Abstract
EDG-6 is a recently cloned member of the endothelial differentiation gene (EDG) G protein-coupled receptor family that is expressed in lymphoid and hematopoietic tissue and in the lung. Homology of EDG-6 to the known sphingosine-1-phosphate (SPP) receptors EDG-1, EDG-3, and EDG-5 and lysophosphatidic acid (LPA) receptors EDG-2 and EDG-4 suggested that its ligand may be a lysophospholipid or lysosphingolipid. We examined the binding of [32P]SPP to HEK293 cells, transiently transfected with cDNA encoding EDG-6. Binding of [32P]SPP was saturable, demonstrating high affinity (KD = 63 nmol/L). Binding was also specific for SPP, as only unlabeled SPP and sphinganine-1-phosphate, which lacks the trans double bond at the 4 position, potently displaced radiolabeled SPP. LPA did not compete for binding of SPP at any concentration tested, whereas sphingosylphosphorylcholine competed for binding to EDG-6, but only at very high concentrations. In addition, SPP activated extracellular signal-regulated kinase (Erk) in EDG-6 transfected cells in a pertussis toxin-sensitive manner. These results indicate that EDG-6 is a high affinity receptor for SPP, which couples to a Gi/o protein, resulting in the activation of growth-related signaling pathways.
Sphingosine-1-phosphate (SPP) is a metabolite of complex sphingolipids that acts as both a second messenger and as a high-affinity ligand for cell surface receptors.1 SPP is produced by sphingosine kinase that is activated in response to a variety of signals, including mitogens such as platelet-derived growth factor (PDGF) and serum,2 G protein–coupled receptor agonists such as carbachol,3 the cytokine TNF-α,4 and ligation of Fc receptors.5Intracellular-formed SPP mediates release of Ca++ from intracellular stores,3,5,6 stimulates several mitogenic and antiapoptotic signaling pathways,7,8 and also contributes to the mitogenic response of fibroblasts to PDGF.2,9 In further support of an intracellular mode of action for SPP, microinjection of SPP mobilizes calcium from internal sources,3 is mitogenic for Swiss 3T3 fibroblasts,9 and inhibits apoptosis of mouse oocytes induced by the antitumor drug doxorubicin.10 Furthermore, overexpression of sphingosine kinase increases intracellular SPP and promotes cell growth and survival.11
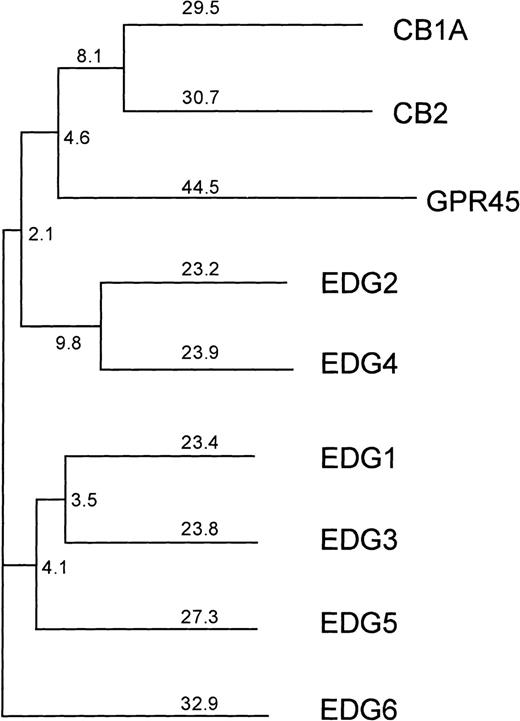
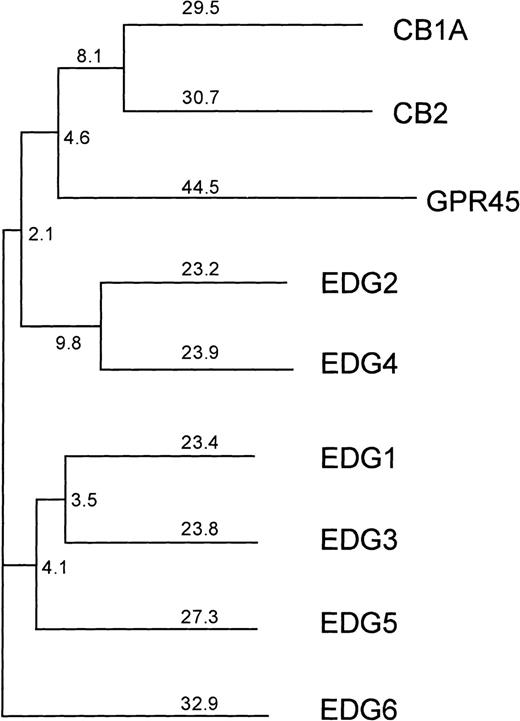
However, because several responses to SPP are at least partially inhibited by pertussis toxin (PTX), which adenosine diphosphate (ADP) ribosylates and specifically inactivates Gi/o proteins, and some require very low concentrations of SPP, it has been suggested that G protein–coupled cell surface receptors (GPCRs) might also be involved (reviewed in Speigel et al12). In agreement, a family of GPCRs, known as the endothelial differentiation gene (EDG) receptors, which specifically bind SPP or the related lipid, lysophosphatidic acid (LPA), has recently been identified.13-18 The EDG family can be divided into 2 subfamilies based on amino acid sequence homology. The subfamily consisting of EDG-1, EDG-3, and EDG-5 display 40% to 45% sequence identity to each other and only 30% to 35% identity to the members of the other subfamily EDG-2 and EDG-4 (Figure1). EDG-1, EDG-3, and EDG-5 have been shown to be SPP receptors,13-16 whereas EDG-2, EDG-4, and EDG-7 are LPA receptors.17-19
Phylogenetic tree of the human lysophospholipid-binding receptors, together with the next closest group of human cannabinoid receptors.
