric acid values were higher in patients assigned rasburicase by about 1 mg/dL, but this did not reach statistical significance. Rasburicase patients presented with a mean uric acid value ± SD of 7.7 ± 3.5 mg/dL versus 6.8 ± 3.4 mg/dL in allopurinol patients. All other metabolic parameters were similar between treatment groups including calcium, potassium, and phosphorus. Finally, the presentation creatinine levels were nearly identical between groups, with the rasburicase patients presenting with a mean creatinine of 0.60 ± 0.33 mg/dL versus 0.61 ± 0.30 mg/dL in allopurinol patients (Table 1).
Drug administration
All 52 patients randomized received at least one dose of their assigned treatments with rasburicase or allopurinol. Patients assigned rasburicase received a dose once every 24 hours, except for one patient with early pre-B cell ALL with a WBC count of 178 × 109/L and a uric acid value of 4.1 mg/dL who received every 12-hour dose × 4 because of investigator preference. A total of 387 doses (median number of 3 doses/d) of oral allopurinol were administered with a median dose of 100 mg (1 tablet) per dose. The mean time from study drug administration to start of chemotherapy (excluding intrathecal therapy) was 21 hours in both treatment groups. The treatment protocol was violated in 2 patients in each arm because chemotherapy was started prior to the initiation of the study drug. Four patients withdrew from the study before completion of randomized drug. One patient assigned to rasburicase withdrew after 4 doses because of grade 4 hemolysis; a relationship to the study drug was not known by the investigator (see “Adverse events and mortality”). Three patients assigned to allopurinol withdrew from study prior to completion. Two of these patients suffered unrelated deaths (see below) during induction chemotherapy after 14 and 8 doses of allopurinol, respectively. The third patient was withdrawn after 14 doses of allopurinol because chemotherapy had not been administered. All patient data were used regardless of study completion in an intent-to-treat analysis.
Control of plasma uric acid
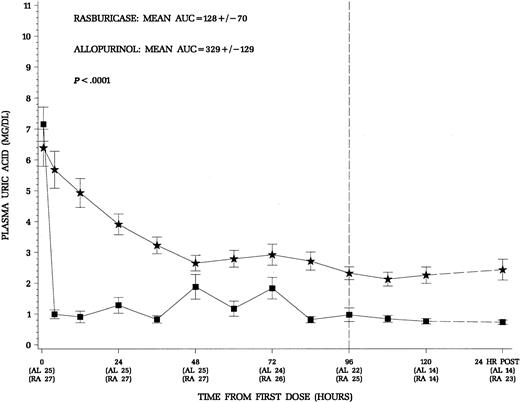
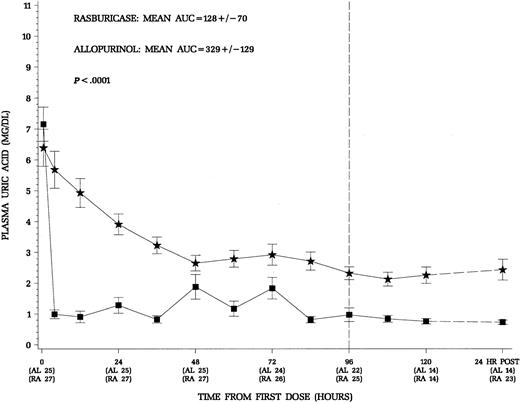
The primary end point of this trial was the control of uric acid through the first 96 hours of therapy with concomitant induction chemotherapy appropriate for the specific disease. Patients treated with rasburicase had a more rapid decline and maintained lower plasma uric acid levels throughout induction chemotherapy (Figure2). The area under the curve for mean uric acid AUC0-96 was used to quantify the exposure to uric acid during the first 96 hours of induction chemotherapy (Figure 2). In an intent-to-treat analysis, the mean AUC0-96 was 128 ± 70 mg/dL.hr for the rasburicase group and 329 ± 129 mg/dL.hr for the allopurinol group (P < .0001). Patients receiving rasburicase on average experienced a 2.6-fold less exposure to uric acid during the first 96 hours of therapy. For the subgroup of 19 hyperuricemic patients any time prior to dosing, there was a similar reduction in uric acid exposure during therapy in patients who received rasburicase (Table2). Although no attempt was made to randomize the small number of patients with Burkitt lymphoma in this trial, these patients are at greatest risk for hyperuricemic nephropathy during initiation of treatment.9 10 As illustrated in Table 2, patients with advanced Burkitt lymphoma had the greatest reduction in exposure to uric acid (∼ 4-fold) when comparing rasburicase treatment to allopurinol during the first 96 hours of therapy. Thus, the advantageous decrease in uric acid was evident across all patients included in the study as well as in treatment subgroups, normouricemic versus hyperuricemic patients, and patients with leukemia or lymphoma.
