Abstract
To determine prognostic factors and treatment outcome, patients with primary progressive Hodgkin lymphoma (HD) registered in the database of the German Hodgkin Lymphoma Study Group (GHSG) were analyzed retrospectively. Detailed records from randomized prospective multicenter trials performed between 1988 and 1998 of 3807 patients recruited in these trials were reviewed. The median age of the 206 patients available was 34 years (range, 16-71). Fifty-seven patients (28%) in intermediate stage and 149 patients (72%) in advanced stage developed progressive disease (PD). One hundred and fifty-three patients (74%) were treated with salvage chemotherapy, 47 patients (23%) with salvage radiotherapy, and 6 patients (3%) did not receive any therapy due to rapid PD. Seventy patients (34%) were treated with high-dose chemotherapy (HDCT) and autologous stem cell transplantation. The 5-year freedom from second failure (FF2F) and overall survival (OS) for all patients were 17% and 26%, respectively. The 5-year FF2F and OS for patients treated with HDCT were 31% and 43%, respectively. In multivariate analysis low Karnofsky performance score at the time of progression (P < .0001), age above 50 years (P = .019), and failure to attain a temporary remission on first-line treatment (P = .0003) were significant adverse prognostic factors for OS. Patients with none of these risk factors had a 5-year OS of 55% compared with 0% for patients with all 3 of these unfavorable prognostic factors. Although HDCT is a reasonable option for selected patients with primary progressive HD, the majority did not receive HDCT. Interestingly, salvage radiotherapy gave promising results in patients with localized PD.
Introduction
The majority of patients with Hodgkin lymphoma (HD) can be cured with conventional chemotherapy or radiotherapy or both.1 However, the outcome of patients with primary progressive disease (PD) defined as progression during induction treatment or within 90 days after the end of treatment is dismal. Treatment results with second-line chemotherapy produces low remission rates, with long-term disease-free survival (DFS) in 0% to 10% of patients with primary progressive HD.2,3 Salvage radiotherapy is an effective treatment for localized relapsed HD, but little is known about radiotherapy in patients with PD. Experiences with high-dose chemotherapy (HDCT) followed by autologous stem cell transplantation (ASCT) from selected series in primary progressive HD indicate a DFS rate of 31% to 42%.4-7 However, patients receiving HDCT undergo a considerable selection. A high proportion of patients are not treated with HDCT because of rapid disease progression, insufficient stem cell harvest, poor performance status, or older age.
We thus retrospectively analyzed patients with primary progressive HD after first-line polychemotherapy who are registered in the database of the German Hodgkin Lymphoma Study Group (GHSG). Patient characteristics, salvage treatment, and prognostic factors were evaluated at the time of progression. The purpose of the present analysis is to assess whether and to which extent long-term DFS can be achieved in patients with primary progressive HD and to determine patient-, disease-, and treatment-related factors correlated with outcome.
Patients and methods
Patient selection
Using the database of the GHSG, patients with primary progressive HD after first-line polychemotherapy were analyzed retrospectively. Recruitment into trials for intermediate (HD5 and HD8) and advanced stages HD (HD6 and HD9) began in 1988 and continued until 1998 (Table 1). A total of 3807 patients were included in these trials. To be eligible, patients between the ages of 16 and 75 years had to have biopsy-proven HD at diagnosis; adequate organ function as defined by a creatinine clearance more than 60 mL/min, serum transaminase levels less than 3 times normal, and bilirubin less than 2 mg/dL; left ventricular ejection fraction more than 0.45; forced expiratory volume in first second [FEV1] or diffusion capacity of carbon monoxide (DLCO) more than 60% of predicted; white blood cell (WBC) counts 3500/μL or greater; hemoglobin level 8 g/dL or greater; and platelets 100 000/μL or greater. Patients were required to test negative for antibodies against human immunodeficiency virus and to be free of active infections. All patients signed informed consent based on the Institutional Review Board Guidelines. Patients in localized stages with risk factors (intermediate stages) received 4 chemotherapy cycles (COPP/ABVD or COPP/ABV/IMEP) and extended field (EF) or involved field (IF) radiotherapy; patients in advanced stages received 8 chemotherapy cycles (COPP/ABVD or COPP/ABV/IMEP or BEACOPP) and localized radiotherapy to residual or initial bulky disease.8-10Primary progressive disease was defined as disease progression during first-line chemotherapy, or only transient response (complete remission [CR] or partial remission [PR] lasting ≤ 90 days) after induction treatment. Criteria for progressive disease were at least one of: (1) 25% or more increase from nadir in the sum of the products of the greatest diameter of any previously identified abnormal node for partial responders or nonresponders or (2) appearance of any new lesion during or less than 90 days after the end of therapy. A rebiopsy at the time of disease progression was recommended. This analysis is based on data extracted from the database and patient files between February and April 1999.