GPR45 is the mammalian orthologue of the Xenopus laevis LPA receptor PSP24.37 The numbers indicate the percentage of divergence. EDG-6 is more closely related to the SPP-binding receptors EDG-1, EDG-3, and EDG-5 than to the LPA-binding receptors EDG-2 and EDG-4.
Phylogenetic tree of the human lysophospholipid-binding receptors, together with the next closest group of human cannabinoid receptors.
GPR45 is the mammalian orthologue of the Xenopus laevis LPA receptor PSP24.37 The numbers indicate the percentage of divergence. EDG-6 is more closely related to the SPP-binding receptors EDG-1, EDG-3, and EDG-5 than to the LPA-binding receptors EDG-2 and EDG-4.
Recently, a new member of the EDG family was cloned and named EDG-6.20 EDG-6 is expressed in lymphoid and hematopoietic tissue as well as the lung.20 Interestingly, EDG-6 does not clearly belong to either the SPP or the LPA subfamily of EDG receptors, as it displays a similar degree of homology to all 5 of the previously identified members. Thus, it was unclear whether EDG-6 is likely to be a receptor for SPP, LPA, or another related lysophospholipid. In this paper, we show that SPP binds specifically to EDG-6 and activates the mitogen-activated protein kinase (MAPK) signal transduction pathway.
Materials and methods
Materials
SPP, sphinganine-1-phosphate (dihydro-SPP), and sphingosylphosphorylcholine (SPC) were purchased from Biomol Research Laboratory Inc (Plymouth Meeting, PA). Lysophosphatidic acid was purchased from Avanti Polar Lipids (Birmingham, AL). γ-[32P]ATP (3000 Ci/mmol) was purchased from Amersham (Arlington Heights, IL). Pertussis toxin (PTX) was from Research Biochemicals International (Natick, MA). Serum and medium were obtained from Biofluids (Rockville, MD). PathDetect Elk trans-Reporting System was from Stratagene (La Jolla, CA).
Cell culture and transfection
Human embryonic kidney cells (HEK293, ATCC CRL-1573) and Chinese hamster ovary cells (CHO-K1) were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum. EDG-6 expression plasmids (RC/CMV containing c-terminal c-myc-tagged or N-terminal hemaglutinin [HA]-tagged EDG-6) were transfected into HEK293 or CHO-K1 cells using Lipofectamine Plus (Life Technologies, Gaithersburg, MD) according to the manufacturer's instructions. The cells were then grown for 2 days to allow expression of receptors before the experiments were performed. In some experiments, cells were cotransfected with pCEFL GFP, which encodes green fluorescent protein. Transfection efficiencies were typically 30% to 35%. CHO-K1 cells stably transfected with c-myc-tagged human EDG-6 were grown in DMEM containing 10% fetal bovine serum and 0.4 g/L G418 sulfate (Biofluids).
Fluorescence-activated cell sorter (FACS) analysis
The human EDG-6 receptor was N-terminal tagged with an HA-epitope (peptide sequence: MGYPYDVPDYAGGP) and C-terminal tagged with a c-myc-epitope. Construction, expression, and flow cytometry analysis were performed as described.21 The N-terminal HA-epitope tag was detected with a fluorescein isothiocyanate (FITC)-labeled anti-HA antibody.
