Abstract
Human fibrinogen-420, (Eβγ)2, was isolated from plasma and evaluated for its ability to form clots and for its susceptibility to proteolysis. Clotting parameters, including cross-linking of subunit chains, of this subclass and of the more abundant fibrinogen-340 (βγ)2, were found to be similar, suggesting little impact of the unique EC domains of fibrinogen-420 on coagulation. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis of plasmic digestion patterns revealed production from fibrinogen-420 of the conventional fibrinogen degradation products, X, Y, D, and E, to be comparable to that from fibrinogen-340 in all respects except the presence of at least 2 additional cleavage products that were shown by Western blot analysis to contain the EC domain. One was a stable fragment (ECX) comigrating with a 34-kd yeast recombinant EC domain, and the other was an apparent precursor. Their release occurred early, before that of fragments D and E. Two bands of the same mobility and antibody reactivity were found in Western blots of plasma collected from patients with myocardial infarction shortly after the initiation of thrombolytic therapy.
Fibrinogen, the precursor of the fibrin clot, is composed of paired sets of 3 types of chains: α, β, and γ. These chains share amino terminal homologies, but the long, random coil at the carboxyl terminus of the conventional α chain (αC) is distinct from the related globular domains at the ends of the conventional β and γ chains (βC and γC, respectively). However, in a subclass of fibrinogen molecules, accounting in humans for 1% to 3% of the total,1 a globular C-terminal domain (αEC), comparable to βC and γC, further elongates the α chain to form an extended α chain isoform (αE).2,3 The αEC, βC, and γC domains share approximately 40% amino acid identity,2 and the folds of their α-carbon backbones are largely superimposable, as recently shown by x-ray crystallography.4-7
The human αE and conventional α chains are identical in sequence through the length of the αC region to Val610, but in αE this valine is followed by Arg611 and the 236 residues encoded by the α gene's 6th exon (Asp612–Gln847)2 that form the globular domain. This αEC domain is the only region of any normal α chain to be glycosylated in human fibrinogen.3,8 From sequence analyses, it is now known that the αE isoform occurs throughout the vertebrate kingdom, and that the αEC domain is the single largest conserved portion of the chain.9-11
Both conventional fibrinogen and αE-containing fibrinogen are symmetrical and heavily disulfide-bonded hexamers.3,8,12 13 We have introduced nomenclature based on size for these 2 human fibrinogen subclasses: fibrinogen-340 for the conventional 340-kd (αβγ)2 form and fibrinogen-420 for (αEβγ)2 with its predicted mass of 420 kd.
The general spatial arrangement of the fibrinogen subunits has been well studied, and the molecule's primary functioning in clot formation and lysis has been characterized in detail.12 13 Briefly, the fibrinogen chain amino termini are clustered in a central E-domain from which 2 coiled coil regions emerge, each ending in a distal carboxyl terminal D-domain that contains the βC and γC globular domains and an interior region of the α chain. A clot begins to form when thrombin cleaves fibrinopeptides A and B from the amino termini of fibrinogen's α and β chains, thereby generating a fibrin monomer. Each newly exposed α-chain N-terminus spontaneously interacts with a complementary polymerization pocket located in the carboxyl-terminal domain of a γ chain from 2 other fibrin molecules, noncovalently associating the fibrin monomers in a half-staggered, double array of protofibrils. Thus organized, the polymers become covalently stabilized by the thrombin-activated transglutaminase, factor XIIIa, which catalyzes establishment of ε-amino-(γ-glutamyl) lysine cross-links between γ chain carboxy termini and, to a lesser extent, between α chains. Both formation and cross-linking of fibrin polymers are promoted by calcium ions, the binding of which also limits susceptibility of fibrin(ogen) to proteases such as plasmin. Under attack by plasmin and other proteases, fibrin clots are ultimately depolymerized, restoring plasma fluidity.
A distinctive role for fibrinogen-420 has yet to be elucidated. Properties of the αEC domains have been explored in mutation studies of recombinant fibrinogen-420 assembly in COS cells8 and in biochemical and structural analyses with a recombinant human αEC domain (rαEC) expressed in the yeast Pichia pastoris.6,14 Like βC and γC,4,7 the αEC domain has a calcium-binding site.6,14 However, in lieu of the negatively charged pockets of βC and γC that allow fibrin monomer polymerization,7,15 αEC has a cleft with neutral residues at its center.6
Physical separation of fibrinogen-420 from fibrinogen-340 molecules in plasma has presented a formidable challenge because of their disproportionate representation and their similarity of structure but for the αEC domains of fibrinogen-420. In the current article, we describe a procedure for the isolation of highly purified fractions of fibrinogen-420 and of fibrinogen-340 from human plasma. We verify fibrinogen-420's native structure as a symmetrical molecule. We show that both species clot in similar fashion and that their degradation by plasmin yields comparable patterns of proteolysis, with 1 notable exception: the conserved αEC domain is released from fibrin(ogen)-420 as a stable cleavage product (αECX).
