Abstract
The activation of kinases of the mitogen-activated protein kinase superfamily initiated by lipopolysaccharide (LPS) plays an important role in transducing inflammatory signals. The pathway leading to the induction of stress-activated protein kinases in macrophages stimulated with LPS was investigated. The activation of Jun N-terminal kinases (JNK) by LPS is herbimycin sensitive. Using specific inhibitors, it was shown that the pathway involves the activation of phosphoinositide 3-kinase (PI 3-K). However, in contrast to previous reports, the small GTPases Cdc42 and Rac are not required downstream of PI 3-K for JNK activation. Instead, the phosphoinositides produced by PI 3-K stimulate protein kinase C (PKC) ζ activation through PDK1. In turn, activation of this atypical PKC leads to the stimulation of phosphatidylcholine phospholipase C (PC-PLC) and acidic sphingomyelinase (ASMase). It is therefore proposed that PKCζ regulates the PC-PLC/ASMase pathway, and it is hypothesized that the resultant ceramide accumulation mediates the activation of the SEK/JNK module by LPS.
Introduction
Lipopolysaccharide (LPS), a conserved component of the gram-negative bacteria cell wall, serves as a potent modulator of macrophage activity. It triggers the activation of multiple intracellular signaling cascades, resulting in the release of immunoregulatory molecules such as TNFα, IL-1, IL-6, and arachidonic acid metabolites. These mediators are essential for the recruitment and activation of immunocompetent cells that concur in fighting bacterial infection.
The precise molecular mechanism by which LPS induces these events is not entirely understood. It is known that LPS complexed with a serum protein (LPS-binding protein) binds to the surface molecule CD14.1 This GPI-anchored protein lacks a cytosolic domain and was therefore postulated to interact with a co-receptor that transduces the signal across the plasma membrane. The molecule performing this function is Tlr-4 (toll-like receptor 4), recently identified as the product of the Lpsdgene.2 Among the first steps in LPS signal transduction is the rapid phosphorylation of various proteins on tyrosyl residues. Signal transduction cascades initiated in this way result in the activation of mitogen-activated protein kinases (MAPK).3-6
The MAPK subgroup JNK is activated preferentially by cellular stress signals such as irradiation, heat shock, osmotic stress, and protein synthesis inhibitors,7 but stimulation by growth factors has also been reported.8,9 Relevant to our study, this pathway is also activated by inflammatory stimuli (LPS3-6; IL-1, TNF-α7) and on infection of cultured cells by various pathogens, including gram-negative bacteria.10,11Targeted disruption of the JNK-kinase, stress and extracellular signal-activated kinase 1 (SEK1), causes defects in the transcriptional activity of AP-1,12 a transcription factor implicated in the regulation of cytokine genes.13 Recent data also implicate JNK in the stabilization14,15 and translation16 of cytokine mRNA. Consistently, T-cell differentiation is defective in Jnk-1-17 andJnk-2-deficient mice.18
The process of JNK activation by LPS in macrophages depends on tyrosine kinase activity.3,6 Among the best-characterized JNK activators that act downstream of tyrosine kinases are members of the PI 3-K family. Thus, it has been reported that PI 3-K participates in endothelial growth factor-mediated activation of the JNK pathway in epithelial cells8 and that it plays a crucial role in JNK activation mediated by c-Kit in bone marrow-derived mast cells.9 In the latter instance, the pathway requires Rac1 as an intermediate. Small GTPases of the Rho-family modulate JNK activity,19,20 and their function has been reported to be necessary for JNK activation in several systems.21-23 The connection between PI 3-K and members of Cdc42/Rac is supported by the fact that the PI 3-K inhibitor, wortmannin, and dominant-negative forms of PI 3-K block cytoskeletal reorganization mediated by Rac.24-27 PI 3-K can interact physically with small GTPases,28 and the phospholipid products of PI 3-K stimulate GDP/GTP exchange on the members of Rho family GTPases directly29 or indirectly through the guanine nucleotide exchange factor Vav.30
Another prominent downstream target of PI 3-K is phosphoinositide-dependent kinase 1 (PDK1), which in turn regulates PKB,31,32 p70S6K,33,34 and protein kinase C (PKC) with its isoenzymes α, β, δ, ε, and ζ.35,36 Both PKCα and ζ are activated in monocytes and macrophages stimulated with LPS.37,38 In particular, PKCζ activation occurs in a PI 3-K–dependent manner.37However, the downstream targets of this signaling pathway remained unidentified.
The lipid second messenger ceramide has also been shown to participate in JNK activation.39-41 Notably, low but consistent amounts of ceramide are produced in the course of macrophage stimulation by LPS.42
Here we show that LPS stimulates the JNK pathway by the successive activation of tyrosine kinases, PI 3-K, PKCζ, and PC-PLC/ASMase. We propose that this cascade generates ceramide that can stimulate the SEK1/JNK module. Unexpectedly, the involvement of Rac, Rho, or Cdc42, which are common mediators in JNK activation in many cell types, is not required in macrophages.
Materials and methods
Cell culture, stimulation, and pretreatment
BAC-1.2F5 cells43 were cultured in Dulbecco modified Eagle's medium supplemented with 10% fetal calf serum and 20% L-cell–conditioned medium as a source of CSF-1. Confluent cells (approximately 5 × 106 cells per 100-mm diameter tissue culture dish) were cultured for 16 hours in medium without CSF-1 and then stimulated with 1.5 μg/mL bacterial LPS (fromSalmonella typhimurium; Sigma, Vienna, Austria) for the indicated times. In selected experiments, macrophages were treated with PC-PLC (from Bacillus cereus; 50 U/mL, 60 minutes; Boehringer Mannheim), C2 ceramide, or the inactive analog dihydro-C2 ceramide (250 μmol/L for 30 minutes, unless otherwise indicated; both from Calbiochem, Darmstadt, Germany) to monitor the effect of these signal transducers on JNK activation. Tyrosine kinases were inhibited by pretreatment with herbimycin A (4 μg/mL, 4 hours; Sigma). Activation of PI 3-K was blocked by pretreatment with wortmannin (100 nmol/L, 20 minutes; Sigma). Inhibition of Rho-family small GTPases (RhoA, Rac1, and Cdc42) was performed by a 60-minute preincubation with toxin B (fromClostridium difficile)44 at a final concentration of 100 ng/mL. Inhibition of PC-PLC activity was performed by preincubating the cells for 60 minutes with 10 μmol/L xanthogenate tricyclodecan-9-yl (D609; Alexis Biochemicals, Laufelfingen, Switzerland). PKC was inhibited by treating the cells with 10 μmol/L bisindoleylmaleimide I (BIM; Calbiochem) for 60 minutes before stimulation.45 Down-regulation of diacylglycerol (DAG)-dependent PKC isoforms was performed by 24-hour treatment with 5 μmol/L tetradecanoyl-phorbol 13-acetate (TPA; Sigma) in dimethyl sulfoxide (DMSO; 14 mmol/L final concentration).