SPP binding assay
[32P]SPP was synthesized enzymatically using recombinant sphingosine kinase as previously described.22Transfected cells were incubated with the indicated concentration of [32P]SPP in 200 μL binding buffer (20 mmol/L Tris-HCl, pH 7.4, 100 mmol/L NaCl, 15 mmol/L NaF, 2 mmol/L deoxypyridoxine, 0.2 mmol/L phenyl-methyl-sulfonyl-fluoride [PMSF], 1 μg/mL aprotinin and leupeptin) for 30 minutes at 4°C. Unlabeled lipid competitors were added as 4 mg/mL fatty acid–free bovine serum albumin (BSA) complexes. Cells were washed twice with ice cold binding buffer containing 0.4 mg/mL fatty acid–free BSA, resuspended in phosphate-buffered saline (PBS), and bound [32P]SPP was quantitated by scintillation counting.14
Extracellular signal-regulated kinase activation
Cells were seeded in 60-mm plates and transfected the following day with EDG-6 expression plasmid and HA-tagged extracellular signal-regulated kinase (Erk)2 (at a 2:1 ratio of HA-Erk2 to EDG-6) with Lipofectamine Plus. After 2 days, cells were treated as indicated and lysed by the addition of 0.5 mL lysis buffer containing 25 mmol/L 4(-2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) pH 7.4, 0.3 mol/L NaCl, 1.5 mmol/L MgCl2, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecylsulfate (SDS), 0.5 mmol/L dithiothreitol (DTT), 20 mmol/L β-glycerophosphate, 0.2 mmol/L ethylenediaminetetraacetic acid (EDTA), 1 mmol/L Na3VO4, 1 mmol/L PMSF, and 10 μg/mL leupeptin for 10 minutes on ice. Lysates were centrifuged 15 minutes at 4°C. Anti-HA (2 μg) (Santa Cruz Biotechnology) was then added to lysates (800 μg protein) and incubated 2 hours at 4°C with rocking. Protein A/G Sepharose beads (Santa Cruz Biotechnology) (20 μL) were added and the incubation continued for an additional hour. The beads were pelleted and washed 3 times in lysis buffer and twice in kinase buffer (12.5 mmol/L HEPES pH 7.4, 10 mmol/L MgCl2, 0.5 mmol/L DTT, 12.5 mmol/L β-glycerophosphate, 0.5 mmol/L NaF, 0.5 mmol/L Na3VO4). The kinase assay was initiated by resuspending the beads in 50 μL of kinase buffer containing 50 μmol/L adenosine triphosphate (ATP), 0.5 mg/mL myelin basic protein (MBP), and 5 000 dpm/pmol γ-[32P]ATP, and incubating 20 minutes at 30°C. The reaction was stopped by the addition of 12 μL of 6 ×-concentrated Laemmli sample buffer and the samples were boiled 5 minutes, separated on 12% SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to nitrocellulose. Nitrocellulose membranes were stained with Ponceau S (Sigma) to visualize protein bands and then exposed to film for autoradiography. Radioactivity was measured in a scintillation counter after cutting the radioactive bands. In some experiments, for the determination of Erk 1/2 phosphorylation, 15 μg of clarified whole cell lysate were resolved by 10% SDS-PAGE, and Erk 1/2 phosphorylation was detected by protein immunoblotting with rabbit polyclonal phospho-specific MAPK IgG (1:1 000, Promega), followed by horseradish peroxidase (HRP)-conjugated goat antirabbit IgG (1:10 000; Amersham Pharmacia Biotech) as a secondary antibody. Erk 1/2 phosphorylation was detected by ECL (Amersham Pharmacia Biotech). Nitrocellulose membranes were stripped and reprobed using rabbit polyclonal anti-Erk2 IgG (Santa Cruz Biotechnology) to confirm equal loading.
Elk1-dependent transcription of a luciferase reporter
For the detection of Elk1-dependent transcription, the PathDetect in vivo signal transduction pathway reporting system (Stratagene) and the Dual-Luciferase reporter assay system (Promega) were used. CHO-K1 cells were seeded in 6-well plates and cotransfected the following day with 50 ng of the fusion activator plasmid pFA-Elk (Stratagene), 500 ng of the firefly luciferase reporter vector pFR-Luc (Stratagene), 100 ng of the Renilla luciferase control reporter vector (Promega), and 50 ng of 1 of the plasmids, pcDNA3.1(+) or pcDNA3.1(+) containing the human EDG-6 receptor or the human EDG-1 receptor. After 30 to 40 hours, cells were washed with PBS and serum-free medium was added. Two to 3 hours later, the cells were stimulated with SPP and incubated for an additional 5 to 6 hours. The medium was removed and the cells were incubated in 300 μL passive lysis buffer (Promega) for 30 minutes at room temperature. Luminescence of 20 μL aliquots was measured with the Berthold Luminat LB 9507 for 10 seconds after injection of 50 μL each of luciferase assay buffer II and Stop & Glo buffer (Promega).
Results
Overexpression of EDG-6 in HEK293 cells
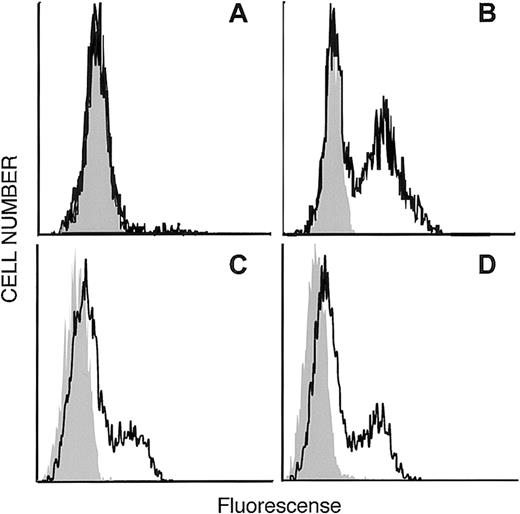
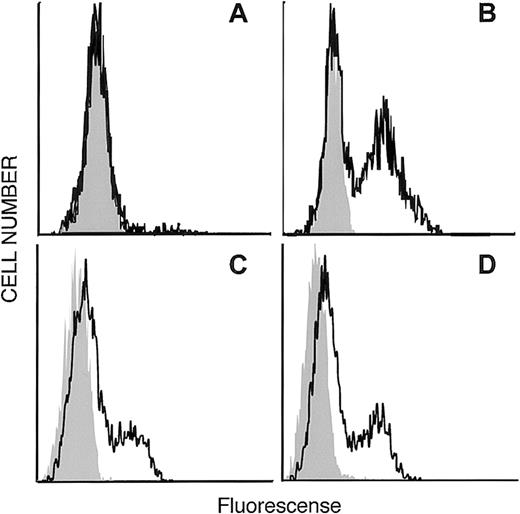