Materials and methods
Materials
Human umbilical cord plasma was donated through the Placental Blood Program of the New York Blood Center under the direction of Pablo Rubinstein. Blood collection and plasma processing for these samples have been described elsewhere.1,16 Plasma samples from patients undergoing thrombolytic therapy with recombinant tissue plasminogen activator or streptokinase were generously provided by Joan Sobel (Columbia Presbyterian Medical Center, NY). The samples were archival, originating from The TIMI Study Group's Phase 1 Trial.17
Human plasmin and α-thrombin were generous gifts of Michael Mosesson (Sinai Samaritan Medical Center, Milwaukee, WI). Recombinant factor XIII was graciously provided by Paul Bishop (ZymoGenetics, Seattle, WA).
Rabbit anti-fibrinogen was purchased from DAKO Corporation (Carpenteria, CA). Rabbit anti-αEC #9395, also known as anti-VI, was generated against a recombinant human αEC domain expressed in Escherichia coli; it has been described previously.2,3 Rabbit anti-α(615-625), a gift from Russ Doolittle (University of California at San Diego, La Jolla, CA), was generated against the synthetic peptide TSPLGKPSLSP. This sequence corresponds to the carboxyl-terminal residues of the α(1-625) chain18,19 before it is processed to the predominant plasma form α(1-610).20 On Western blots, the antibody reacted strongly with fibrinogen in spent medium from HepG2 culture, obtained as described previously 3; these cells are known to secrete a significant proportion of α(1-625)-containing fibrinogen.21
Mouse monoclonal anti-αEC #29-1 was also generated against a recombinant human αEC expressed in E coli and is specific for an epitope at the domain's C-terminus.14 Monoclonal anti-α(603-610) antibody F-48, kindly provided by Gary Matsueda (Bristol–Myers Squibb, Princeton, NJ), was generated against the synthetic octapeptide, GHAKSRPV, representing the common α chain carboxyl terminus; it is specific for processed but nondegraded α chains in plasma fibrinogen.22 Monoclonal anti-α chain antibody, 1D4, supplied by Bohdan Kudryk (New York Blood Center, New York, NY), has been described previously.23 24
Column chromatography
Human fibrinogen (fraction I-2) was prepared from umbilical cord plasma according to Mosesson and Sherry25 and dialyzed against 0.005 mol/L Tris phosphate, pH 8.6. (In all Tris phosphate buffers, the molarity refers to phosphate26.) The material (30 ml at a concentration of 4 mg/ml) was applied to a Mono Q HR 10/10 anion exchange column (Pharmacia, Piscataway, NJ) that previously had been equilibrated with the same buffer. Bound protein was eluted using a stepwise gradient starting from 0.005 mol/L Tris phosphate, pH 8.6, to a final 0.5 mol/L Tris phosphate, pH 4.2. Eluted protein was collected in 2.5-mL fractions. For storage and further analysis, pooled fractions were either dialyzed against 125 mmol/L NaCl, 25 mmol/L HEPES (pH 7.4) or concentrated and exchanged to the buffer using a YM10 ultrafiltration membrane within an Amicon stirred cell (Amicon, Beverly, MA).
SDS-PAGE and Western blot analysis
Samples were prepared for electrophoresis in Laemmli sample buffer in the absence or presence of 0.1 mol/L dithiothreitol27and were separated on SDS-PAGE using a Mini-Protean II Electrophoresis Cell (Bio-Rad, Hercules, CA). Protein was stained with Gel Code Blue Stain Reagent (Pierce, Rockford, IL). Electrophoretic transfer onto 0.2-μm nitrocellulose membranes was performed with a Mini Trans-Blot Cell (Bio-Rad). Membranes were incubated with either primary mouse monoclonal or rabbit polyclonal antibodies followed by horseradish peroxidase-labeled secondary antibody, either goat antimouse IgG (Pierce) or goat antirabbit IgG (Pierce), as appropriate. To visualize enzyme activity, signals were developed by enhanced chemiluminescence (SuperSignal Chemiluminescent Substrate, Pierce) and filmed.