Cell lysis, immunoprecipitation, and Western blotting
Cells were lysed in solubilization buffer (10 mmol/L Tris-base, 50 mmol/L sodium chloride, 30 mmol/L sodium pyrophosphate, 50 mmol/L sodium fluoride, 1% Triton X-100, pH 7.0) supplemented with 1 mmol/L phenylmethylsulfonyl fluoride (PMSF), 100 μmol/L sodium vanadate, 1 mmol/L dithiothreitol, and protease inhibitors (aprotinin, 3 μg/mL; pepstatin and leupeptin, 0.5 μg/mL each). For immunoblotting, 30-40 μg whole cell extracts were separated by 10% SDS-PAGE and transferred onto nitrocellulose membranes. For immunoprecipitation, 500-600 μg whole cell extracts were incubated in the presence of protein A beads (Amersham, Pharmacia, Freiburg, Germany) with anti-PKCζ (Santa Cruz Biotechnology, Santa Cruz, CA) for 16 to 18 hours at 4°C. After incubation, beads were collected and washed 3 times with lysis buffer. The immunocomplexes were eluted by boiling in SDS sample buffer and subjected to Western blot analysis. Membranes were blocked for 8 to 16 hours at 4°C in TTBS (10 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 0.1% Tween-20) supplemented with 4% bovine serum albumin (BSA, fraction V; Sigma) and probed with the appropriate primary antibodies diluted in 1% BSA in TTBS before incubation with peroxidase-conjugated secondary antibodies and detection by the enhanced chemiluminescence system (Pierce). The primary antibodies used in this study recognize selectively the phosphorylated forms of JNK1/2 (anti-phJNK, T183/Tyr185), SEK1 (anti-phSEK, T223; all from New England BioLabs, Schwalbach, Germany), and PKCζ (recognizing the PDK1 phosphorylation site T410 within the C-loop)36 or their unmodified forms (Santa Cruz Biotechnology).
In vitro glucosylation of Rho-family GTPases
Cells were lysed by sonication in hypotonic buffer (25 mmol/L HEPES, pH 7.5, 2 mmol/L MgCl2, 100 μmol/L PMSF, 40 μg/mL aprotinin, 25 μg/mL leupeptin, 80 μg/mL benzamidin). After the removal of nuclei and debris by centrifugation, lysates of untreated or toxin B-stimulated BAC-1.2F5 cells (100 μg) were incubated with Clostridium difficile toxin B (fragment CDB546; 100 nmol/L) in 20 μL glucosylation buffer (50 mmol/L HEPES, pH 7.5, 100 mmol/L KCl, 2 mmol/L MgCl2, 1 mmol/L MnCl2, 100 μg/mL BSA) supplemented with 20 μmol/L [14C]-UDP-glucose for 60 minutes at 37°C. Recombinant GST-Rac (1μg) was used as a positive control. Labeled proteins were analyzed by SDS-PAGE and subsequently by phosphorimaging (Molecular Dynamics, Freiburg, Germany).
Measurement of PC-PLC and ASMase activity
PC-PLC and ASMase activity of whole cell extracts was determined as previously described.46 Briefly, cells (2.5 × 106) were scraped in 2-mL ice-cold phosphate-buffered saline and centrifuged for 10 minutes at 400 rpm, 4°C. Three hundred microliters of Triton X-100 (0.01% for PC-PLC, 0.2% for ASMase activity measurements) was added to the pellet, and the samples were incubated on ice for 10 minutes before sonication. Fifteen micrograms of lysate was incubated for 2 hours at 37°C either in PC-PLC buffer (50 mmol/L Tris-Cl, pH 7.3, 6.3 mmol/L CaCl2, 150 mmol/L ammonium sulfate, plus 50 nCi L-3-phosphatidyl[N-methyl-14C]choline, [14C]PC; 80 μL total volume) or in ASMase buffer (250 mmol/L sodium acetate, pH 5.0, 0.2% Triton X-100, plus 50 nCi methyl-[14C]sphingomyelin; 50 μL total volume). Labeled lipids were from Amersham. The PC-PLC assay was terminated by extracting the lipids with CHCl3:CH3OH (1:2 vol/vol, 180 μL), 0.9% NaCl (60 μL), and CHCl3 (60 μL). The aqueous and organic phases containing [14C]phosphocholine ([14C]PCho) and [14C]PC, respectively, were separated and quantitated by liquid scintillation. The ASMase assay was terminated by extracting the lipids with CHCl3:CH3OH (1:1 vol/vol, 400 μL) and water (180 μL). The amount of [14C]PCho produced was quantitated by liquid scintillation counting. PC-PLC and ASMase activity were expressed as percentages of substrate hydrolyzed.