Figure 1 shows a phylogenetic tree depicting the relationship of EDG-6 to other EDG family members, as well as the next most closely related group of receptors, the cannabinoid receptors. The LPA-receptors EDG-2 and EDG-4, as well as the SPP-receptors EDG-1, EDG-3, and EDG-5, are clearly distinct from each other and form their own subgroups. Within these 2 EDG-subgroups, EDG-6 has a slightly higher homology to the first and the seventh transmembrane domains of the SPP subgroup than to the LPA subgroup. EDG-6 has a 44% overall identity to EDG-1, 46% to EDG-3, and 42% to EDG-5, and less homology to the LPA subgroup, 39% to EDG-4 and 37% to EDG-2.20These homologies suggest that EDG-6 could be an SPP receptor. To examine this possibility, we transfected HEK293 cells, which do not express EDG-6, as determined by RT-PCR (data not shown), and have no detectable binding sites for SPP,9 14 with C-terminal c-myc epitope tagged or N-terminal HA-tagged human EDG-6 cDNA. Expression of EDG-6 protein on the cell surface was examined by flow cytometric analysis with anti–c-myc or anti-HA antibodies. FACS analysis showed that intact HEK293 cells transfected with EDG-6-myc were indistinguishable from untransfected cells (Figure 2A). However, when EDG-6-myc–transfected HEK293 cells were permeabilized, a distinct shift in the fluorescence was detected, indicating that permeabilization allowed access of the c-myc antibody to the C-terminus that is located at the cytoplasmic tail of EDG-6 (Figure2B). HEK293 cells transfected with N-terminal HA-tagged EDG-6 showed a shifted fluorescence without permeabilization (Figure 2C), indicating cell surface expression of HA-tagged EDG-6, as the N-termini of G protein–coupled receptors are located extracellularly. Interestingly, this peak was also slightly enhanced by cell permeabilization (Figure2D), suggesting that some HA-tagged EDG-6 may be expressed intracellularly.
Expression of the N-terminal HA-tagged and the C-terminalmyc-epitope–tagged human EDG-6 receptor . (A, C) Intact and (B, D) permeabilized HEK293 cells transfected with C-terminalmyc-epitope–tagged human EDG-6 (A, B) or with N-terminal HA-tagged human EDG-6 (C, D). FACS analysis was performed as described in “Materials and methods.” Grey areas indicate the signal without the first antibody (A, B) or autofluorescence of unstained cells (C, D), respectively.
Expression of the N-terminal HA-tagged and the C-terminalmyc-epitope–tagged human EDG-6 receptor . (A, C) Intact and (B, D) permeabilized HEK293 cells transfected with C-terminalmyc-epitope–tagged human EDG-6 (A, B) or with N-terminal HA-tagged human EDG-6 (C, D). FACS analysis was performed as described in “Materials and methods.” Grey areas indicate the signal without the first antibody (A, B) or autofluorescence of unstained cells (C, D), respectively.
Binding of SPP to EDG-6
Having established that human EDG-6 is expressed on the surface of transiently transfected HEK293 cells, it was of interest to determine whether SPP binds to EDG-6. In agreement with previous reports,9,13 14 no specific SPP binding was detected in HEK293 cells transfected with the vector alone, whereas HEK293 cells transfected with c-myc–tagged human EDG-6 (HEK293–EDG-6) displayed dramatically increased binding of [32P]SPP, which was competed by 1000-fold molar excess of either unlabeled SPP or dihydro-SPP to a level similar to that seen in untransfected cells (Figure 3). Neither SPC nor LPA effectively competed with [32P]SPP for binding to HEK293–EDG-6 cells at 1000-fold excess (1 μmol/L). A computer curve fitting of binding isotherms indicated that SPP binding to EDG-6 was saturable and displayed moderately high affinity (KD = 63 nmol/L) (Figure 4).
SPP binds specifically to HEK293 cells expressing EDG-6. HEK293 cells were transiently transfected with an expression plasmid containing the EDG-6 open reading frame (solid bars) or with vector alone (white bars); and binding of 0.5 nmol/L [32P]SPP was measured in the absence (total) or presence of a 1000-fold excess of the indicated lipids as competitors as described in “Materials and methods.” Results are means ± SD of triplicate determinations. Similar results were obtained in at least 3 independent experiments.
SPP binds specifically to HEK293 cells expressing EDG-6. HEK293 cells were transiently transfected with an expression plasmid containing the EDG-6 open reading frame (solid bars) or with vector alone (white bars); and binding of 0.5 nmol/L [32P]SPP was measured in the absence (total) or presence of a 1000-fold excess of the indicated lipids as competitors as described in “Materials and methods.” Results are means ± SD of triplicate determinations. Similar results were obtained in at least 3 independent experiments.
SPP binds to EDG-6 with high affinity . HEK293 cells were transfected with EDG-6 and specific binding of [32P]SPP at the indicated concentrations was determined. “Specific binding” is binding in the absence of unlabeled SPP minus binding in the presence of excess unlabeled SPP. Results are means ± SD of triplicate determinations. The binding curve and the KD (63 nmol/L) were calculated using Deltagraph for Macintosh.