Mean (± SE) plasma uric acid concentrations over time for all patients.
Squares denote patients who received rasburicase (n = 27) and stars allopurinol (n = 25). The 24 hours.post levels reflect 24 hours after the last dose of study drug. Patients who received rasburicase demonstrated more rapid decline and maintained lower plasma uric acid levels throughout the study period. The area under the serial plasma uric acid concentration curve through the first 96 hours of therapy was significantly less for patients receiving rasburicase (P < .0001).
Mean (± SE) plasma uric acid concentrations over time for all patients.
Squares denote patients who received rasburicase (n = 27) and stars allopurinol (n = 25). The 24 hours.post levels reflect 24 hours after the last dose of study drug. Patients who received rasburicase demonstrated more rapid decline and maintained lower plasma uric acid levels throughout the study period. The area under the serial plasma uric acid concentration curve through the first 96 hours of therapy was significantly less for patients receiving rasburicase (P < .0001).
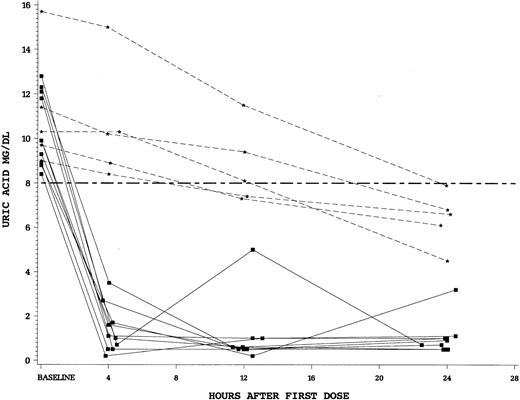
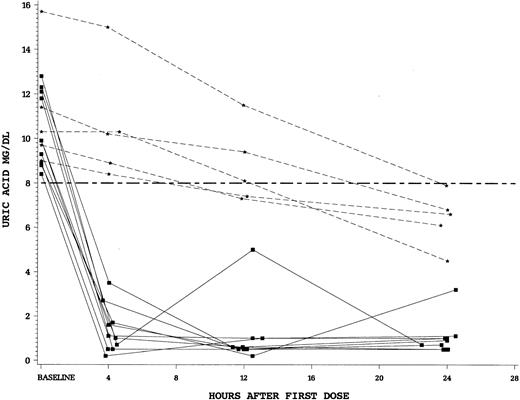
The rapidity of control of uric acid by study drug was a secondary end point in this trial. We found a striking difference between the single dose of intravenous rasburicase at 0.20 mg/kg versus standard doses of oral allopurinol. For patients randomized to rasburicase, there was an 86% reduction in plasma uric acid levels after 4 hours of the first dose compared to only 12% for allopurinol (P < .0001). Finally, we examined the fall in plasma uric acid in patients who were hyperuricemic (uric acid > 8 mg/dL at T = 0). Patients hyperuricemic at baseline and treated with rasburicase (n = 10) all achieved a uric acid concentration of less than 8 mg/dL in less than 4 hours. In contrast, no patients hyperuricemic at start of allopurinol (n = 5) achieved a uric acid level less than 8 mg/dL by 4 hours (Figure 3).
