Abstract
Absence of band 3, associated with the mutation Coimbra (V488M) in the homozygous state, caused severe hereditary spherocytosis in a young child. Although prenatal testing was made available to the parents, it was declined. Because the fetus stopped moving near term, an emergency cesarean section was performed and a severely anemic, hydropic female baby was delivered. She was resuscitated and initially kept alive with respiratory assistance and hypertransfusion therapy. Cord blood smears revealed erythroblastosis, poikilocytosis, and red cells with stalk-like elongations. Band 3 and protein 4.2 were absent; spectrin, ankyrin, and glycophorin A were significantly reduced. Renal tubular acidosis was detected by the age of 3 months. Nephrocalcinosis appeared soon thereafter. After 3 years of follow-up the child is doing reasonably well on a regimen that includes regular blood transfusions and daily bicarbonate supplements. The long-term prognosis remains uncertain given the potential for hematologic and renal complications.
Introduction
Band 3, also known as the red cell anion exchanger 1, is encoded by the EPB3 gene. Band 3 Coimbra (GTG→ATG; V488M) represents a mutation at the beginning of the fourth transmembrane domain.1 Insertion of the fourth transmembrane domain is a prerequisite for incorporation of transmembrane domains 1 through 32 and therefore band 3 Coimbra must represent a membrane insertion defect. In addition, anN-terminally truncated band 3 isoform exists in renal tubular intercalar A cells.
In the heterozygous state, band 3 Coimbra causes typical hereditary spherocytosis (HS) and is associated with partial deficiency of band 3 and of protein 4.2 (the latter is deficient as a secondary phenomenon).1 In addition, certain mutations of theEPB3 gene are responsible for dominant distal renal tubular acidosis (DRTA).3-5 In these cases, the amount of erythrocyte band 3 is normal and hematologic manifestations are absent. A mutation causing band 3 deficient-HS and partial DRTA,6or a higher basal urinary pH,7 in the heterozygous state has been observed.
Severe HS resulting from the absence of band 3 has been observed in a natural strain of cattle8 and in 2 band 3 null mouse strains engineered through targeted recombination.9,10 In one strain glycophorin A was absent,10,11 and a susceptibility with respect to thrombotic complications was noted.12 Severe HS in humans was first reported by Ribeiro and colleagues.13 We herein provide a full account of a case of severe HS associated with total absence of band 3, as well as follow-up over a subsequent 3-year period.
Study design
Several cousins were found to be heterozygous for band 3 Coimbra, including the patient's mother who has had 3 pregnancies. In her first pregnancy (1989), the fetus stopped moving near term, and a stillborn baby with hydrops fetalis was delivered. We have almost no information on the fetus, but we may reasonably surmise that it was homozygous for band 3 Coimbra. In her second pregnancy (1994), prenatal diagnosis, taking advantage of an NlaIII site created by mutation Coimbra (not shown), concluded that the fetus was homozygous for the mutation. This led to medical termination of pregnancy after approval by the Coimbra Maternity Ethical Committee. The placenta had a normal appearance, but on histologic examination, a deficient formation of the capillaries and a marked siderosis were observed. The male fetus had cervical edema and no obvious malformation. Liver and spleen samples were unsuitable for analysis.
The parents declined prenatal diagnosis in the third pregnancy (1996). By week 34 of gestation, a pericardial effusion was recorded. At week 36, there was significant ascites and anasarca, and the fetus stopped moving. An emergency cesarean section was performed. The hydropic female newborn had intense pallor, generalized edema, prominent ascites, and massive hepatosplenomegaly (weight, 2445 g; Apgar scores, 2, 8, and 9).