Results
Herbimycin and wortmannin block LPS-induced activation of SEK and JNK
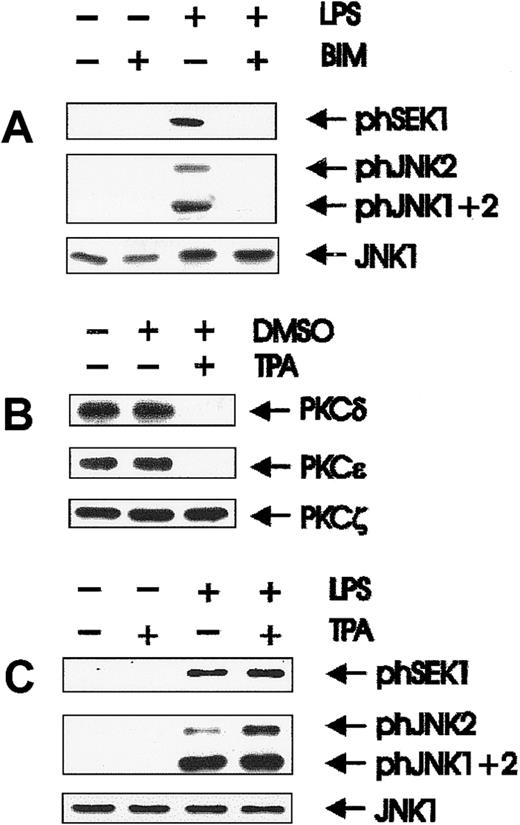
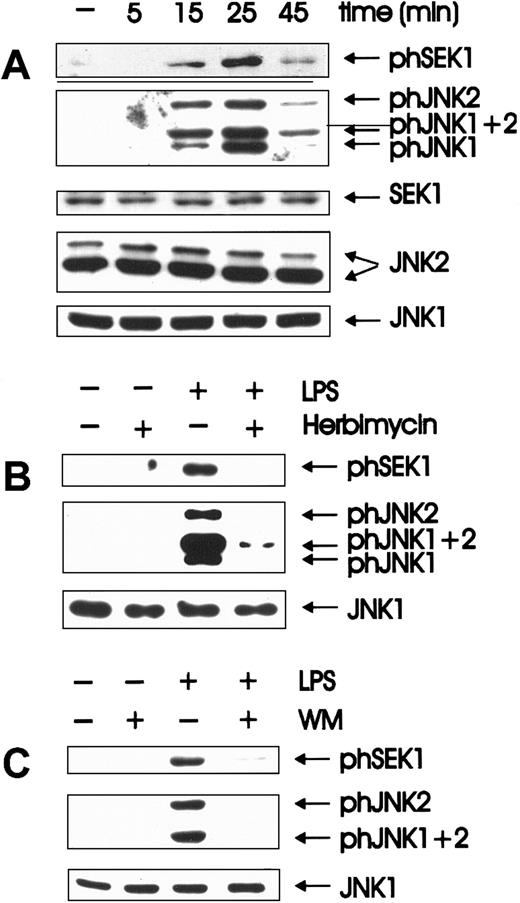
Quiescent BAC-1.2F5 cells were stimulated with LPS for different time periods. Activation states of the relevant kinases were assessed in whole cell extracts by immunoblotting with antibodies that specifically recognize the phosphorylated, activated form of each enzyme (Figure 1A). The identity of the isoforms detected by the phosphospecific antibodies was determined by immunoblotting lysates of LPS-treated, bone marrow-derived macrophages from JNK1−/− or JNK2−/− mice (gift of Dr Erwin Wagner, I.M.P., Vienna, Austria; data not shown). As a control for equal loading, the membranes were stripped and reprobed with antibodies against unmodified SEK1/JNK. SEK/JNK were phosphorylated with activation/inactivation kinetics comparable to those published for JNK.3 Peak activation occurred after 25 minutes and then decayed. Inactivation was complete by 1 hour, and no further changes were observed over a period of 4 hours (data not shown). These kinetics of activation resembled those of the other MAPK subfamily, ERK (extracellularly regulated kinases).4
Herbimycin- and wortmannin-sensitive activation of SEK and JNK is an early event in LPS-induced signal transduction.
(A) Quiescent BAC-1.2F5 cells were stimulated with 1.5 μg/mL LPS at 37°C for different times before solubilization. The presence of the phosphorylated, active forms of SEK (phSEK) and JNK (phJNK) and of their unmodified forms was detected by immunoblotting with the corresponding antibodies. The band labeled phJNK1 + 2 consists of co-migrating, phosphorylated forms of both kinases. (B) Inhibition of tyrosine kinases was performed by treating the cells with herbimycin A (4 μg/mL for 4 hours) before stimulation with LPS (15 minutes). (C) Cells were incubated with wortmannin (WM, 100 nmol/L, 20 minutes) before a 15-minute stimulation with 1.5 μg/mL LPS.
Herbimycin- and wortmannin-sensitive activation of SEK and JNK is an early event in LPS-induced signal transduction.
(A) Quiescent BAC-1.2F5 cells were stimulated with 1.5 μg/mL LPS at 37°C for different times before solubilization. The presence of the phosphorylated, active forms of SEK (phSEK) and JNK (phJNK) and of their unmodified forms was detected by immunoblotting with the corresponding antibodies. The band labeled phJNK1 + 2 consists of co-migrating, phosphorylated forms of both kinases. (B) Inhibition of tyrosine kinases was performed by treating the cells with herbimycin A (4 μg/mL for 4 hours) before stimulation with LPS (15 minutes). (C) Cells were incubated with wortmannin (WM, 100 nmol/L, 20 minutes) before a 15-minute stimulation with 1.5 μg/mL LPS.
Herbimycin-dependent kinases have previously been implicated in the activation of JNK3 6 by LPS. We could confirm the inhibition of LPS-mediated JNK activation by herbimycin A; in addition, SEK activation was also herbimycin sensitive (Figure 1B).
PI 3-K–dependent JNK activation has been reported after the stimulation of tyrosine kinase8,9,47 and of G-protein–coupled receptors.22 To test whether the activation of JNK by LPS was similarly regulated in BAC-1.2F5 macrophages, the cells were pretreated with wortmannin (100 nmol/L) before LPS stimulation. Wortmannin severely inhibited LPS-mediated activation of SEK and JNK (Figure 1C). Identical results were obtained using a second PI 3-K inhibitor, LY294002 (data not shown). We therefore postulate that PI 3-K acts as a downstream effector of LPS-stimulated tyrosine kinase(s) in the pathway to JNK activation.