Plasma uric acid concentrations during 24 hours after first dose of study drug for individual patients with uric acid levels more than 8 mg/dL at time 0.
Squares (solid lines) denote individual patients who received rasburicase and stars (dashed lines) allopurinol. Hyperuricemic patients who received rasburicase (n = 10) all achieved a plasma uric acid level less than 8 mg/dL within 4 hours of first drug dose. None of the hyperuricemic allopurinol patients (n = 5) achieved control by 4 hours.
Plasma uric acid concentrations during 24 hours after first dose of study drug for individual patients with uric acid levels more than 8 mg/dL at time 0.
Squares (solid lines) denote individual patients who received rasburicase and stars (dashed lines) allopurinol. Hyperuricemic patients who received rasburicase (n = 10) all achieved a plasma uric acid level less than 8 mg/dL within 4 hours of first drug dose. None of the hyperuricemic allopurinol patients (n = 5) achieved control by 4 hours.
Renal outcome
The study sample size was too small to determine a difference in the incidence of renal failure or required renal support. One patient who received allopurinol required hemodialysis during the study period. This patient was a 12-year-old with stage IV Burkitt lymphoma who had renal failure, hypocalcemia, and a maximum serum phosphorous of 20.2 mg/dL on day 2 of standard cytoreductive chemotherapy with cyclophosphamide, vincristine, and prednisone. He underwent hemodialysis followed by hemofiltration for 6 days with recovery of renal function. In contrast, a 15-year-old girl with a bulky presentation of pre-B ALL in the rasburicase group presented with frank renal insufficiency with a creatinine of 2.5 mg/dL at baseline and a uric acid concentration of 16.5 mg/dL. This patient's renal function improved during therapy without the need for any type of dialysis or hemofiltration.
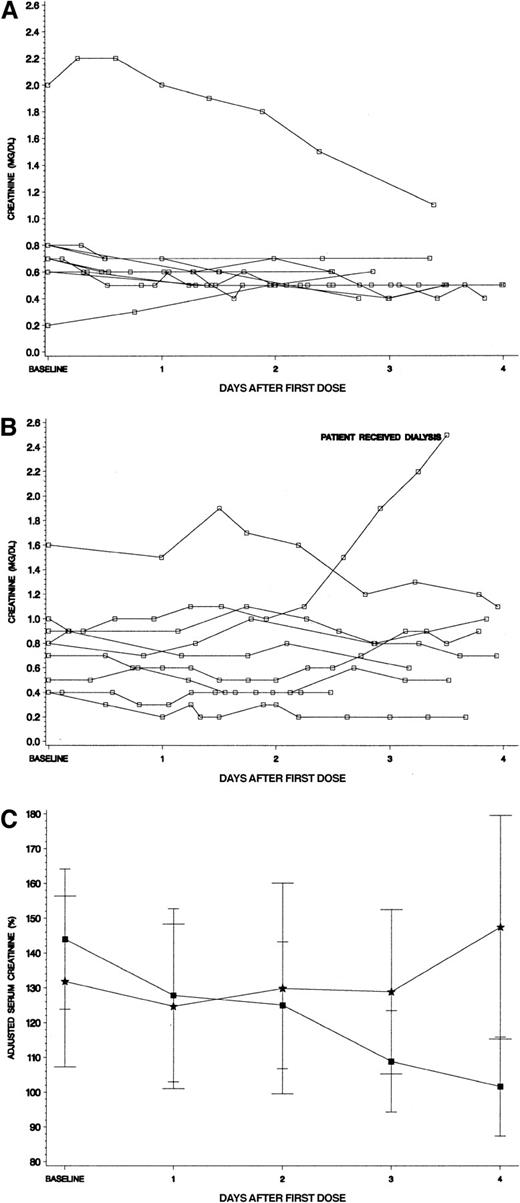
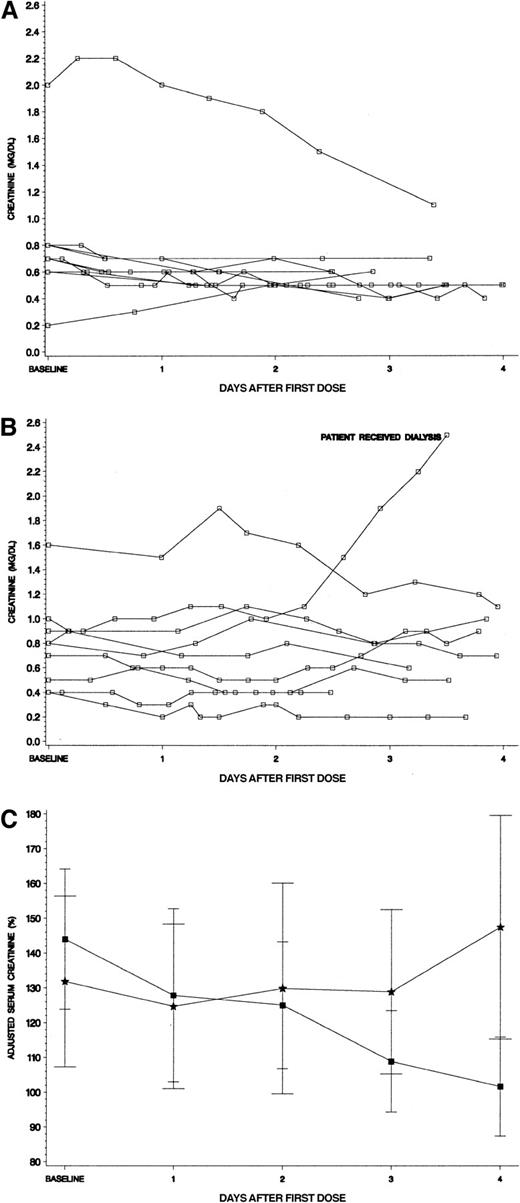