Salvage therapy
Data on salvage chemotherapy included the type of regimen and the number of cycles. Sensitivity to conventional dose salvage chemotherapy defined as more than 50% decrease in the sum of the products of the greatest diameter of measurable lesions was assessed after 1 to 3 chemotherapy courses. Data relating to salvage radiotherapy included field and size, that is, total nodal irradiation (TNI), mantle field (MF), EF, IF radiation, respectively, and dose delivered. Data on HDCT included type of hematopoietic graft, preparative regimen, and chemosensitivity before transplantation.
Staging procedures after salvage treatment
Before salvage therapy, the extent of disease was assessed by chest x-ray, abdominal sonography, computed tomography, and bone marrow biopsy. Restaging was performed after salvage chemotherapy or radiotherapy. After the end of the salvage therapy and prior to ASCT, all sites of initial disease were reassessed by adequate methods including bone marrow biopsy for patients who had bone marrow involvement before salvage therapy.
Response definition
A CR was defined as the disappearance of all clinical and radiographic evidence of disease for at least 1 month. PR was defined as a greater than 50% reduction in the product of the largest diameter and its perpendicular of measurable disease lasting more than 1 month. Any response less than PR was considered a treatment failure.
Statistics
Freedom from second treatment failure (FF2F) was measured from the date of entry into the salvage protocol until progression, relapse, or death from any cause. Overall survival (OS) was measured from the date of entry into the salvage protocol until death from any cause. Demographics and disease characteristics were summarized using descriptive statistics. OS and FF2F rates were estimated according to the method of Kaplan and Meier.11 The prognostic significance of various factors was tested by multivariate Cox regression analysis for each of the outcome variables (FF2F and OS).12 All patients who received salvage therapy with curative intent were included.
The factors tested were as follows:
prior to progression: primary treatment protocol, attainment of a temporary remission on first-line treatment;
at progression: age (continuous variable), gender, Karnofsky performance score, B symptoms, stage, localization of PD, time of occurrence of progression (during first-line treatment versus 1-3 months after end of treatment);
after progression: sensitivity to conventional salvage chemotherapy.
In the main analysis, only factors available at (or before) progression were included. Sensitivity to conventional salvage chemotherapy was included only after assessing the significance of the above factors.
Candidate factors were examined by stepwise procedures: removal and entry levels of significance were 0.1 and 0.05, respectively. AllP values are 2-sided. Due to the presence of multiple comparisons (10 factors were tested), significance should be finally assessed, following Bonferroni,13 using the more stringent threshold P value of .05/10 = .005. Once the selection of factors had been made, each selected factor was simplified into 2 categories (age ≥ 50 or < 50 years, Karnofsky performance score ≥ 90 or < 90, and failure to attain a temporary remission on first-line treatment or not). The chosen Cox regression model was then fitted again using all cases with complete data in the selected factors. Model predictions for OS rates at 3 years were calculated for each combination of factor categories. All statistical analyses were done using SPSS 6.1. (SPSS, Chicago, IL).
Results
Patient characteristics
A total of 239 patients with primary progressive HD were identified. Patients were excluded from the final analysis due to non-Hodgkin lymphoma (NHL) histology at PD (n = 12), insufficient first-line therapy (n = 6), refusal of salvage therapy (n = 8), palliative radiotherapy (defined as radiation not delivered to all involved sites; n = 6), and lack of data on salvage therapy (n = 1). Thus, 206 patients were available for the final analysis. Patient characteristics are listed in Table2.