SPP binds to EDG-6 with high affinity . HEK293 cells were transfected with EDG-6 and specific binding of [32P]SPP at the indicated concentrations was determined. “Specific binding” is binding in the absence of unlabeled SPP minus binding in the presence of excess unlabeled SPP. Results are means ± SD of triplicate determinations. The binding curve and the KD (63 nmol/L) were calculated using Deltagraph for Macintosh.
Because many studies indicate that SPC and LPA can also bind to SPP receptors, we examined whether SPC or LPA might bind to EDG-6 with very low affinity. Competition binding experiments were performed using up to 10 μmol/L unlabeled lipids. As expected, SPP competed for [32P]SPP binding very effectively, with 50% of the total binding competing at 46 nmol/L unlabeled SPP (Figure5), close to the measured KD. Dihydro-SPP was less effective than SPP, competing 50% of the total binding at 210 nmol/L. SPC was able to compete for binding of [32P]SPP, but only at very high concentrations in the micromolar range (Figure 5B). In contrast, the related lysophospholipid LPA did not compete for [32P]SPP binding to HEK293–EDG-6 cells at any concentration tested.
Specificity of lipid binding to EDG-6 . HEK293 cells were transfected with EDG-6 and binding of 0.5 nmol/L [32P]SPP was determined in the presence of the indicated concentrations of unlabeled lipid competitors. Results are means ± SD of triplicate determinations. Competition curves were fit and Kis calculated using Deltagraph program for Macintosh. Below, shown are the Ki values and correlation coefficients (r2) of the curve fits for SPP, dihydro-SPP, and SPC. LPA did not compete at any concentration tested.
Specificity of lipid binding to EDG-6 . HEK293 cells were transfected with EDG-6 and binding of 0.5 nmol/L [32P]SPP was determined in the presence of the indicated concentrations of unlabeled lipid competitors. Results are means ± SD of triplicate determinations. Competition curves were fit and Kis calculated using Deltagraph program for Macintosh. Below, shown are the Ki values and correlation coefficients (r2) of the curve fits for SPP, dihydro-SPP, and SPC. LPA did not compete at any concentration tested.
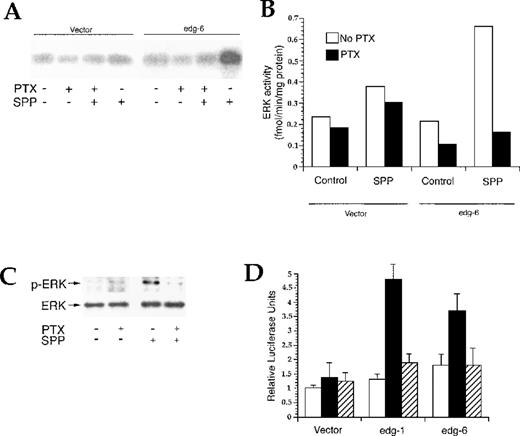
Binding of SPP to EDG-6 activates Erk
The MAPK Erk is activated by a wide variety of G protein–coupled receptors,23 including the known SPP receptors, EDG-1,13,15 EDG-3,24 and EDG-5.25 To determine whether EDG-6 also activates Erk, HEK293 cells were cotransfected with EDG-6 and HA-tagged Erk2. SPP, markedly enhanced myelin basic protein phosphorylation in anti–HA-Erk2 immunoprecipitates from EDG-6 transfected HEK293 cells. However, Erk2 was also stimulated by SPP in vector-transfected cells, albeit to a lesser degree (data not shown). To circumvent the activation of Erk by SPP in untransfected HEK293 cells, which could be due to the presence of EDG-3 and EDG-5,2,9,13,26 we used CHO-K1 cells, which only express low levels of EDG-524 and do not show Erk activation by SPP.24,25 Moreover, CHO cells have previously been used successfully to examine Erk activation by the binding of SPP to cells overexpressing EDG-1, EDG-3, or EDG-5.24,25,27 Expression of EDG-6 in CHO-K1 cells in the absence of SPP did not stimulate Erk, whereas SPP markedly activated HA-Erk2 in these cells, and as expected, SPP had only a slight effect on Erk2 activity in vector-transfected CHO-K1 cells (Figure 6A). Pretreatment with PTX completely eliminated SPP-stimulated Erk2 activity (Figure 6A). Moreover, in CHO-K1 cells stably expressing EDG-6, 100 nmol/L SPP activated endogenous Erk, as indicated by enhanced Erk phosphorylation (Figure 6C). SPP-stimulated Erk phosphorylation was completely PTX-sensitive. To examine whether Erk activation resulted in increased transcription, we used CHO-K1 cells and the PathDetect Elk trans-Reporting System, which detects Elk1-dependent transcription of a luciferase reporter. An advantage of this system is that the GAL4 DNA binding domain is a yeast transcriptional activator that is not recognized by mammalian transcription factors and thus yields very low backgrounds. Indeed, SPP did not enhance luciferase reporter activity in CHO cells transfected with empty pcDNA3.1 vector (Figure 6D), in agreement with the low endogenous expression of SPP EDG receptors in these cells. In contrast, SPP significantly increased luciferase activity after transfection with human EDG-1 or human EDG-6. Thus, similar to EDG-1,13 15 EDG-6 also mediates SPP-induced MAPK activation, leading to phosphorylation and activation of Elk1.