Fibrinogen clottability
Clottability of the purified fibrinogen fractions was determined as previously described28 using thrombin (1 U/mL) and 125 mmol/L NaCl, 25 mmol/L HEPES, (pH 7.4), and 5 mmol/L CaCl2.
Polymerization turbidity curves
Polymerization of fibrinogen species was evaluated by measuring turbidity changes with time at 350 nm using a Lambda 2 spectrophotometer (Perkin Elmer, Norwalk, CT) equipped with a Peltier temperature-regulated cuvette holder. Measurements were made at 25°C in 100-μL quartz cuvettes. Data were collected with a sampling interval of 0.2 seconds and analyzed using UVWINLAB software.
Factor XIIIa-catalyzed cross-linking
Cross-linking reactions were carried out in 125 mmol/L NaCl, 25 mmol/L HEPES (pH 7.4), and 5 mmol/L CaCl2 at room temperature. Reactions were initiated by the addition of thrombin (0.5 U/mL) to a mixture containing fibrinogen (0.36 mg/mL), either fibrinogen-420 or fibrinogen-340, and recombinant human factor XIII (10 μg/mL). Reactions were stopped at specified times by the addition of Laemmli sample buffer and boiling.
Digestion with plasmin
Proteolysis by plasmin was conducted with substrates (0.45 mg/mL) in a buffer containing 125 mmol/L NaCl, 25 mmol/L HEPES, pH 7.4, and 5 mmol/L CaCl2 at 37°C. Proteolysis was initiated by the addition of plasmin to a final concentration of 0.03 U/mL. At specified times, aliquots were removed and the reaction was stopped by mixing with Laemmli sample buffer and boiling.
Results
Purification of E-fibrinogen
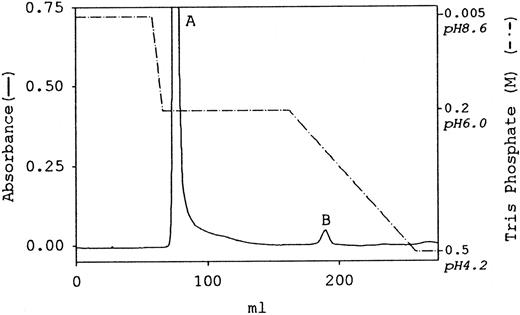
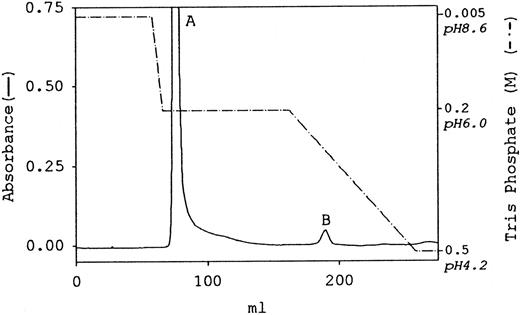
The paucity of positively charged amino acids in the αEC domains of fibrinogen-4202 6 implies that the molecule will be more negatively charged than the more abundant fibrinogen-340. This difference was the rationale for attempting separation of the 2 species by anion exchange chromatography. As shown by the elution profile in Figure 1, separation of human fibrinogen (fraction I-2) into 2 separate, unequal peaks was accomplished using a Mono Q column with a stepwise gradient of Tris phosphate. A steep step from the starting buffer, 0.005 mol/L Tris phosphate, pH 8.6, to 0.2 mol/L Tris phosphate, pH 6, was immediately followed by elution of most of the protein in a single major peak (peak A). Foothills of peak A eluted during maintenance of the step for 12-column volumes. A distinct second peak (peak B) eluted approximately midway during the subsequent 12-column volume linear gradient, ending at 0.5 mol/L Tris phosphate, pH 4.2.
Separation of fibrinogen species by Mono Q anion exchange chromatography . Human fibrinogen (fraction I-2) was purified from umbilical cord plasma and then subjected to column chromatography as described in “Materials and methods.” The elution profile is plotted with absorbance at 280 nm as a solid line (scale on the left), and the stepwise gradient in Tris phosphate is indicated by the broken line (scale on the right). The major and minor peaks are labeled A and B, respectively.
Separation of fibrinogen species by Mono Q anion exchange chromatography . Human fibrinogen (fraction I-2) was purified from umbilical cord plasma and then subjected to column chromatography as described in “Materials and methods.” The elution profile is plotted with absorbance at 280 nm as a solid line (scale on the left), and the stepwise gradient in Tris phosphate is indicated by the broken line (scale on the right). The major and minor peaks are labeled A and B, respectively.