The median age of the patients at progression was 34 years (range, 16-71 years). There were 112 males (54%) and 94 females (46%) with the following histologies at first diagnosis: nodular sclerosis, 140 (68%); mixed cellularity, 46 (22%); lymphocyte depleted, 11 (5%); lymphocyte predominant, 5 (3%); and unclassified, 4 patients (2%). Progression was proven by biopsy in 118 patients (57%) or demonstrated unequivocally on radiographic studies. Fifty-seven patients (28%) had intermediate stage and 149 patients (72%) advanced stage at first diagnosis. In 148 patients (72%), a remission (PR or CR) had been documented during staging examinations after 4 or 8 cycles of first-line chemotherapy. Stage at progression by Ann Arbor criteria was stage I in 24 patients (11%), stage II in 64 (31%), stage III in 37 (18%), and stage IV in 81 patients (40%). Twenty-one patients (10%) had extranodal disease, 21 (10%) had bone involvement, 28 (14%) had liver involvement, 45 (22%) had lung involvement, and 7 (3%) had bone marrow involvement. Twenty-three patients (11%) had more than one organ involvement. Forty-eight patients (23%) had Karnofsky performance scores less than 90, and 97 patients (47%) had B symptoms at the time of progression.
Initially, 111 patients (54%) had been treated with COPP/ABVD at initial diagnosis, 57 patients (28%) with COPP/ABV/IMEP, and 38 patients (18%) with BEACOPP (15% BEACOPP baseline; 3% BEACOPP escalated). Eighty-one patients (37%) had received both chemotherapy and radiotherapy during first-line treatment.
Time until progression and localization of disease progression
Disease progression occurred after a median of 7 months after first diagnosis; 143 (70%) patients had disease progression during first-line treatment. Twenty patients (9%) had progressive disease within the first month, 27 patients (13%) within the second month, and 16 patients (8%) within the third month after the end of first-line treatment. PD occurred in initial areas in 91 patients (44%), in initial areas and new areas in 97 patients (47%), and only in new areas in 15 patients (7%). Data on disease localization were missing in 3 patients (2%).
Salvage treatment
After documented progress, 153 patients (72%) received second-line chemotherapy. Forty-seven patients (22%) were treated with salvage radiotherapy with intent to cure, defined as radiation delivered to all involved sites. Another 6 patients (3%) did not receive any therapy due to rapid progressive disease and died 1 to 8 months after first diagnosis. Seventy-eight patients (51%) received Dexa-BEAM as salvage chemotherapy (dexamethasone, carmustine, etoposide, cytarabine, melphalan), 15 patients (10%) CEVD (cyclophosphamide, etoposide, vindesine, dexamethasone). Several other second-line salvage chemotherapy regimens were used, mostly based on etoposide, ifosfamide, ciplatin, or high-dose Ara-C such as IMVP-16 (ifosfamide, methotrexate, etoposide), IEV (ifosfamide, epirubicin, etoposide), and DHAP (dexamethasone, cytarabine, cisplatin). Fifty patients (33%) treated with salvage chemotherapy had stage I/II and 103 (67%) had stage III/IV at the time of disease progression. The overall response rate after salvage chemotherapy was 43% (37% CR and 6% PR). Salvage radiotherapy as second-line treatment with curative intention included TNI in 9 patients, EF radiotherapy in 15 patients, MF radiotherapy in 14 patients, and IF radiotherapy in 9 patients. The median dose of radiotherapy delivered was 40 Gy (range, 30.6-50 Gy). Thirty-eight patients (81%) treated with salvage radiotherapy had stage I/II, 8 patients (17%) stage III, and 1 patient stage IV at the time of disease progression. Eleven patients (23%) had B symptoms, 7 (15%) had a Karnofsky status below 80, and 7 (15%) were over 50 years old. The overall response rate after salvage radiotherapy was 66% (62% CR and 4% PR).
High-dose chemotherapy and stem cell transplantation
Seventy patients (33%) were treated with HDCT and ASCT. Of those, 62 patients received HDCT after CR or PR on conventional salvage therapy and 8 patients were treated with HDCT in chemoresistant progression. Twelve of these 70 patients had relapsed after radiotherapy as initial salvage treatment and 58 patients had initially been treated with salvage chemotherapy. The source of stem cells was bone marrow in 21% and peripheral blood in 79% of the cases. Nine patients received radiotherapy to residual lymphoma after HDCT. The HDCT regimens used were BEAM in 38 patients (54%) (carmustine [BCNU], etoposide, cytarabine, and melphalan) and CVB in 22 patients (31%) (cyclophosphamide, etoposide, and carmustine [BCNU]). Two patients (3%) received a total body irradiation–containing regimen, and 8 patients (12%) were treated with other regimens (Table2).