Red blood cell indices in cord blood were: red blood cells, 1.07 T/L; hemoglobin, 52 g/L; hematocrit, 15.7%; mean corpuscular volume, 147 fL; mean corpuscular hemoglobin, 49 pg; mean corpuscular hemoglobin concentration, 31 g/dL; and reticulocytes, 9.57%. Red blood cells exhibited a wide variety of abnormal morphologies, some presenting with long spike-like elongations (Figure 1A). Blood transfusion, intensive resuscitation, and assisted respiratory ventilation were immediately initiated after birth. During 6 weeks in the neonatal intensive care unit, the baby required continuous ventilation, drainage of the ascitic fluid, and exchange transfusion (taking severe hyperbilirubinemia into account). A hypertransfusion regimen was started because pretransfusion hemoglobin levels were rarely above 6.5 g/L. Erythroblasts and nucleated red blood cells with scant and irregular cytoplasm were present. Iron chelating treatment was initiated when the serum ferritin concentration reached 800 ng/L (8-12 hours of intravenous infusion of desferroxiamine ([40 mg/kg] twice a week).
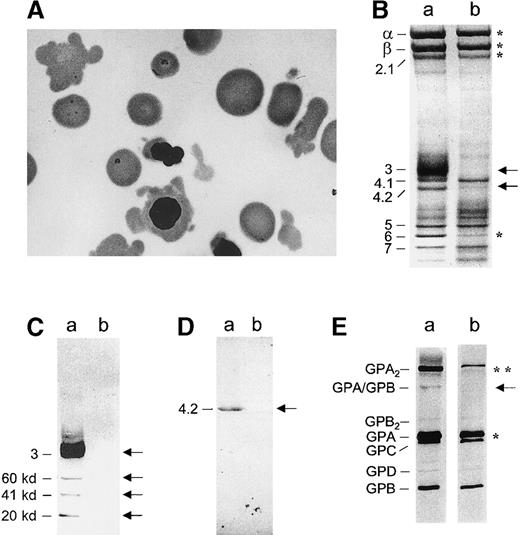
Peripheral blood smear, red cell membrane proteins, and glycophorins before transfusion.
(A) Red blood cells with bizarre shapes, spherocytes, and erythroblasts with cytoplasmic elongations. (B) Coomassie blue staining. (C) Western blotting of band 3 using a monoclonal antibody (Sigma, St Louis, MO). (D) Western blotting of protein 4.2 using our own polyclonal antibody. (E) Silver staining of glycophorins; a: control; b: patient (venous blood prior to transfusion). (B) In the patient, band 3 and protein 4.2 were missing (denoted by ←); Spectrin α and β chains, ankyrin were strongly reduced, as was band 6 (*). Unlike the membrane of mice with targeted inactivation of the band 3 gene,9 10 the patient's membrane retained negligible amounts (4%) of hemoglobin (versus 1% in controls); residual hemoglobin would not significantly alter the quantification of membrane proteins. The amounts of protein 4.1 and actin were found to be rather similar to those of controls. (C) Band 3 was present and accompanied by known proteolytic fragments (60, 41, and 22 kd) in the control. In the patient, band 3 was totally missing (←). (D) Protein 4.2 was totally missing as well (←). (E) There was a strong reduction of the GPA dimer (**) and a less pronounced decrease of the GPA monomer (*). The heterodimer GPA/GPB was nearly undetectable (←). No obvious differences were recorded as for the other glycophorins.
Peripheral blood smear, red cell membrane proteins, and glycophorins before transfusion.
(A) Red blood cells with bizarre shapes, spherocytes, and erythroblasts with cytoplasmic elongations. (B) Coomassie blue staining. (C) Western blotting of band 3 using a monoclonal antibody (Sigma, St Louis, MO). (D) Western blotting of protein 4.2 using our own polyclonal antibody. (E) Silver staining of glycophorins; a: control; b: patient (venous blood prior to transfusion). (B) In the patient, band 3 and protein 4.2 were missing (denoted by ←); Spectrin α and β chains, ankyrin were strongly reduced, as was band 6 (*). Unlike the membrane of mice with targeted inactivation of the band 3 gene,9 10 the patient's membrane retained negligible amounts (4%) of hemoglobin (versus 1% in controls); residual hemoglobin would not significantly alter the quantification of membrane proteins. The amounts of protein 4.1 and actin were found to be rather similar to those of controls. (C) Band 3 was present and accompanied by known proteolytic fragments (60, 41, and 22 kd) in the control. In the patient, band 3 was totally missing (←). (D) Protein 4.2 was totally missing as well (←). (E) There was a strong reduction of the GPA dimer (**) and a less pronounced decrease of the GPA monomer (*). The heterodimer GPA/GPB was nearly undetectable (←). No obvious differences were recorded as for the other glycophorins.