To more closely examine the relationship of renal function to treatment arm, we studied creatinine levels over the first 96 hours of therapy for the hyperuricemic patients. Panels A and B in Figure4 show the actual creatinine values for hyperuricemic patients by study drug received. From infancy through adolescence, “normal” serum creatinine levels can vary greatly (∼3-fold) by age and sex.20 We therefore adjusted each patient's creatinine level by expressing it as a percentage of the published mean for age and gender to facilitate comparison in the hyperuricemic patients by study drug (Figure 4C). The hyperuricemic patients who received rasburicase had a baseline creatinine of 144% of their age/sex-defined mean. A steady decline occurred through the 4 days of therapy to an adjusted creatinine level of 102%. In contrast, the hyperuricemic allopurinol group had a baseline creatinine of 132%, which worsened over the 4 days of therapy to 147% of mean (Figure 4C).
Serum creatinine levels for hyperuricemic patients.
(A) Serum creatinine levels for hyperuricemic patients who received rasburicase (n = 10). All serum creatinine levels for patients were plotted in reference to initiation of study drug. No patients received assisted renal support, including a 15-year-old girl whose creatinine level was more than 2 mg/dL at baseline (see text for details). (B) Serum creatinine levels for hyperuricemic patients who received allopurinol (n = 9). All serum creatinine levels for patients were plotted in reference to initiation of study drug. One patient who began therapy with a normal creatinine for age developed renal failure requiring hemodialysis and hemofiltration for several days (see text for details). (C) Adjusted creatinine levels (percent ± SE of age/sex defined mean) for patients who were hyperuricemic at presentation. Squares denote patients who received rasburicase and stars represent allopurinol. Each creatinine level was adjusted by dividing the serum creatinine value by the published mean creatinine for patient's age and gender × 100%. Normal mean creatinine levels were the following: less than 1 year = 0.3 mg/dL; 1 to 12 years = 0.5 mg/dL; 12 to 16 years = 0.75 mg/dL; males 16 years or older = 0.95 mg/dL, females 16 years or older = 0.85 mg/dL.20 The adjusted creatinine declined steadily during initiation of chemotherapy only for the rasburicase group.