JNK and SEK activation is a Cdc42/Rac-independent process
PI 3-K–mediated JNK activation has been shown to be dependent on Rac, a member of Rho-family small GTPases.9,22 To test whether Cdc42, Rac, or Rho participated in LPS-mediated JNK activation, we pretreated BAC-1.2F5 cells with toxin B, which glucosylates and inactivates these GTPases.44 In BAC-1.2F5 cells, toxin B inhibits phagocytosis completely4 and, on longer incubation, reduces the adherence of these macrophages to the substrate. Therefore, toxin B perturbs Rho-family–dependent cytoskeletal rearrangements in these cells. However, toxin B pretreatment did not prevent SEK or JNK activation by LPS (Figure2A). We could not corroborate the toxin B result by overexpressing dominant-negative alleles of CDC42 and Rac in BAC-1.2F5 cells because of the extremely low transfection efficiency that can be achieved in this cell line. We therefore tested directly whether toxin B efficiently glucosylated Cdc42 and Rac in BAC-1.2F5 macrophages in vivo. To this end, we performed in vitro glucosylation assays using lysates from untreated or toxin B–treated cells as substrates. In this assay, [14C]-UDP-glucose is transferred to the Rho-type GTPases present in cell lysates by toxin B in vitro. Rho-family GTPases in lysates of untreated cells were efficiently glucosylated in vitro; in contrast, toxin B failed to glucosylate in vitro Cdc42, Rac, or Rho in the lysates of toxin B–pretreated cells (Figure 2B). These data indicate that Rho-type GTPases had been successfully glucosylated (and therefore inactivated) by the in vivo treatment of macrophages with toxin B. Hence, unexpectedly, a novel pathway independent of functional Cdc42/Rac operates in macrophages stimulated with LPS.
Toxin B efficiently glucosylates Cdc42 and Rac without affecting LPS-mediated SEK and JNK activation.
(A) The Rho-family GTPase inhibitor toxin B does not affect JNK activation by LPS; quiescent BAC-1.2F5 cells were incubated with toxin B (Tox B, 100 ng/mL) for 60 minutes before stimulation with 1.5 μg/mL LPS for 15 minutes. The presence of phosphorylated forms of SEK and JNK, and of JNK1 as a loading control, was detected by immunoblotting with the corresponding antibodies. (B) In vivo treatment with toxin B efficiently glucosylates Rho subtype proteins in BAC-1.2F5 cells. Cells were either left untreated or pretreated with toxin B (100 ng/mL for 60 minutes). Lysates (100 μg) or recombinant GST-Rac (1 μg) were incubated in vitro with recombinant toxin B (fragment CDB546) and UDP-[14C]glucose (20 μmol/L) for 60 minutes at 37°C. Glucosylated proteins were analyzed by SDS-PAGE and phosphorimaging. The identity of the GTPases was confirmed by immunoblotting.
Toxin B efficiently glucosylates Cdc42 and Rac without affecting LPS-mediated SEK and JNK activation.
(A) The Rho-family GTPase inhibitor toxin B does not affect JNK activation by LPS; quiescent BAC-1.2F5 cells were incubated with toxin B (Tox B, 100 ng/mL) for 60 minutes before stimulation with 1.5 μg/mL LPS for 15 minutes. The presence of phosphorylated forms of SEK and JNK, and of JNK1 as a loading control, was detected by immunoblotting with the corresponding antibodies. (B) In vivo treatment with toxin B efficiently glucosylates Rho subtype proteins in BAC-1.2F5 cells. Cells were either left untreated or pretreated with toxin B (100 ng/mL for 60 minutes). Lysates (100 μg) or recombinant GST-Rac (1 μg) were incubated in vitro with recombinant toxin B (fragment CDB546) and UDP-[14C]glucose (20 μmol/L) for 60 minutes at 37°C. Glucosylated proteins were analyzed by SDS-PAGE and phosphorimaging. The identity of the GTPases was confirmed by immunoblotting.
Atypical PKCζ is involved in LPS-mediated activation of the SEK/JNK pathway
Results obtained with toxin B excluded Cdc42/Rac as potential downstream effectors of PI 3-K. Because various PKC isoforms have been shown to be regulated by PI 3-K in vitro and in vivo,35,36we examined whether PKCs were involved in LPS-mediated SEK/JNK activation. BAC-1.2F5 cells express the novel DAG-dependent PKC isoforms δ and ε and the atypical PKCζ,45 all of which can be inhibited by BIM.48 Activation of SEK and JNK was completely suppressed after pretreatment with BIM (Figure3A). Sustained treatment (up to 24 hours) with 5 μmol/L TPA, which causes the effective degradation of the DAG-dependent PKC isoforms δ and ε (Figure 3B), did not affect SEK and JNK activation (Figure 3C). Therefore, the effect of BIM on JNK activation must result from the inhibition of an atypical PKC.
Involvement of PKC in LPS-initiated SEK and JNK activation.
(A) The PKC inhibitor BIM inhibits SEK/JNK activation by LPS; quiescent BAC-1.2F5 cells were treated with the PKC inhibitor BIM (10 μmol/L, 60 minutes) before stimulation with 1.5 μg/mL LPS for 15 minutes. The presence of phosphorylated SEK/JNK and of unmodified JNK1 was detected with the corresponding antibodies. (B) Prolonged TPA treatment of BAC-1.2F5 cells down-regulates DAG-dependent PKCs; cells were either left untreated or incubated with TPA (5 μmol/L in DMSO) or with DMSO alone for 24 hours. PKC δ, ε, and ζ were detected by immunoblotting. (C) Down-regulation of DAG-dependent PKC does not affect SEK/JNK activation by LPS; down-regulation of DAG-dependent PKCs was performed as described in B before stimulation with 1.5 μg/mL LPS for 15 minutes, cell lysis, and immunodetection of phosphorylated SEK/JNK and of unmodified JNK1.
Involvement of PKC in LPS-initiated SEK and JNK activation.
(A) The PKC inhibitor BIM inhibits SEK/JNK activation by LPS; quiescent BAC-1.2F5 cells were treated with the PKC inhibitor BIM (10 μmol/L, 60 minutes) before stimulation with 1.5 μg/mL LPS for 15 minutes. The presence of phosphorylated SEK/JNK and of unmodified JNK1 was detected with the corresponding antibodies. (B) Prolonged TPA treatment of BAC-1.2F5 cells down-regulates DAG-dependent PKCs; cells were either left untreated or incubated with TPA (5 μmol/L in DMSO) or with DMSO alone for 24 hours. PKC δ, ε, and ζ were detected by immunoblotting. (C) Down-regulation of DAG-dependent PKC does not affect SEK/JNK activation by LPS; down-regulation of DAG-dependent PKCs was performed as described in B before stimulation with 1.5 μg/mL LPS for 15 minutes, cell lysis, and immunodetection of phosphorylated SEK/JNK and of unmodified JNK1.