Characterization of fibrinogen species in peaks A and B
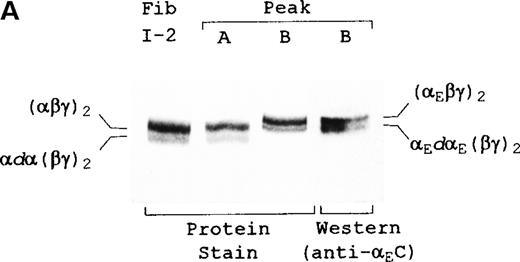
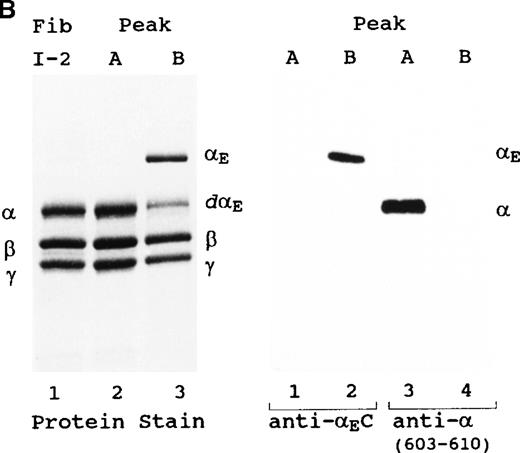
The first 3 fractions of peak A were pooled and compared, by SDS-PAGE and Western blot analysis, with a concentrated pool of the central 3 fractions of peak B (Figure 2). The 2 bands visible in peak A (Figure 2A) corresponded directly to the 2 most abundant species in the original fibrinogen I-2 fraction—intact and partially degraded forms of the conventional α chain-containing fibrinogen; the heterogeneity reflects the well-known susceptibility of the α chains to carboxyl terminal proteolysis.29 After its disulfide chains were reduced (Figure 2B, left panel, lane 2), peak A resolved into the intact conventional α, β, and γ bands and into minor bands corresponding to the partially degraded α chains. Comigration of I-2 fibrinogen and peak A bands throughout these analyses suggests that column separation did not affect the initial ratio of intact to partially degraded molecules.
Characterization of fibrinogen species in peaks A and B. (A) Unreduced samples and (B) reduced samples. Fibrinogen (Fib I-2) represents the material added, and peak A and peak B represent the material eluted from the anion exchange column of Figure 1. Western blot analysis was performed with either polyclonal anti-αEC #9395 or monoclonal anti-α(603-610). Samples in the upper panel were electrophoresed on 4-15% SDS-PAGE gels; proteins in the lower panels were separated on homogeneous 12% SDS-PAGE gels. Positions of various hexamers (A) and individual chains (B) are indicated. dα and dαE refer to degraded α and αE, respectively. All designated α gene-derived species were recognized by 1D4, an antibody specific for an epitope in the center of the αC region (not shown).
Characterization of fibrinogen species in peaks A and B. (A) Unreduced samples and (B) reduced samples. Fibrinogen (Fib I-2) represents the material added, and peak A and peak B represent the material eluted from the anion exchange column of Figure 1. Western blot analysis was performed with either polyclonal anti-αEC #9395 or monoclonal anti-α(603-610). Samples in the upper panel were electrophoresed on 4-15% SDS-PAGE gels; proteins in the lower panels were separated on homogeneous 12% SDS-PAGE gels. Positions of various hexamers (A) and individual chains (B) are indicated. dα and dαE refer to degraded α and αE, respectively. All designated α gene-derived species were recognized by 1D4, an antibody specific for an epitope in the center of the αC region (not shown).
In the late-eluting peak B, anti-αEC identified 2 bands (Figure 2A, lane 4) that corresponded directly to the 2 bands detected by protein staining (lane 3). The proportionality between these 2 bands of αE-fibrinogen, approximately 3:1 (upper:lower), was roughly comparable to that of the 2 bands in peak A (lane 2), suggesting that they might also represent intact and partially proteolyzed forms.