The majority of patients receiving salvage chemotherapy were excluded from high-dose programs because of progressive disease on salvage chemotherapy (60%), life-threatening severe toxicity on salvage treatment (11%), insufficient stem cell harvest (1%), or age above 60 years (1%).
Survival
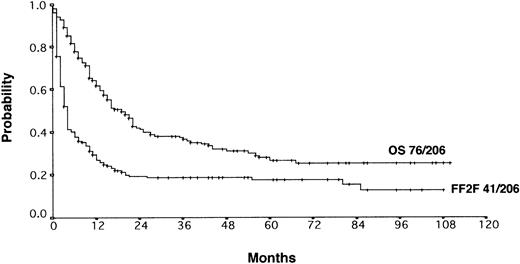
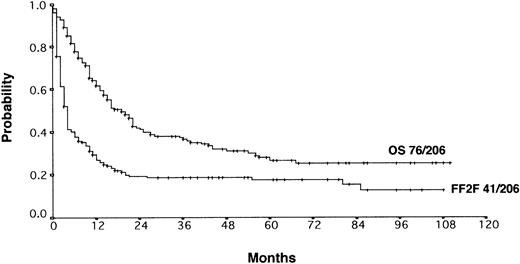
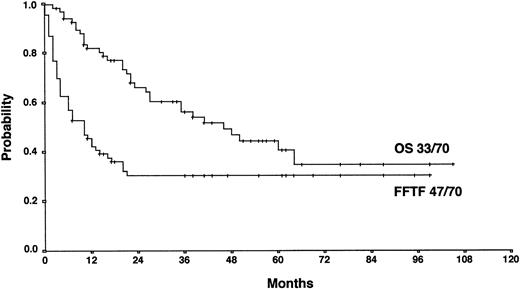
With a median observation time of 52 months, the actuarial 5-years FF2F and OS for all patients after initiation of salvage therapy were 17% and 26%, respectively (Figure 1). Currently, 77 patients are alive with a follow-up between 3 and 110 months. Among those who did not survive, 112 patients (53%) died as a result of disease progression, 19 patients (9%) died due to life-threatening severe toxicity on salvage treatment; 3 patients (1%) died from secondary acute myelogenous leukemia or myelodysplastic syndrome, and 1 patient (< 1%) died after myocardial infarction.
Actuarial OS and FF2F after disease progression for all patients (n = 206).
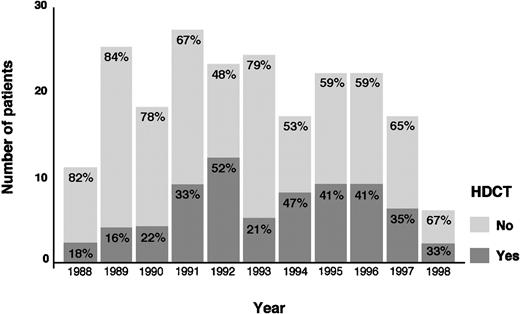
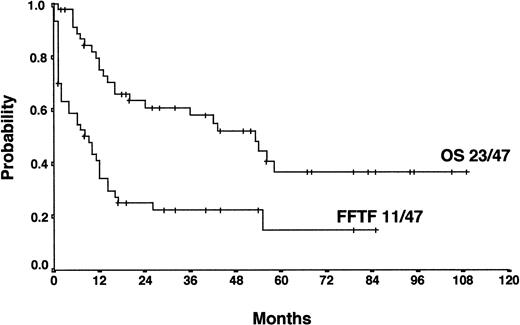
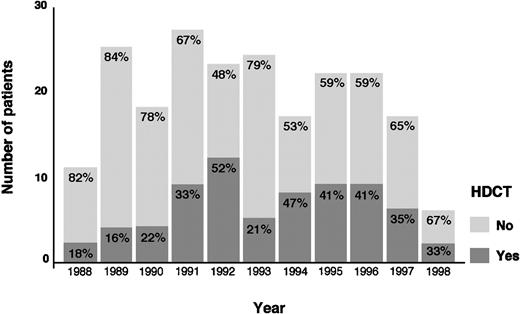
The actuarial FF2F and OS for 70 patients treated with HDCT and ASCT were 31% and 43%, respectively (Figure2). Sixty-two patients (89%) treated with HDCT had reached a remission (PR/CR) with conventional salvage therapy before HDCT. Of 8 patients treated with HDCT in chemoresistant disease, 3 are alive and in continuous CR 6 to 61 months after HDCT. Although the use of HDCT for patients with primary progressive HD increased from 1988 to 1998, less than 50% of all cases underwent transplantation even in the late 1990s (Figure3).