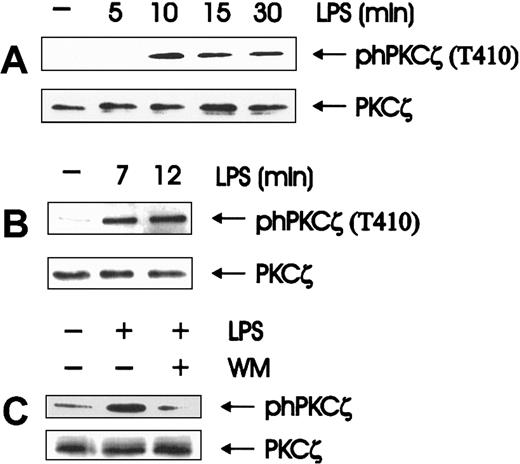
In fact, atypical PKCs would be a natural target of the phosphoinositides generated by PI 3-K. PKCζ, the only atypical PKC expressed in BAC-1.2F5 macrophages (data not shown), can be activated by PI(3,4,5)P3, PI(1,4,5)P3, and PI(3,4)P2 directly49 and indirectly through PDK1.35,36 PKCζ activation was monitored by immunoblotting with antibodies that specifically recognize phosphorylation of the PDK1 site T410 in the C-loop of the PKCζ isoform.36 Western blot analysis of whole cell extracts showed that the T410 phosphorylation was stimulated by LPS, reached a maximum after 10 minutes of stimulation, and slowly decayed (Figure4A). We confirmed the identity of the PKC isoform activated by immunoprecipitating PKCζ and analyzing its phosphorylation status (Figure 4B). T410 is the PKCζ residue phosphorylated by the PI-3K target PDK1.35,36 Its phosphorylation on LPS treatment of macrophages confirms the previously reported involvement of PI-3K in LPS signaling.50Consistently, LPS-mediated PKCζ phosphorylation/activation, like JNK activation, was abolished by wortmannin pretreatment (Figure 4C). Taken together, the data implicate PKCζ as the enzyme involved downstream of PI 3-K in relaying the LPS signal to the JNK module and indicate that its activation is mediated by PDK1.
Wortmannin-sensitive activation of PKCζ is an early event in LPS-induced signal transduction.
(A, B) LPS stimulates the phosphorylation of PKCζ on the PDK1 target residue T410; quiescent BAC-1.2F5 cells were stimulated with 1.5 μg/mL LPS for the indicated time points. The presence of phosphorylated PKCζ was assessed by immunoblot analysis of either whole cell extracts (A) or anti-PKCζ immunoprecipitates (B). (C) Wortmannin affects LPS-stimulated PKCζ phosphorylation. BAC-1.2F5 cells were incubated with wortmannin (WM, 100 nmol/L, 20 minutes) before a 15-minute stimulation with 1.5 μg/mL LPS. Anti-PKCζ blots of whole cell extracts (A, C) or immunoprecipitates (B) are shown as a loading control.
Wortmannin-sensitive activation of PKCζ is an early event in LPS-induced signal transduction.
(A, B) LPS stimulates the phosphorylation of PKCζ on the PDK1 target residue T410; quiescent BAC-1.2F5 cells were stimulated with 1.5 μg/mL LPS for the indicated time points. The presence of phosphorylated PKCζ was assessed by immunoblot analysis of either whole cell extracts (A) or anti-PKCζ immunoprecipitates (B). (C) Wortmannin affects LPS-stimulated PKCζ phosphorylation. BAC-1.2F5 cells were incubated with wortmannin (WM, 100 nmol/L, 20 minutes) before a 15-minute stimulation with 1.5 μg/mL LPS. Anti-PKCζ blots of whole cell extracts (A, C) or immunoprecipitates (B) are shown as a loading control.
Involvement of PC-PLC and ASMase in signaling to JNK
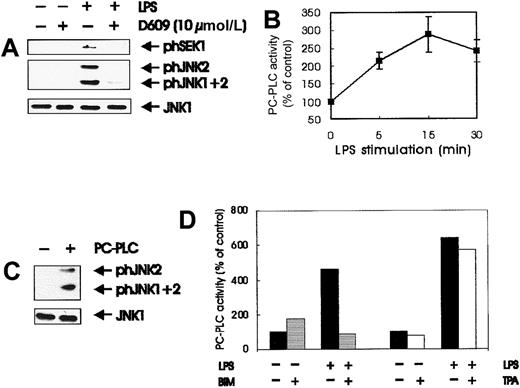
High concentrations of the PC-PLC inhibitor D609 decrease LPS-mediated stimulation of Raf, MEK, and ERK.45 To investigate whether phospholipase activation was important for the stimulation of SEK and JNK by LPS, we treated quiescent BAC-1.2F5 cells with D609 (10 μmol/L) before LPS stimulation. The inhibitor severely blunted kinase activation (Figure 5A). PC-PLC was activated by LPS with kinetics consistent with those observed for SEK/JNK activation (Figure 5B), and the addition of bacterial PC-PLC to macrophages caused the activation of JNK, confirming that this signal transducer is in principle able to stimulate the JNK pathway (Figure 5C). Like SEK/JNK activation, stimulation of PC-PLC by LPS could be blocked completely by pretreating the cells with the PKC inhibitor BIM but was completely resistant to down-regulation of DAG-dependent PKCs by sustained TPA treatment (Figure 5D). These data indicate that PC-PLC is involved in JNK activation by LPS. As previously reported in other systems (reviewed in Exton51), in LPS-stimulated macrophages PC-PLC is a downstream target of PKC.
Involvement of PC-PLC in LPS-induced JNK activation.
(A) PC-PLC inhibitor affects SEK/JNK activation; quiescent BAC-1.2F5 cells were pretreated with the PC-PLC inhibitor D609 (10 μmol/L, 60 minutes) before stimulation with 1.5 μg/mL LPS for 15 minutes. The presence of phosphorylated SEK and JNK was detected by immunoblotting with the corresponding antibodies. The inhibitor did not have any effect on the basal level of kinase phosphorylation. An anti-JNK1 blot is shown as loading control. (B) LPS stimulates PC-PLC; quiescent BAC-1.2F5 cells were stimulated with 1.5 μg/mL LPS for different times before solubilization. PC-PLC activity was determined in whole cell extracts as described in “Materials and methods.” Results are expressed as the percentage increase with respect to the control values. The plot in B represents the mean of 3 independent experiments, and vertical bars represent the standard errors of the mean. (C) Bacterial PC-PLC activates JNK in macrophages; quiescent BAC-1.2F5 cells were stimulated with 50 U/mL bacterial PC-PLC for 60 minutes before solubilization. The presence of phosphorylated JNK was detected by immunoblotting with the corresponding antibodies. An anti-JNK1 blot is shown as a loading control. (D) BIM pretreatment, but not down-regulation of DAG-dependent PKC, affects the activation of PC-PLC by LPS. Quiescent BAC-1.2F5 cells were left untreated (black bars) or were treated with BIM (10 μmol/L, 60 minutes; gray bars); alternatively, down-regulation of DAG-dependent PKC was performed as described in Figure 3B (white bars). Cells were stimulated with LPS for 15 minutes before lysis and determination of PC-PLC activity in whole cell extracts. Results are expressed as the percentage increase with respect to the control values.