On reduction of the disulfide bonds in peak B, it became clear that the αE-fibrinogens collectively contained not only αE, β, and γ chains but also a minor band migrating at approximately 70 kd (Figure 2B, left panel, lane 3), just above the 68-kd α chain of peak A (lane 2). Presumably derived from a larger species, the band was tentatively designated a degradation product (dαE) of the intact approximately 110-kd αE chain. It closely corresponded to the expected size of the αE sequence remaining after cleavage of the αEC domain
This chain assignment was confirmed by Western blot analysis with discriminating antibodies: anti-αEC #9395, which recognizes an epitope(s) unique to the extended C-terminus of αE chains, and anti-α(603-610), which is specific for the last residues of intact α chains (Figure 2B, right panel). Anti-αEC recognized peak B's 110-kd band but not its 70-kd band (lane 2), consistent with the latter being an αE chain without its carboxy terminal domain. Anti-α(603-610) recognized only peak A's intact α chain (lane 3) and not any of the bands in peak B (lane 4). Although intact αE includes the same sequence as the terminal residues 603-610 of conventional α, it presumably escapes recognition by this antibody because the peptide bond between Val610 and Arg611 of αEC eliminates the free carboxyl epitope. By the same logic, the immunoreactive differences between the 68-kd α chain and the 70-kd band of peak B suggest that the latter is indeed an αE chain cleaved at a site downstream from Val610. In this context, it should be noted that in peak B fibrinogen-420, no significant contribution of α(1-625), the conventional α chain's non-processed form,18 19 was detected by Western analysis with anti-α(615-625) (data not shown). Had it been present, the α(1-625) chain would have comigrated with the 70-kd band designated dE and, like it, escaped recognition by anti-α(603-610).
It is noteworthy that, even after heavily overloading the gels, no αE-containing material was detectable in peak A by Western blot analysis with anti-αEC, suggesting an essentially complete separation of the 2 subclasses. Although a portion of the α or αE chains in each peak is degraded, resulting in the minor bands labeled αdα(βγ)2 and αEdαE(βγ)2 in Figure 2A, for simplicity we hereafter refer to the pooled subfractions by the nomenclature for the intact species—fibrinogen-340 for peak A and fibrinogen-420 for peak B.
Thrombin-catalyzed fibrin polymerization
When incubated with human thrombin, both fibrinogen-420 and fibrinogen-340 were found to be more than 90% clottable, forming clots that were sufficiently solid that they remained in place in inverted cuvettes. Parameters of thrombin-induced clot formation were compared by monitoring turbidity as a function of time. As seen in Figure3, the turbidity curves obtained for fibrinogen-420 and fibrinogen-340 are typical for clot formation: an initial delay, followed by a rapid rise in turbidity that culminates in a plateau. The lag period represents the time required for fibril formation, and the maximum slope reflects the rate of fibril assembly during the phase of lateral associations and branching,30,31 whereas the plateau value attained by each species is related to the average fiber thickness in the clot.32 By all 3 measures, the curves for fibrinogen-420 and fibrinogen-340 were similar, suggesting fundamentally comparable roles in the clotting process, though a functional impact of the observed differences cannot be excluded.
Polymerization with fibrinogen-420 and fibrinogen-340. Polymerization was initiated by the addition of thrombin (0.1 U/mL) at time 0 to substrate at 0.1 mg/mL: either fibrinogen-340 (A) or fibrinogen-420 (B). Polymer formation was measured as change in turbidity at 350 nm with time as described in “Materials and methods.” Data in each panel are averages obtained from 4 separate trials. Bars indicate the standard deviation of the mean. For fibrinogen- 340 and fibrinogen-420, respectively, the lag periods were 8.0 ± 4.0 and 9.8 ± 4.6 minutes, and the maximum slopes were 7.3 ± 2.7 and 5.2 ± 3.7 × 10−5seconds−1.
Polymerization with fibrinogen-420 and fibrinogen-340. Polymerization was initiated by the addition of thrombin (0.1 U/mL) at time 0 to substrate at 0.1 mg/mL: either fibrinogen-340 (A) or fibrinogen-420 (B). Polymer formation was measured as change in turbidity at 350 nm with time as described in “Materials and methods.” Data in each panel are averages obtained from 4 separate trials. Bars indicate the standard deviation of the mean. For fibrinogen- 340 and fibrinogen-420, respectively, the lag periods were 8.0 ± 4.0 and 9.8 ± 4.6 minutes, and the maximum slopes were 7.3 ± 2.7 and 5.2 ± 3.7 × 10−5seconds−1.