Activation of Erk by binding of SPP to EDG-6. (A) CHO-K1 cells were transiently cotransfected with an HA-tagged Erk2 and EDG-6 or an empty vector. Cells were treated without or with 200 ng/mL PTX for 3 hours, then stimulated with vehicle or 100 nmol/L SPP for 5 minutes. HA-Erk2 was immunoprecipitated from whole cell lysates and assayed for kinase activity using MBP as a substrate as described in “Materials and methods.” Results are typical of 2 independent experiments. (B) 32P incorporation into MBP was determined by scintillation counting. Data are expressed as picomole per minute per milligram and are the means of 2 separate experiments. White bars indicate the absence of PTX; solid bars, the presence of PTX. (C) CHO-K1 cells stably transfected with EDG-6 were treated without or with PTX for 3 hours, then stimulated with vehicle or 100 nmol/L SPP for 5 minutes, and Erk activation was determined by Western blot analysis with phospho-specific anti-Erk antibody. Blots were then stripped and reprobed with Erk antibody to determine total Erk levels. (D) Binding of SPP to EDG-6 stimulates Elk-1. CHO-K1 cells were cotransfected with the reporter plasmid pFR-Luc and the pcDNA vector alone or with either EDG-6 or EDG-1 inserts. Cells were treated without (white bars) or with 1 μmol/L SPP (solid and hatched bars) in the absence (solid bars) or presence of PTX (hatched bars) for 5 to 6 hours, and luciferase activity was measured as described in “Materials and methods.” Data are means ± SEM.
Activation of Erk by binding of SPP to EDG-6. (A) CHO-K1 cells were transiently cotransfected with an HA-tagged Erk2 and EDG-6 or an empty vector. Cells were treated without or with 200 ng/mL PTX for 3 hours, then stimulated with vehicle or 100 nmol/L SPP for 5 minutes. HA-Erk2 was immunoprecipitated from whole cell lysates and assayed for kinase activity using MBP as a substrate as described in “Materials and methods.” Results are typical of 2 independent experiments. (B) 32P incorporation into MBP was determined by scintillation counting. Data are expressed as picomole per minute per milligram and are the means of 2 separate experiments. White bars indicate the absence of PTX; solid bars, the presence of PTX. (C) CHO-K1 cells stably transfected with EDG-6 were treated without or with PTX for 3 hours, then stimulated with vehicle or 100 nmol/L SPP for 5 minutes, and Erk activation was determined by Western blot analysis with phospho-specific anti-Erk antibody. Blots were then stripped and reprobed with Erk antibody to determine total Erk levels. (D) Binding of SPP to EDG-6 stimulates Elk-1. CHO-K1 cells were cotransfected with the reporter plasmid pFR-Luc and the pcDNA vector alone or with either EDG-6 or EDG-1 inserts. Cells were treated without (white bars) or with 1 μmol/L SPP (solid and hatched bars) in the absence (solid bars) or presence of PTX (hatched bars) for 5 to 6 hours, and luciferase activity was measured as described in “Materials and methods.” Data are means ± SEM.
Discussion
EDG-6 displays high homology (37%-46% amino acid identity) to the previously identified members of the EDG family of G protein–coupled receptors. Although by homology it cannot be clearly grouped with either the EDG-1, EDG-3, and EDG-5 subfamily, which bind SPP, or the EDG-2 and EDG-4 subfamily, which bind LPA, evidence presented here indicates that EDG-6 is an SPP receptor. SPP binds specifically to EDG-6 expressed on HEK293 cells, although with a lower affinity (63 nmol/L) than to EDG-1 (8 nmol/L),13EDG-3 (23 nmol/L), or EDG-5 (27 nmol/L).14 Thus, residues conserved among EDG-1, EDG-3, and EDG-5 but not in EDG-6 may contribute to the increased affinity of SPP binding. Nevertheless, the affinity of EDG-6 for SPP is high enough to indicate that SPP could be a physiologically relevant ligand for EDG-6, as the concentration of SPP in plasma and serum is about 200 nmol/L and 500 nmol/L, respectively.28
Dihydro-SPP, which binds to EDG-1, EDG-3, and EDG-5 as potently as SPP, also binds to EDG-6, although with approximately a 5-fold lower affinity than SPP. Interestingly, SPC binds to EDG-6 with a 100-fold lower affinity than SPP (4.6 μmol/L). Similarly, SPC was recently shown to mobilize Ca++ in Xenopus oocytes expressing EDG-1, EDG-3, or EDG-5 at low micromolar concentrations, whereas SPP was active at nanomolar concentrations.29 Moreover, SPC induced increased [Ca++]i in HEL cells expressing EDG-127 or EDG-3,24 and in K562 cells expressing EDG-5,25 in each case with approximately a 100-fold lower efficacy than SPP. Thus, SPC may be a low affinity agonist for all 4 of the currently identified EDG SPP receptors. However, it is also possible that these effects were not mediated by SPC itself, as it was recently found that commercial preparations of SPC are contaminated with highly potent alkenyl-glycero-3-phosphates.30 In contrast, SPC activated IK(Ach) in guinea pig atrial myocytes with EC50s of 1.5 nmol/L.31 Moreover, SPC activates signaling pathways different from those stimulated by SPP.32 33 Thus, another sphingolipid receptor may exist that has a high affinity for SPC.