Hyperchloremic metabolic acidosis was detected by the age of 3 months. The infant received oral sodium bicarbonate (8 mEq/kg daily) and subsequently also received monopotassium phosphate. Soon after the discovery of blood acidosis, ultrasound showed the onset of nephrocalcinosis. Correcting the acidosis led to the normalization of calciuria. Nephrocalcinosis subsequently remained stable without impairment of glomerular filtration.
Psychomotor development was slightly delayed, but there appeared to be no increased susceptibility to infection, no neurologic abnormality, no hearing impairment, and no thrombotic tendency. At the age of 3, the child was doing reasonably well.
Red cells were obtained from umbilical cord and peripheral blood before the first transfusion. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting of membrane proteins and DNA analysis were carried out as described before,1 with minor modifications. Characterizations of the renal acidosis were based on standard methodologies.14-16
Results and discussion
Polymerase chain reaction amplification of DNA from the child's white blood cells followed by NlaIII digestion demonstrated homozygosity for mutation Coimbra. Band 3 and protein 4.2 were absent. Spectrin α and β chains and ankyrin were reduced (by about 43% and 57%, respectively) in comparison to protein 4.1, which was taken as a reference (Figure 1B-D). Band 6 was strongly diminished (Figure 1B), and glycophorin A was markedly decreased (Figure 1E). These features matched those described in 1 band 3 null mouse strain with a number of small differences.10 11
The urine anion gap was positive, and the value of plasma K+ was normal to decreased. The lowest urine pH after furosemide administration was 6.6. The urine/blood Pco2 gradient was less than 20 mm Hg after NaHCO3 loading, the fractional excretion of HCO3− at normal plasma HCO3−concentration was 4%, and there was a high Ca++urinary excretion (urinary Ca++/creatinine ratio 1.2 mmol/mmol). These tests indicated a distal acidification defect (Table1).
The child would probably have died had not an extensive knowledge of the family history and of the underlying mutation led to preparation for intensive care. The prognosis is uncertain, however. The transfusion demand will remain the same, necessitating the control of iron overload. Bone marrow transplantation with a compatible donor would represent the most satisfactory solution. In the meantime, a partial or total splenectomy is being considered to reduce transfusion requirements. DRTA has been corrected with oral HCO3−supplementation, but nephrocalcinosis will persist and may cause renal failure.
A similar case of HS with missing band 3 has been identified.17 No DRTA occurred in this instance because the frameshift mutation only caused deletion of exon 2 and spared the renal isoform of band 3. This work shows that intensive treatment may keep a patient with total band 3 deficiency alive. Nevertheless, potential hematologic and renal complications can be quite severe. Thus, medical termination of pregnancy, as was done with the previous gestation, remains an alternative.
Acknowledgments
We thank the family studied here for their kind cooperation, the Prenatal Diagnosis Unit of the Maternidade Bissaya Barreto for assistance during the pregnancy, and Dr M. J. Julião for performing the postmortem examination of the fetus after the second pregnancy.
Supported by the Forum Hematológico, the Centre National de la Recherche Scientifique (URA 1171), the Institut National de la Santé et de la Recherche Médicale (CRE 930405), the Université Claude-Bernard Lyon-1′, the Association pour la Recherche sur le Cancer, the Association Française contre les Myopathies, and the Conseil de la Région Rhône-Alpes. Presented in part at the 39th Annual Meeting of the American Society of Hematology, San Diego, December 5-9, 1997, and published by Blood 90:265a, 1997 (suppl, abstract).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
M. L. Ribeiro, Unidade de Hematologia Molecular, Serviço de Hematologia, Hospital Pediátrico, Centro Hospitalar de Coimbra, 3001 Coimbra Codex, Portugal; e-mail:leticiar@mail.telepac.pt.