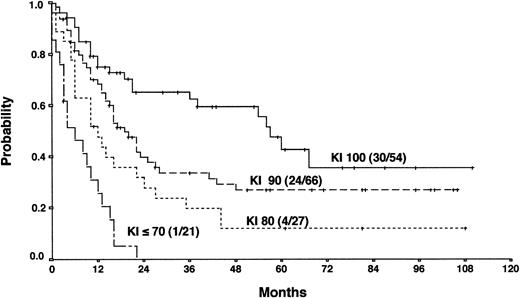
Actuarial OS according to the Karnofsky performance score (KI) at the time of PD.
Actuarial OS according to the Karnofsky performance score (KI) at the time of PD.
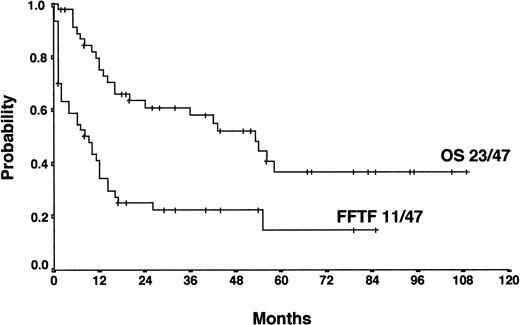
For patients treated with salvage radiotherapy, FF2F and OS after 4 years were 22% and 52%, respectively (Figure4). Currently, 11 patients (23%) are alive and in continuous CR after salvage radiotherapy with a median of 34 months follow-up (range, 6-79 months). Twenty-five patients (54%) who relapsed (18 patients) or who were refractory (7 patients) to salvage radiotherapy were treated with a salvage chemotherapy regimen. Of those, 12 patients received HDCT and ASCT. Among these patients, 7 are alive and in continuous CR.
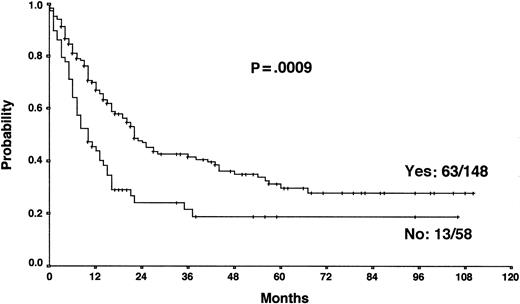
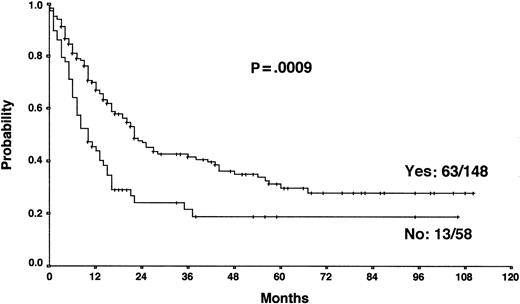
Actuarial OS according to the achievement of a temporary remission with first-line chemotherapy.
Actuarial OS according to the achievement of a temporary remission with first-line chemotherapy.
Prognostic factors
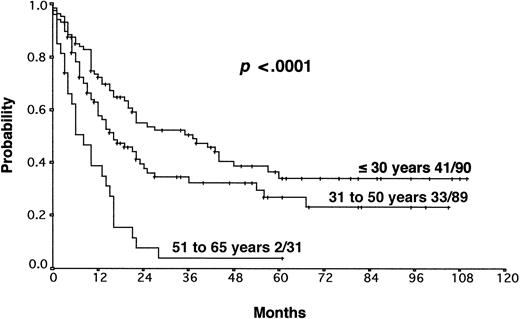
The results from the univariate analysis are listed in Table3. In multivariate analysis, chemosensitive disease on salvage chemotherapy (P < .0001), Karnofsky performance score at the time of progression (P < .0001), age (P = .0026), and attainment of a temporary remission before progression (P = .0034) were significant prognostic factors for OS (Table 4). Backward and forward selection procedures both identified identical significant factors. As shown in Figure 5, the most predictive factor was the Karnofsky performance score. Patients with Karnofsky performance scores of 90 or above had an OS of 34%, whereas patients with Karnofsky scores less than 90 had an OS of 6%. Age was also an important prognostic factor, with 4-year survival of 35% for patients under 30 years but virtually no chance of even 2-year survival for patients older than 50 years (Figure 6). Patients achieving remission before first progression had an 18% higher survival probability than the remainder (Figure7). For FF2F, only the Karnofsky performance score was consistently significant (P = .0046). OS for patients progressing after intermediate or advanced stage at first diagnosis did not show any difference (P = .15).