Binding of SPP to EDG-6 activates Erk, leading to the activation of the transcription factor Elk1. Activation of the MAPK Erk2 by EDG-6 in CHO-K1 cells was sensitive to PTX, suggesting that this response is mediated through a Gi/o protein. Similarly, EDG-1, EDG-3, and EDG-5 have all been shown to link to PTX-sensitive G proteins to mediate MAPK activation.13,24 25 However, all 3 previously described EDG SPP receptors have also been shown to have PTX-insensitive effects as well, suggesting that EDG receptors can couple to multiple G proteins.
EDG-6 is expressed mainly in lymphocytes that would therefore be constantly exposed to high concentrations of SPP in serum. Thus, SPP signaling through EDG-6 may be maintained at a constant moderate level. The increase in release of SPP seen when platelets are activated may further enhance EDG-6 signaling. However, it is possible that EDG-6 signaling may be down-regulated by constant exposure to SPP because it has previously been shown that ligand binding to GPCRs induces internalization of receptors34 and SPP induces rapid trafficking of EDG-1.13 26 It remains to be determined whether regulation of surface expression of EDG-6 is an important mechanism in determining how subsets of resting or activated lymphocytes respond to different SPP levels in the periphery or in tissue.
Although the biologic significance of SPP signaling through EDG-6 in lymphocytes and dendritic cells is poorly understood, the well-characterized growth-related or cytoskeleton-associated activities of SPP suggest that EDG-6 could be involved in various immunologic responses. Recently, it was shown that SPP protects human T-lymphoblastoma cells, which express EDG-3 and EDG-5, from apoptosis induced by Fas or ceramide.35 Moreover, invasion of T-lymphoma cells into a fibroblast monolayer is dependent on SPP receptor-mediated RhoA and phospholipase C signaling pathways that lead to pseudopod formation and enhanced infiltration.36 Thus, members of the EDG family may synergize with signaling pathways initiated by cytokines. Lysosphingophospholipids may play a critical role as potent autocrine and paracrine mediators in specific microenvironmental settings of normal and pathophysiologic immune responses.
Acknowledgment
We thank Dr S. Milstien for helpful discussion.
Supported by research grants from the National Institutes of Health (GM43880 and CA61774) to S.S. and a postdoctoral fellowship (1 F32 GM19209-01A1) to J.V.B.
J.R.V.B. and M.H.G. contributed equally to this study.
Reprints:Sarah Spiegel, Department of Biochemistry and Molecular Biology, Georgetown University Medical Center, 353 Basic Science Bldg, 3900 Reservoir Rd, NW, Washington, DC 20007; e-mail: spiegel@bc.georgetown.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Fig. 3. SPP binds specifically to HEK293 cells expressing EDG-6. HEK293 cells were transiently transfected with an expression plasmid containing the EDG-6 open reading frame (solid bars) or with vector alone (white bars); and binding of 0.5 nmol/L [32P]SPP was measured in the absence (total) or presence of a 1000-fold excess of the indicated lipids as competitors as described in “Materials and methods.” Results are means ± SD of triplicate determinations. Similar results were obtained in at least 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2624/4/m_bloo00833003x.jpeg?Expires=1768085481&Signature=Qy0LqJvau6wmxBvxu9eRSPPavjywn862TaWlKc8pGu7oSGe4uNLsn7nsqe~E8UAYFG9Xed0~y3lorygP5HR3rnhyQUfYZowZGqsqbjw-FE9RKcBHU-2~FOvumq3gclJAuK8ge18UXy9L0l4w5Jm2cPiYtVnbrAVe-822ukyLl2cj7qybnPZXEMd5crzvJ~XJ01SWOkJAZ~NNfH5UoFa6JiyRh5nYCDOzW-Gr1dzrPDVXElgi89lklOirm1AlybXzmS80Dpc5Eif4y16xJSXuTQ9CPQ9vOVqqJ1b2SpMHwyVCEMGp~LkmS0A6WjKFP-sCLMU9l9ubqGHdw4AvGmkO2w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. SPP binds to EDG-6 with high affinity . HEK293 cells were transfected with EDG-6 and specific binding of [32P]SPP at the indicated concentrations was determined. “Specific binding” is binding in the absence of unlabeled SPP minus binding in the presence of excess unlabeled SPP. Results are means ± SD of triplicate determinations. The binding curve and the KD (63 nmol/L) were calculated using Deltagraph for Macintosh.