Involvement of PC-PLC in LPS-induced JNK activation.
(A) PC-PLC inhibitor affects SEK/JNK activation; quiescent BAC-1.2F5 cells were pretreated with the PC-PLC inhibitor D609 (10 μmol/L, 60 minutes) before stimulation with 1.5 μg/mL LPS for 15 minutes. The presence of phosphorylated SEK and JNK was detected by immunoblotting with the corresponding antibodies. The inhibitor did not have any effect on the basal level of kinase phosphorylation. An anti-JNK1 blot is shown as loading control. (B) LPS stimulates PC-PLC; quiescent BAC-1.2F5 cells were stimulated with 1.5 μg/mL LPS for different times before solubilization. PC-PLC activity was determined in whole cell extracts as described in “Materials and methods.” Results are expressed as the percentage increase with respect to the control values. The plot in B represents the mean of 3 independent experiments, and vertical bars represent the standard errors of the mean. (C) Bacterial PC-PLC activates JNK in macrophages; quiescent BAC-1.2F5 cells were stimulated with 50 U/mL bacterial PC-PLC for 60 minutes before solubilization. The presence of phosphorylated JNK was detected by immunoblotting with the corresponding antibodies. An anti-JNK1 blot is shown as a loading control. (D) BIM pretreatment, but not down-regulation of DAG-dependent PKC, affects the activation of PC-PLC by LPS. Quiescent BAC-1.2F5 cells were left untreated (black bars) or were treated with BIM (10 μmol/L, 60 minutes; gray bars); alternatively, down-regulation of DAG-dependent PKC was performed as described in Figure 3B (white bars). Cells were stimulated with LPS for 15 minutes before lysis and determination of PC-PLC activity in whole cell extracts. Results are expressed as the percentage increase with respect to the control values.
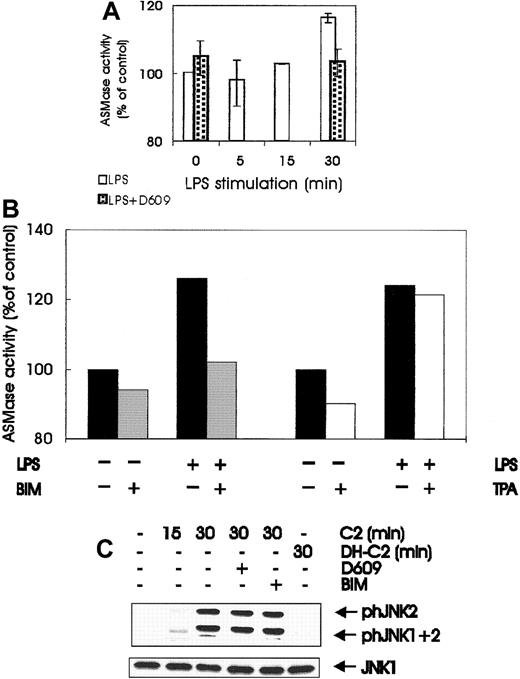
LPS treatment of BAC-1.2F5 cells also increased the activity of ASMase, the PC-PLC downstream target responsible for ceramide generation.52 ASMase activity increased steadily during the first 30 minutes of LPS stimulation, and the extent of activation that was achieved correlated well with the small but significant increase in ceramide observed by others in LPS-treated macrophages.42 Like SEK/JNK stimulation, ASMase activation was abrogated by pretreatment with the PC-PLC inhibitor D609 (Figure6A) and by treatment with the PKC inhibitor BIM (Figure 6B). As in SEK/JNK and PC-PLC activation, down-regulation of DAG-dependent PKCs by prolonged TPA treatment did not affect LPS-mediated ASMase activation (Figure 6B). Taken together, the data in Figures 5 and 6 imply that LPS stimulation of macrophages generates ceramide42 by a PC-PLC/ASMase pathway operating downstream of PKC, and they strongly suggest that this lipid second messenger is responsible for SEK/JNK activation. Exogenous C2 ceramide, but not dihydro-C2 ceramide, was capable of stimulating JNK phosphorylation when added to BAC-1.2F5 cells, though to a lesser extent than LPS (Figure 6C). Ceramide-mediated JNK stimulation was insensitive to pretreatment with D609 or BIM, demonstrating that these inhibitors do not generally prevent JNK stimulation but act specifically on the LPS-induced JNK activation.
Involvement of ASMase and ceramide in JNK activation by LPS.
(A) LPS stimulates ASMase; quiescent BAC-1.2F5 cells were either left untreated or pretreated with D609 (10 μmol/L, 60 minutes) to block PC-PLC before stimulation with 1.5 μg/mL LPS for different times before solubilization. ASMase activity was determined in whole cell extracts as described in “Materials and methods.” Results are expressed as the percentage increase with respect to the control values. The plot represents the mean of 2 independent experiments, and vertical bars represent the range of the samples. (B) BIM pretreatment, but not down-regulation of DAG-dependent PKC, affects the activation of ASMase by LPS. Quiescent BAC-1.2F5 cells were left untreated (black bars) or were treated with BIM (10 μmol/L, 60 minutes; gray bars); alternatively, down-regulation of DAG-dependent PKC was performed as described in Figure 3B (white bars). Cells were stimulated with LPS for 15 minutes before lysis and determination of ASMase activity in whole cell extracts. Results are expressed as the percentage increase with respect to the control values. (C) Exogenous ceramide stimulates JNK in macrophages; BAC-1.2F5 cells were left untreated or were pretreated with D609 (10 μmol/L, 60 minutes) to block PC-PLC or with BIM (10 μmol/L, 60 minutes) to inhibit PKC. Cells were then stimulated with C2 ceramide (C2, 250 μmol/L) or with the inactive analog dihydro-C2 ceramide (DH-C2, 250 μmol/L), as indicated. The presence of phosphorylated JNK was detected by immunoblotting with the corresponding antibodies. An anti-JNK1 blot is shown as a loading control.