Serum creatinine levels for hyperuricemic patients.
(A) Serum creatinine levels for hyperuricemic patients who received rasburicase (n = 10). All serum creatinine levels for patients were plotted in reference to initiation of study drug. No patients received assisted renal support, including a 15-year-old girl whose creatinine level was more than 2 mg/dL at baseline (see text for details). (B) Serum creatinine levels for hyperuricemic patients who received allopurinol (n = 9). All serum creatinine levels for patients were plotted in reference to initiation of study drug. One patient who began therapy with a normal creatinine for age developed renal failure requiring hemodialysis and hemofiltration for several days (see text for details). (C) Adjusted creatinine levels (percent ± SE of age/sex defined mean) for patients who were hyperuricemic at presentation. Squares denote patients who received rasburicase and stars represent allopurinol. Each creatinine level was adjusted by dividing the serum creatinine value by the published mean creatinine for patient's age and gender × 100%. Normal mean creatinine levels were the following: less than 1 year = 0.3 mg/dL; 1 to 12 years = 0.5 mg/dL; 12 to 16 years = 0.75 mg/dL; males 16 years or older = 0.95 mg/dL, females 16 years or older = 0.85 mg/dL.20 The adjusted creatinine declined steadily during initiation of chemotherapy only for the rasburicase group.
Adverse events and mortality
Frequent adverse events were common among the study patients including fever, pain, and mucositis secondary to their disease and chemotherapeutic regimens. Two patients assigned allopurinol died during the study period. A 6-year-old girl with T-cell leukemia died of pseudomonal sepsis on day 11 of study, and a 2-year-old girl with acute myelogenous leukemia died of an intracerebral hemorrhage on day 2 of study. Therapy was discontinued for one patient receiving rasburicase because of hemolysis. This 11-year-old boy with pre-B precursor ALL had evidence of disseminated intravascular coagulopathy, which resolved concomitantly with discontinuation of the rasburicase; the relationship to rasburicase is unknown. Testing of the patient's blood and the 6 red blood cell donors from whom he received transfusions did not show any evidence of underlying G-6-PD deficiency. No patients experienced anaphylactic events due to rasburicase. Predose and end-of-study samples for the qualitative determination of antibodies to urate oxidase protein were received from 23 of 27 patients assigned rasburicase. None of the samples contained detectable amounts of antibodies to rasburicase.
Discussion
The use of urate oxidase is not a new therapeutic modality for the treatment of hyperuricemia associated with hematologic malignancies.21,22 The aspergillus-derived protein (nonrecombinant urate oxidase, Uricozyme) has been used in France and Italy for almost 3 decades. A report from France using the nonrecombinant urate oxidase protein in children with advanced Burkitt lymphoma/mature B-cell ALL demonstrated only a 1.7% incidence of dialysis during induction chemotherapy.23 This compares favorably to a recent report in the United States in which 21% of children with advanced Burkitt lymphoma treated with allopurinol, hydration, and alkalinization required hemodialysis during induction chemotherapy and 6 patients died during induction therapy secondary to renal/metabolic complications.10 Pui and colleagues treated 134 children with acute leukemia and Burkitt lymphoma with the nonrecombinant urate oxidase.24 They found a more rapid and significantly greater decrease in blood uric acid levels than historical controls treated with similar chemotherapy and allopurinol. However, 4.5% of the children given aspergillus-purified urate oxidase developed allergic reactions, manifested primarily by urticaria, bronchospasm, and hypoxia.24 The present study is the first randomized comparison of recombinant urate oxidase (rasburicase) versus allopurinol in pediatric patients with hematologic malignancies at high risk for tumor lysis.