The factors Karnofsky performance score, age, and previous remission were each dichotomized into 2 categories: Karnofsky performance score of 90 or above or less than 90, age 50 years or less or more than 50 years, with or without previous remission. Table5 shows the model predictions for 3-year OS rates for each combination of factor categories. These predictions are compared with the Kaplan-Meier estimates for the respective patients group for all combinations with at least 10 cases. For patients over 50 years the numbers of cases with each of the 4 combinations was too small; when pooled, these 22 older patients had a Kaplan-Meier estimate of only 4% OS at 3 years. The results are valid in the context of the treatment strategies actually chosen for these progressing patients. Obviously, choice and administration of treatment may play an important role alongside prognostic factors.
Discussion
Several important findings emerge from this analysis. (1) The clinical outcome for patients registered in the GHSG database with primary progressive HD is poor. The 5-year FF2F is 17% and the 5-year OS is 26% for a total of 206 patients evaluable. (2) Prognostic factors for OS being predictive at the time of disease progression include Karnofsky performance score, age, and achievement of a temporary remission with first-line treatment. Patients with none of these risk factors had a predicted 3-year OS of 55% compared with 0% for patients with 3 of these unfavorable prognostic factors. (3) Salvage radiotherapy can give promising results in a subset of patients with localized PD.
One of the most adverse prognostic factors in HD is primary disease progression to an initial chemotherapy regimen. Patients with primary PD have a particular poor response to second- or third-line induction treatment. Their probability of long-term survival with conventional chemotherapy is low. The 5-year OS ranges between 0% and 10% in small series reported.2,3 In an attempt to improve the outcome for patients with primary progressive HD, HDCT followed by ASCT has been increasingly used.4-7
We retrospectively analyzed Hodgkin lymphoma patients with PD registered in the GHSG database to determine prognostic factors and outcome after salvage treatment. Another aim of this study was to identify patients who are candidates for more aggressive treatment modalities. The collection of data was based on the definition of primary progressive disease as disease progression during induction treatment or within 90 days after the end of treatment.2,6,7 14
Our results show that the long-term outcome of patients with primary progressive HD is disappointing. The 5-year FF2F and OS rates for all patients were 17% and 26%, respectively. This predominantly poor prognosis emphasizes the need for more effective first-line treatments that reduce primary progression rates.10 The 5-year OS of patients progressing after presenting in intermediate or advanced stages at first diagnosis did not differ significantly, indicating that one of the most important determinants of outcome in primary treatment is less important when disease progression occurs. An important predictive factor correlated with OS was the Karnofsky performance score at the time of disease progression. The 5-year probability of OS with Karnofsky performance scores of 90% or higher and less than 90% were 34% and 6%, respectively. The age of the patient was also an important factor for OS. Unfortunately, older age and poor performance status may discourage the use of more aggressive treatment modalities in these patients. Moreover, attainment of a temporary remission with first-line chemotherapy was a significant predictor for OS. Patients never achieving a temporary remission during first-line chemotherapy had an 18% lower survival probability than the remainder, indicating the biologic aggressiveness of the disease in this subgroup. The model predictions for each combination of factor categories show that the predicted 3-year OS for patients with none of these risk factors was 55% compared with 0% for patients with 3 of these unfavorable prognostic factors. Therefore, for patients presenting with reduced Karnofsky performance score, age 50 years or older, and with no temporary remission on first-line therapy, palliative rather than aggressive treatment modalities should be used.