Factor XIIIa-catalyzed cross-linking
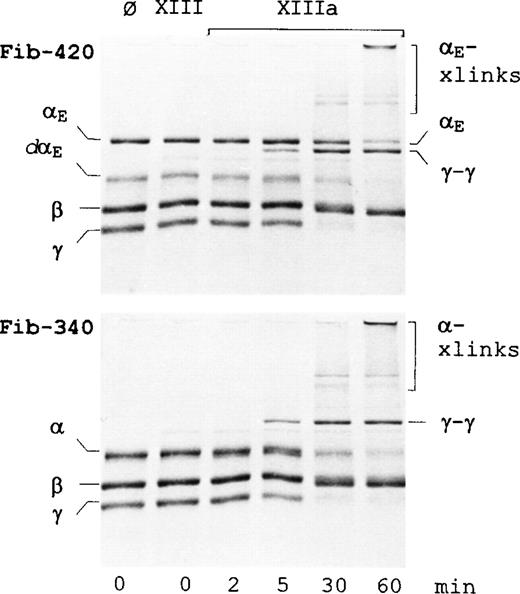
The kinetics of factor XIIIa cross-linking of fibrinogen-420 and fibrinogen-340 is compared in Figure4. Cross-linking of the γ chains in both preparations was essentially complete within 30 minutes, as evidenced both by the disappearance of the band corresponding to the γ chain and the concomitant appearance of γ-dimer. Cross-linking of the α chain in fibrinogen-340 (evident from its gradual disappearance and the emergence of higher molecular weight species) occurred at a rate lagging that of the γ chain, as expected.33 A similarly delayed cross-linking occurred for the αE anddαE chains of fibrinogen-420. This observation contrasts with findings in lamprey fibrinogen where cross-linking of the αE homologue (α′) was considerably more efficient than for the α chain.34 The disparity may be the result of differences in the αC regions of the lamprey fibrinogen α chain and the αE chain homologue, which are atypically derived from separate genes.10 11
Time course of factor XIIIa-catalyzed cross-linking. Cross-linking reactions with either fibrinogen-420 (upper panel) or fibrinogen-340 (lower panel) were carried out as described in “Materials and methods.” Lane 1 contains substrate alone. Lanes 2 to 6 contain substrate with either nonactivated factor XIII (lane 2) or thrombin-activated factor XIIIa (lanes 3-6), incubated for 2 minutes (lane 3), 5 minutes (lane 4), 30 minutes (lane 5), or 60 minutes (lane 6). Proteins were separated on homogenous 12% SDS-PAGE gels under reducing conditions and stained. Positions of individual and cross-linked fibrinogen chains are indicated. dαErefers to degraded αE, αE-xlinks refers to cross-linked αE chains, and α-xlinks refers to cross-linked α chains.
Time course of factor XIIIa-catalyzed cross-linking. Cross-linking reactions with either fibrinogen-420 (upper panel) or fibrinogen-340 (lower panel) were carried out as described in “Materials and methods.” Lane 1 contains substrate alone. Lanes 2 to 6 contain substrate with either nonactivated factor XIII (lane 2) or thrombin-activated factor XIIIa (lanes 3-6), incubated for 2 minutes (lane 3), 5 minutes (lane 4), 30 minutes (lane 5), or 60 minutes (lane 6). Proteins were separated on homogenous 12% SDS-PAGE gels under reducing conditions and stained. Positions of individual and cross-linked fibrinogen chains are indicated. dαErefers to degraded αE, αE-xlinks refers to cross-linked αE chains, and α-xlinks refers to cross-linked α chains.
Plasmic digestion of fibrinogen-420
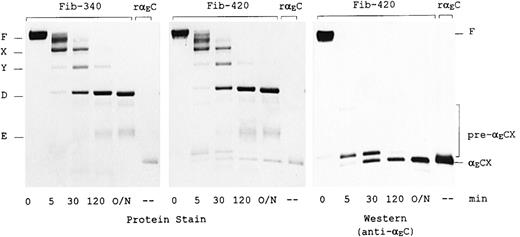
Comparison of plasmic digestion of fibrinogen-420 and fibrinogen-340 by SDS-PAGE revealed similar kinetics for production of the conventional fibrinogen degradation products: fragments X, Y, D, and E (Figure 5, left and middle panels). However, accumulation of at least 2 additional products was observed in the plasmic digest of fibrinogen-420: 1 band (αECX) comigrating with rαEC, the 34-kd yeast recombinant αEC domain,14 and another of slower mobility, which appears to be its immediate precursor (Figure 5, middle panel). Both products were detected in immunoblots using antibodies specific for the αEC domain (Figure 5, right panel). In addition, some short-lived pre-αECX species of slower mobility were observed. Cleavage of fibrinogen-420 to yield the pre-αECX species was a particularly early event in the digestion, occurring well before the appearance of significant quantities of fragments D and E. Quantitation, using rαEC as a standard, suggested that the final product, αECX, accumulated in molar proportion to the amount of αEC present in the intact fibrinogen-420 (Figure 5, middle panel), indicating a degree of stability comparable to that of the core fragments D and E. Stabilization of the domain against further digestion by plasmin requires the presence of calcium (data not shown), an observation first noted for the recombinant domain.14
Plasmin digestion of fibrinogen-420 and fibrinogen-340. The first 5 lanes in each panel contain purified fibrinogen (3.4 μg/lane), either fibrinogen-420 or fibrinogen-340; the sixth contains 0.5 μg recombinant human αEC (rαEC), which migrates at 34 kd.14 Proteins were separated on 4% to 15% SDS-PAGE gels under nonreducing conditions. Left and middle panels: Gelcode blue stain. Right panel: Western blot analysis of fibrinogen-420 using monoclonal anti-αEC #29-1. Positions of fibrinogen (F) and fragments X, Y, D, and E are indicated, as are those of the αE-containing cleavage products αECX and its precursors (pre-αECX); the larger precursors can only be seen in overexposures.