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2624/4/m_bloo00833004x.jpeg?Expires=1768085481&Signature=q3WIqwvIIFOzcU7~KDoQ-SwmZ92Z8XzgapS6Bsrr1KR8MdUoruua4cWl5QLJFZlVvHcSTQ-BmchZbmiYiYNGafkKL~x~BcCmwkl3F4siyLfeYaYowyrMmjKpsdpiIhwU12wMXX98gGfHtC65yzxx~dX~c0Vyc82Nexp~tDrC-nDfpxQ6OGzMKJBOQJz8t8zNS4RTc55xHAbwj86cQjcgJ9F55HEsF~kRVgeRVKrVaSVBYW1tO2pVSt1Rfc9ZfqkRaWZPuFVF~-g0VlOQYu-T-B2g7YvN7C3ul9IpAAfcssHVvioJThbTsvZH8bzl~hA88tAHs9iWaAb~dvKbetHWXQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Specificity of lipid binding to EDG-6 . HEK293 cells were transfected with EDG-6 and binding of 0.5 nmol/L [32P]SPP was determined in the presence of the indicated concentrations of unlabeled lipid competitors. Results are means ± SD of triplicate determinations. Competition curves were fit and Kis calculated using Deltagraph program for Macintosh. Below, shown are the Ki values and correlation coefficients (r2) of the curve fits for SPP, dihydro-SPP, and SPC. LPA did not compete at any concentration tested.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2624/4/m_bloo00833005x.jpeg?Expires=1768085481&Signature=CN2XSTHHRDpa~jYvsDPAJTFspwJ0EJj0m976~VabCXkbMAD-WlbCEIilMRXvEkcay9kO2aPKylpxzzDYjTWcXmCLmbrt4zlZtCmOfqWZi18Q8CYffTumGoo6SOBUq7t9fqJ~rXd1obiYwu2fVyDZkhZF2VhNAr9T~2ZuIJJ0b2stMv7PD1WzxdKi10tnnL-FnbjSmoccUFfOj5as5Fc7ooUz28XWEWvz-LXn-rDeoFYIoKbtldoY3Mfa9bLwnpT-aEMWSswN~xfRppupKoQ6FTxq4WypZzX0JpSBNZdW7xllGv605ZN4Ix-tqfm83gsYYnhDJSR7AjorHakIxK4oYA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 3. SPP binds specifically to HEK293 cells expressing EDG-6. HEK293 cells were transiently transfected with an expression plasmid containing the EDG-6 open reading frame (solid bars) or with vector alone (white bars); and binding of 0.5 nmol/L [32P]SPP was measured in the absence (total) or presence of a 1000-fold excess of the indicated lipids as competitors as described in “Materials and methods.” Results are means ± SD of triplicate determinations. Similar results were obtained in at least 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2624/4/m_bloo00833003x.jpeg?Expires=1768085482&Signature=ed6DcrTfYY1uv1wUNyvUD-1-abDq5w~6CFv75jXAb5JtcS-JCGY5sH1YJ-N3Zsh1mAkMa8pZxoWtKwPqVprDESDqT~86diFV3JX0cyGb07Iv6rbqXBHSMRbUs77lQ3Gh2sdblqZgNuTCypTw1s3p~QKY5TyP4EePYqG898xquMfO~AU2GIYtyTEZg0zNh7CL8LbnDqYLU8vE1~4f2vKBy8zIvB-a1xzbsRxDvwcmPD6e81Yp0pZoSVzlsh2OBdNATOtmvzIgjuVDFduu647~hRsvHdlhKXWZP2nAccQp9baLGHKZ6AhaaVUnrXr9hCGIW7JlOOPf~xl47GBTL2LMAg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. SPP binds to EDG-6 with high affinity . HEK293 cells were transfected with EDG-6 and specific binding of [32P]SPP at the indicated concentrations was determined. “Specific binding” is binding in the absence of unlabeled SPP minus binding in the presence of excess unlabeled SPP. Results are means ± SD of triplicate determinations. The binding curve and the KD (63 nmol/L) were calculated using Deltagraph for Macintosh.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2624/4/m_bloo00833004x.jpeg?Expires=1768085482&Signature=f2YBisVGurYm6YCWKMoDGZ0yqqIAAU0TctT2Gg6jdZ4CTD0hvJBgYL5-UJGbaFlUhXmCBF6war~A3trbgDEwyhqDISWWs~jtqUlVb-Q9BYBhM4GUE1B3It5c-J0bQcUsLvwkcRfiQ9NVeEJKAz09kCj1Zf3G3RTOfE6wZlBilSxKZ9-7uiWVaeiDRgMAAfOll2S2~Sj8EKxwFMOqO9jAp~BZR0o5~MPCop4FaNPzjoF6BJ76EgA9Cv-LXMJNw488n0-M94cmnr099CMrT0LHGMAhNUVZ21yw1OkGK-RPQts6Zu~NdJsv5uQ5wj44RdwZ4RCA7SflZ83GNB-ytoUTXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Specificity of lipid binding to EDG-6 . HEK293 cells were transfected with EDG-6 and binding of 0.5 nmol/L [32P]SPP was determined in the presence of the indicated concentrations of unlabeled lipid competitors. Results are means ± SD of triplicate determinations. Competition curves were fit and Kis calculated using Deltagraph program for Macintosh. Below, shown are the Ki values and correlation coefficients (r2) of the curve fits for SPP, dihydro-SPP, and SPC. LPA did not compete at any concentration tested.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/95/8/10.1182_blood.v95.8.2624/4/m_bloo00833005x.jpeg?Expires=1768085482&Signature=PgxAzhKERz1Tecpoym9QdAlJuoaBOwSi1RB05wqLQWD7RGMg6fxJpk-0mjZ7kuo89CyfyJ2NSQutBUAkbBvYmWkn-aQEbVZb-GbneCcCljit~9203n-pDSqy-u6IJTAjnHg7TWx9Okp9-rW4MwPyU6Zms~xL~Zqcmp~NWRwrSsRt~wWWnlaR7Y-TEMPnaWZ1l7GzSdumh3uJNnhX5G7hYUL8qHeM~9fSHA5C59I8Dzztg1w3Z8y8uv1RyghYCBxcICQ750KtcpasQNhoUvQPln1KhZvTy9p85DGkL4zId0Ds1yVUY~LCvl1hX4RWvJYDFFO-isFThrCGVjMWQebQ5A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)