The results of HDCT/ASCT in primary progressive HD reported from selected series are promising, with 5-year failure-free and overall survival (progression-free survival [PFS]) of 31% to 42%, respectively.4-7 More recent studies reported improved outcome after HDCT/ASCT for refractory HD when compared with historical controls treated with conventional chemotherapy.15,16Thus, HDCT is clearly an effective treatment for these patients. However, patients receiving HDCT undergo a selection bias. A proportion of patients are not included in HDCT programs because of rapid disease progression, older age, poor performance status, or insufficient stem cell harvest. Furthermore, the comparison is biased because the administration of HDCT occurred during the follow-up period. The presence of bias in OS is evident when we consider that some patients failed to receive HDCT simply because they died early. To avoid bias, a multivariate analysis was performed using the landmark method.14 Patients receiving HDCT within 6 months of progression were compared with those not receiving HDCT within this period; patients surviving less than 6 months were excluded. Allowing for relevant patient characteristics and for chemosensitivity in salvage therapy, the course of the HDCT group was not significantly better than that of the other group (P = .091). Therefore, the particularly good results of the HCDT patients may be due to patient selection and to the above-mentioned bias; we cannot conclude that treatment with HDCT has improved the results.
As noted in several other series, chemosensitivity to conventional salvage chemotherapy was also a significant prognostic factor in our analysis.5,17 Patients who achieve a second PR or CR to conventional salvage chemotherapy are expected to be in a favorable position to proceed to HDCT.17,18 Nevertheless, the role of conventional salvage chemotherapy before HDCT has not been clearly defined. It has been used in the past for chemosensitivity testing and has been given for several cycles to induce tumor regression, often while logistic arrangements are underway for HDCT.19 Chopra and coworkers established the effectiveness of HDCT in producing long-term PFS even in patients failing an aggressive pretransplant salvage treatment.5 Several other studies have confirmed that some patients with disease resistant to conventional therapy may benefit from HDCT, but long-term survival or PFS rates (or both) in such patients are low, ranging from 10% to 30%.5,13,14,20 However, as recently reported by our group, a substantial proportion of chemoresistant patients are not included in HDCT programs.21 In this present analysis, only 34% of all patients received HDCT. The relatively low rate of using HDCT can only in part be explained by the infrequent use of HDCT during the first years of our observation period starting in 1988 or chemoresistance to conventional salvage chemotherapy as an exclusion criteria from HDCT programs. Although the use of HDCT for patients with primary progressive HD increased from 1988 to 1998, less than 50% of all cases were treated with HDCT in the late 1990s. In our analysis, a high proportion of chemoresistant patients rapidly succumb to progressive disease. Thirty-one percent of the patients not receiving HDCT had rapidly progressive disease and died within 1 to 6 months after disease progression. Life-threatening severe toxicity on salvage treatment occurred in 11% of the patients. Insufficient stem cell harvest, poor performance status, and older age had also contributed to ineligibility for HDCT. Moreover, the subgroup of patients never achieving temporary remission with front-line chemotherapy had very often rapid disease progression.
Salvage radiotherapy is a valid treatment alternative in patients who have not been given radiation previously or with relapse outside the initial radiation field. In the present analysis, patients treated with salvage radiotherapy were similar regarding age and Karnofsky status to those receiving chemotherapy, but they more often had early stage progressive disease without B symptoms. The potential increase of pulmonary morbidity associated with mediastinal radiotherapy before HDCT limits the use of radiotherapy as a cytoreductive treatment prior to HDCT.22 Salvage radiotherapy offers a potentially curative option with low morbidity for a subset of selected patients. This approach has been reported in several series mostly for relapsed patients.22-25 Wirth and colleagues reported the experience of salvage radiotherapy in 51 patients with relapsed and refractory HD of whom 18 had a PR and only 4 had PD.24Five-year failure-free survival (FFS) and OS were 26% and 57%, respectively.
In view of the surprisingly good results observed in our study, radiotherapy might be used more frequently as salvage therapy for primary progressive HD. Due to the high percentage (70%) of patients who developed disease progression during first-line treatment, only a minority of patients had received prior radiotherapy. In present studies of first-line treatment of HD, radiotherapy is incorporated less frequently and with lower dosages. Therefore, incorporating salvage radiotherapy in salvage treatment programs seems to be feasible in this group of patients in future trials. Moreover, for patients with localized HD radiotherapy might be a curative treatment approach even for patients with poor performance status or older age or both.
Our results show that HDCT is an effective treatment for selected patients with primary progressive HD. Prognostic factors evaluated at the time of disease progression might help to identify patients for whom either aggressive or palliative treatment strategies should be used.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
References
Author notes
Andreas Josting, First Department of Internal Medicine, University Hospital Cologne, Joseph-Stelzmann-Str. 9, 50924 Cologne, Germany.