Plasmin digestion of fibrinogen-420 and fibrinogen-340. The first 5 lanes in each panel contain purified fibrinogen (3.4 μg/lane), either fibrinogen-420 or fibrinogen-340; the sixth contains 0.5 μg recombinant human αEC (rαEC), which migrates at 34 kd.14 Proteins were separated on 4% to 15% SDS-PAGE gels under nonreducing conditions. Left and middle panels: Gelcode blue stain. Right panel: Western blot analysis of fibrinogen-420 using monoclonal anti-αEC #29-1. Positions of fibrinogen (F) and fragments X, Y, D, and E are indicated, as are those of the αE-containing cleavage products αECX and its precursors (pre-αECX); the larger precursors can only be seen in overexposures.
Immunologic identification of ECX in vivo
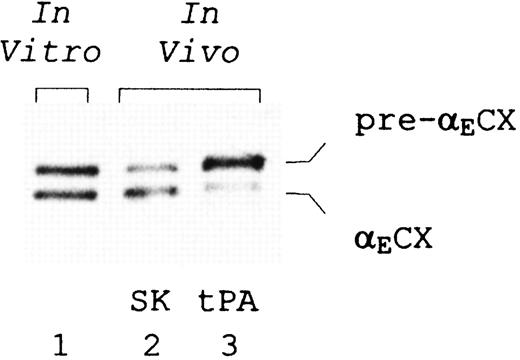
The observed in vitro stability of αECX in the presence of plasmin prompted us to examine whether the fragment could also be detected in such lytic states in vivo. The generation of fibrin(ogen) degradation products is found in clinical states associated with activation of the fibrinolytic system. In particular, relatively high concentrations of fibrinolytic products have been detected in the plasma of patients with myocardial infarction during thrombolytic therapy with tissue plasminogen activator or streptokinase, as a result of the lytic state created during treatment. Figure 6 shows a representative Western blot analysis, with an anti-αEC antibody, of plasma obtained from patients with myocardial infarction 30 minutes after treatment with either streptokinase or tissue plasminogen activator. Indeed, 2 bands are detected that comigrate with αECX and its immediate precursor from plasmic digests of purified fibrinogen-420 in vitro.
Presence of E-containing plasmin cleavage products in vivo. In vitro: a 30-minute time point from plasmin digestion of purified fibrinogen-420 (see Figure 5). In vivo: plasma samples were collected from patients with myocardial infarction 30 minutes into treatment with either streptokinase (SK) or tissue plasminogen activator (tPA). Proteins were separated on 10% SDS-PAGE gels under nonreducing conditions, Western blotted, and detected using monoclonal anti-αEC #29-1. Positions of the split products, αECX and its precursor pre-αECX, are indicated.
Presence of E-containing plasmin cleavage products in vivo. In vitro: a 30-minute time point from plasmin digestion of purified fibrinogen-420 (see Figure 5). In vivo: plasma samples were collected from patients with myocardial infarction 30 minutes into treatment with either streptokinase (SK) or tissue plasminogen activator (tPA). Proteins were separated on 10% SDS-PAGE gels under nonreducing conditions, Western blotted, and detected using monoclonal anti-αEC #29-1. Positions of the split products, αECX and its precursor pre-αECX, are indicated.
Discussion
This study is the first reported purification of fibrinogen-420 from human plasma, enabling a structural and functional characterization of this αE-containing fibrinogen subclass that constitutes a minor percentage of the circulating fibrinogen. With well-separated subfractions of fibrinogen-420 and the more abundant α-fibrinogen, fibrinogen-340, we have demonstrated the overall similar behavior of these fibrinogen subclasses in clot formation and proteolytic susceptibility and have shown that plasmin attack rapidly releases the αEC domain of fibrinogen-420 as an entity, αECX, resistant to further degradation in vitro. Furthermore, the αECX fragment is also detectable in the plasma of patients undergoing thrombolytic therapy. On the basis of these findings, we propose that an important function(s) is discharged by the αEC domainindependent of its parent fibrinogen molecule.