Involvement of ASMase and ceramide in JNK activation by LPS.
(A) LPS stimulates ASMase; quiescent BAC-1.2F5 cells were either left untreated or pretreated with D609 (10 μmol/L, 60 minutes) to block PC-PLC before stimulation with 1.5 μg/mL LPS for different times before solubilization. ASMase activity was determined in whole cell extracts as described in “Materials and methods.” Results are expressed as the percentage increase with respect to the control values. The plot represents the mean of 2 independent experiments, and vertical bars represent the range of the samples. (B) BIM pretreatment, but not down-regulation of DAG-dependent PKC, affects the activation of ASMase by LPS. Quiescent BAC-1.2F5 cells were left untreated (black bars) or were treated with BIM (10 μmol/L, 60 minutes; gray bars); alternatively, down-regulation of DAG-dependent PKC was performed as described in Figure 3B (white bars). Cells were stimulated with LPS for 15 minutes before lysis and determination of ASMase activity in whole cell extracts. Results are expressed as the percentage increase with respect to the control values. (C) Exogenous ceramide stimulates JNK in macrophages; BAC-1.2F5 cells were left untreated or were pretreated with D609 (10 μmol/L, 60 minutes) to block PC-PLC or with BIM (10 μmol/L, 60 minutes) to inhibit PKC. Cells were then stimulated with C2 ceramide (C2, 250 μmol/L) or with the inactive analog dihydro-C2 ceramide (DH-C2, 250 μmol/L), as indicated. The presence of phosphorylated JNK was detected by immunoblotting with the corresponding antibodies. An anti-JNK1 blot is shown as a loading control.
Discussion
Bacterial LPS is one of the most potent immunomodulatory substances known. Macrophages represent the main cellular target of LPS in the body, and their response mediates the positive and the negative pathophysiological effects of this powerful stimulus. In this paper we concentrated on describing the cascade that leads to the activation of the SEK/JNK module after LPS stimulation in macrophages. We find that a pathway involving the sequential activation of PI 3-K, PKCζ, PC-PLC, and ASMase is responsible for SEK/JNK activation. Unexpectedly, stimulation of these kinases is independent of the function of Cdc42/Rac.
Herbimycin- and wortmannin-sensitive pathway mediates Cdc42/Rac-independent SEK/JNK activation by LPS
Herbimycin-sensitive kinases have been implicated in PI 3-K50 and JNK3,6 activation by LPS in macrophages. Herbimycin A and PI 3-K inhibitors severely blunted LPS-mediated activation of SEK/JNK. PI 3-K has been reported previously to mediate JNK activation by tyrosine kinase and G-protein–coupled receptors.8,9,22 However, in all cases in which this has been investigated, dominant-negative forms of Cdc42/Rac blocked PI 3-K–dependent JNK activation. In contrast, we show that toxin B, an efficient bacterial inhibitor of these GTPases, does not prevent the stimulation of the SEK/JNK module by LPS (Figure 2). Both toxin B and wortmannin completely blocked phagocytosis by BAC-1.2F5 cells4 and, on longer incubations, reduced their adherence to the substrate (data not shown). Furthermore, a pathway involving Cdc42/Rac and PI 3-K mediates CD14-dependent, LPS-stimulated monocyte adherence.53 Thus, these signal transducers act in concert to regulate the cytoskeleton of monocytes/macrophages, whereas they do not cooperate to implement JNK activation. It is possible that macrophages contain different PI 3-K isoforms, which are all stimulated by LPS but operate in distinct pathways to accomplish different outcomes.
PKCζ-mediated activation of PC-PLC/ASMase is responsible for SEK/JNK activation by LPS
Conventional, novel, and atypical isoforms of PKC have been implicated in MAPK stimulation.54-57 PI 3-K participates in the activation of PKC isoenzymes.35,36 In the course of LPS stimulation, DAG-dependent PKC isoforms are in fact activated as a result of the PI 3-K–mediated stimulation of PLD-dependent phosphatidylcholine hydrolysis, and they act as intermediates in ERK activation.4 Here, we show that the atypical PKCζ is stimulated by LPS in a wortmannin-sensitive manner. PI 3-K–dependent activation of PKCζ is well documented in the literature.35-37,57 It may occur through the direct binding of PI 3-K–generated phosphoinositides to PKCζ49 or through the phosphorylation of T410 in the activation loop by PDK1.35 36 We demonstrate directly that LPS stimulates phosphorylation of the PDK1 site (Figure 4). Therefore, the latter mechanism operates in LPS-stimulated macrophages.
Activated PKCζ participates in the activation of the SEK/JNK module. This is interesting because, though this enzyme has been shown to modulate the JNK pathway target AP-1,58,59 activation of this isoform has mostly been connected with the stimulation of the ERK pathway.54,55,57,60 The regulation of phosphatidylcholine breakdown is one of the functions of PKC enzymes (reviewed in Exton51). More recent studies point to a role of ceramide generated through the PC-PLC/ASMase pathway as an activator of PKCζ,61,62 though this is somewhat controversial.63 In LPS-stimulated macrophages, both PC-PLC and ASMase are activated (Figures 5B, 6A), and their stimulation is inhibited by BIM but not by prolonged TPA treatment (Figures 5D,6B). Thus, in macrophages PKCζ appears to function in the stimulation of the PC-PLC/ASMase system. The generation of ceramide in LPS-treated macrophages has been reported,42 and the modest increases observed are in good agreement with the level of ASMase activation detected in our study. It should be kept in mind that these relatively small fluctuations were detected in whole cell lysates, and they might be much more impressive in the context of the specific subcellular microenvironment in which activation occurs. In this context, sphingomyelin hydrolysis reportedly occurs in caveolae.64In further support of our hypothesis, the inhibition of ASMase with D609 correlates with the lack of SEK/JNK activation by LPS, and the treatment of macrophages with exogenous bacterial PC-PLC and with exogenous ceramide is sufficient to cause JNK activation. This demonstrates that both signal-transducing molecules are, in principle, able to couple to JNK in macrophages.