In the present study, mean uric acid AUC0-96 was statistically significantly lower in the rasburicase group (128.1 mg/dL.hr) than in the allopurinol group (328.5 mg/dL.hr, P < .0001). Mean percent change from baseline uric acid concentration was −86.0% for the rasburicase group and −12.1% in the allopurinol group at 4 hours following the first dose of the study drug (P < .0001). In patients with hyperuricemia at baseline, median time to first demonstration of control of uric acid (plasma uric acid concentration < 8.0 mg/dL) was 4.0 hours following the first dose for the rasburicase group (n = 10) and 23.9 hours after the first dose for the allopurinol group (n = 5). These results demonstrate that rasburicase is a more potent and faster-acting hypouricemic agent than oral allopurinol. Unfortunately, intravenous allopurinol was investigational at the time of initiation of study and was unavailable for comparison to rasburicase.25 Future studies may want to compare the intravenous formulation of allopurinol to rasburicase.
Moreover, the incidence of renal failure requiring assisted renal support (dialysis or hemofiltration) during this study was null in the rasburicase group compared to one patient in the allopurinol group. This patient had a plasma creatinine level within normal range at baseline and developed renal failure secondary to tumor lysis syndrome during allopurinol dosing. In contrast, one patient in the rasburicase group, with a serum creatinine level of 2.2 mg/dL, improved without assisted renal support.
Hyperuricemic patients who received rasburicase improved their adjusted creatinine levels from 144% to 102% of mean for age and gender, from baseline through day 4 of study, respectively. In contrast, hyperuricemic patients who received allopurinol had worsening adjusted creatinine levels from 132% to 147% of mean, from baseline through day 4 of study (P = .147 comparing change from baseline through day 4 by treatment group). This corresponds to a period where cytoreductive chemotherapy had begun and suggests that the more rapid control of plasma uric acid may have translated into superior renal function during chemotherapy for patients treated with rasburicase. The study was not designed to compare renal function outcome, the incidence of renal failure, or the need for assisted renal support between the 2 treatment arms. Therefore, the number of hyperuricemic patients may have been too small to demonstrate any statistically significant differences.
In summary, this study supports the conclusion that rasburicase is a safe and effective alternative to allopurinol to rapidly control plasma uric acid concentrations in patients with hematologic malignancies at high risk for tumor lysis during induction/reduction chemotherapy. A larger number of patients would be required to determine if the more rapid control and reduction of plasma uric acid with rasburicase will also result in a significant decrease in metabolic complications and morbidity due to tumor lysis syndrome, or the need for additional renal support (dialysis or hemofiltration).
We are indebted to the support of Vicki Toan and Linda Rahl for the editorial assistance in the preparation of this manuscript. We would also like to thank Francine High and James Grabicki from Sanofi-Synthelabo for their assistance in the study and helpful comments regarding the manuscript.
Department of Pediatric Hematology/Oncology at North Texas Hospital for Children at Medical City, Dallas, TX; Department of Pediatric Hematology/Oncology at Children's Hospital and Medical Center, Seattle, WA; Jonathan Jaques Children's Cancer Center at Miller Children's Hospital, Long Beach, CA; Department of Pediatrics at Mott Children's Hospital, Ann Arbor, MI; Department of Pediatric Hematology/Oncology at Riley Hospital for Children, Indianapolis, IN; Department of Pediatric Hematology/Oncology at Doernbecher Children's Hospital, Portland, OR; Sanof-Synthelabo, Inc, Malvern, PA; and the Department of Pediatrics at the Babies and Children's Hospital, Columbia University, New York, NY.
Submitted September 25, 2000; accepted January 23, 2001.
Supported by a grant from Sanofi-Synthelabo, Malvern, PA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Mitchell S. Cairo, Blood and Marrow Transplantation, Pediatric Cancer Research, Babies and Children's Hospital, Columbia University, 161 Fort Washington Ave, Irving 7, New York, NY 10032; e-mail: mc1310@columbia.edu.