The protocol described in this article binds fraction I-2 of human fibrinogen to a Mono Q anion exchange column, eluting it in a stepwise fashion to effect a clean separation of αE-fibrinogen (fibrinogen-420) from α-fibrinogen (fibrinogen-340). Our SDS-PAGE analysis shows that, after purification, fibrinogen-420 is as intact as fibrinogen-340, with roughly 20% of the molecules degraded (see Figure2). The degradation, whether caused by plasmin or other proteases,35 occurs before the column chromatography step, either in vivo or during generation of fibrinogen fraction I-2.
By differential antibody reactivity, we have shown that conventional α chains are not incorporated into αE-fibrinogen from human plasma; the band in reduced αE-fibrinogen that migrates spuriously near the position of conventional α chains is a distinct αE-chain derivative that has lost a significant portion of its C-terminal domain (see Figure 2). Thus, the native structure of αE-fibrinogen is indeed symmetrical (αEβγ)2 rather than mixed, ααE(βγ)2, and reflects a nonstochastic assembly process as noted in an earlier study.8
The closely related structures of fibrinogen-420 and fibrinogen-340 originally led us to investigate whether the αEC domains of fibrinogen-420 might alter the fibrinogen molecule's primary behavior in clotting and fibrinolysis. The analyses of polymerization and cross-linking presented here (Figures 3, 4) show that the presence of the αEC domains on a fibrinogen molecule does not grossly affect these functions. The findings support previous studies showing that rαEC, the recombinant αEC expressed in yeast, lacks a polymerization pocket and does not participate in cross-linking.14 The findings are also consistent with electron micrographic images of clots derived from either fibrinogen-340 or fibrinogen-420 (unpublished observations).
Despite similarities to βC and γC, the αEC domain appears to be specialized for a different and as yet unknown function, based on several considerations. (1) While still attached to the fibrinogen core via its “αC” tether, αEC undoubtedly enjoys more degrees of spatial freedom than βC or γC and, consequently, greater availability of its binding sites to other macromolecules. (2) This location also appears designed to ensure more rapid release of the αEC domain, given the extreme susceptibility of the αC region to proteolysis.36 (3) Proteolytic release of monomeric αECX (Figure 5) provides definitive evidence that the αEC domains of fibrinogen-420 have no disulfide attachments, either to each other or to the core of the molecule, a finding consistent with the results of mutational analysis of recombinant fibrinogen-4208 and trypsin digests of α′-fibrinogen, the counterpart to αE-fibrinogen in lamprey.34 (4) During fibrin(ogen)olysis, the αEC domains are released as monomers, unlike the βC and γC domains, which remain anchored together in the proteolytic fragment D. (5) Finally, the binding clefts of the βC and γC domains contain charged/polar amino acid pairs that engage the polymerization “knobs” during fibrin assembly,5,7,15 whereas the corresponding cleft in the αEC domain has neutral residues at its center, suggesting a different purpose.6
The αEC domain is derived from exon VI, the largest conserved segment of the entire fibrinogen α gene.11 In light of no discernible effect of the domain on coagulation, we suspect that preservation of the αE chains among higher vertebrates reflects the ability of αE-fibrinogen to deliver the αECX fragment to a location critical to its mission. In the recent literature, a growing number of comparable proteolytic products exhibit potent effects unrelated to the primary function of their parent molecules, which often serve to localize fragment release to sites of tissue repair, wound healing, and angiogenesis.37 Recent experiments with recombinant forms expressed in E coli38 and yeast (unpublished observations) suggest that the domain is capable of supporting integrin-mediated cell adhesion. Current investigation is focused on further exploring the role of αECX in this context.
Acknowledgments
We thank Joan Sobel of Columbia Presbyterian Medical Center for providing access to archival plasma samples and Bohdan Kudryk and Alessandra Bini for many helpful discussions. We are particularly grateful to Ludy Dobrilla for her cooperation in supplying cord plasma. We also thank Peter J. Baker for his capable technical assistance.
Supported in part by grants from the National Institutes of Health (HL 51050), the American Heart Association, and the Hugoton Foundation.
Reprints:Gerd Grieninger, New York Blood Center, 310 East 67th Street, New York, NY 10021; e-mail: ggrien@nybc.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.