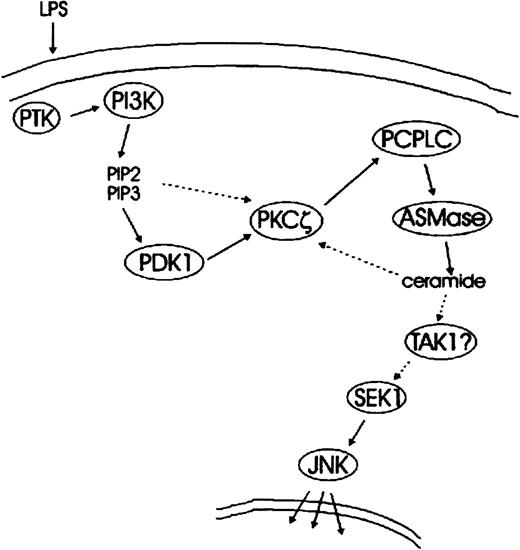
We thus propose a model in which the PI 3-K–mediated activation of PKCζ causes PC-PLC/ASMase activation. This results in the stimulation of the SEK/JNK module, likely through ceramide generation (Figure7). Ceramide generated by PC-PLC/ASMase might also feed back into the stimulation of PKCζ, thus amplifying the pathway (dotted line in Figure 7). Although the actual link between ceramide generation and SEK/JNK activation remains unidentified, the TAK1 kinase, a SEK1 activator stimulated by endogenous and exogenous ceramides,65 would be a suitable candidate. Interestingly, activation of the JNK target AP-1 by the CD28 stimulation of T cells has been suggested to depend on both PI 3-K and ASMase.66 It is therefore possible that a pathway similar to the one described here operates in other systems as well.
Schematic representation of LPS-mediated signal transduction pathways leading to JNK activation—a working model.
LPS-stimulated tyrosine kinase(s) activate PI 3-K, which in turn generates the phosphoinositides PI(3,4)P2 and PI(3,4,5)P3. Activation of the downstream target PKCζ occurs through PDK1-mediated phosphorylation of T410 in the C-loop of PKCζ. The possibility of an allosteric activation of PKCζ by PI(3,4)P2, PI(3,4,5)P3, or both is indicated by a dotted arrow. PKCζ stimulates PC-PLC, which generates DAG required for the activation of ASMase. This in turn results in the production of the second messenger ceramide and in SEK/JNK activation. A candidate link between PC-PLC/ASMase pathway and SEK/JNK module is TAK1, a ceramide-stimulative SEK1 kinase. Speculative steps are indicated by question marks, and possible feedback mechanisms are depicted by dotted lines.
Schematic representation of LPS-mediated signal transduction pathways leading to JNK activation—a working model.
LPS-stimulated tyrosine kinase(s) activate PI 3-K, which in turn generates the phosphoinositides PI(3,4)P2 and PI(3,4,5)P3. Activation of the downstream target PKCζ occurs through PDK1-mediated phosphorylation of T410 in the C-loop of PKCζ. The possibility of an allosteric activation of PKCζ by PI(3,4)P2, PI(3,4,5)P3, or both is indicated by a dotted arrow. PKCζ stimulates PC-PLC, which generates DAG required for the activation of ASMase. This in turn results in the production of the second messenger ceramide and in SEK/JNK activation. A candidate link between PC-PLC/ASMase pathway and SEK/JNK module is TAK1, a ceramide-stimulative SEK1 kinase. Speculative steps are indicated by question marks, and possible feedback mechanisms are depicted by dotted lines.
The data reported here extend our understanding of LPS signal transduction and show for the first time that a pathway comprising PI 3-K, PKCζ, and PC-PLC/ASMase leads to JNK activation independently of CDC42/Rac.
Acknowledgments
We thank Klaus Aktories (University of Freiburg) for support, Pavel Kovarik for constructive discussion and continuous support, and Thomas Decker (Vienna Biocenter) for critically reading this manuscript.
This work was supported by grants #P10766-MED and #P13252-MOB of the Austrian Research Fund (M.B.) and by a grant of the Associazione Italiana per la Ricerca sul Cancro (R.T.).
Submitted September 28, 1999; accepted June 5, 2000.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Manuela Baccarini, Section of Cell Biology and Microbiology, Institute of Microbiology and Genetics, Vienna Biocenter, Dr-Bohrgasse 9, 1030 Vienna, Austria; e-mail:manuela@gem.univie.ac.at.

![Fig. 2. Toxin B efficiently glucosylates Cdc42 and Rac without affecting LPS-mediated SEK and JNK activation. / (A) The Rho-family GTPase inhibitor toxin B does not affect JNK activation by LPS; quiescent BAC-1.2F5 cells were incubated with toxin B (Tox B, 100 ng/mL) for 60 minutes before stimulation with 1.5 μg/mL LPS for 15 minutes. The presence of phosphorylated forms of SEK and JNK, and of JNK1 as a loading control, was detected by immunoblotting with the corresponding antibodies. (B) In vivo treatment with toxin B efficiently glucosylates Rho subtype proteins in BAC-1.2F5 cells. Cells were either left untreated or pretreated with toxin B (100 ng/mL for 60 minutes). Lysates (100 μg) or recombinant GST-Rac (1 μg) were incubated in vitro with recombinant toxin B (fragment CDB546) and UDP-[14C]glucose (20 μmol/L) for 60 minutes at 37°C. Glucosylated proteins were analyzed by SDS-PAGE and phosphorimaging. The identity of the GTPases was confirmed by immunoblotting.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/96/7/10.1182_blood.v96.7.2592/5/m_h81900212002.jpeg?Expires=1769288933&Signature=w-pGaOKs--RIPo8TbrHZuFd7Wmt8HzDsAugetQKG14oYf9Dnk-PJPqmGGbeYpvn0F4~X1wUnYg5A3lX5nUJObKjVYsT8ID8jz4ltqevuhy5ryuS1YOJE6CxJNuyeACu1kDUc5bjGli59T5G8GstZkpxQFNFN-YF2HFlFXURkGoEi-x64JOQUrhrUQYulphhNzVVCmOoOMF8qLSub8m9AdqNeJicOaDbZYd0Gbb5YkhEv-x3fZ2K1f0zRIDZz9-6BRFdjKb~FpLtURVlxlo7RY6LX75UQbe4SXMWA8fNVRuYOwAM~7HlaiPBPqhtmfE3IzBfH5xFX0hSi0xTPmV7KOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)