A recent paper by Marshall et al suggests that decreased production of interleukin-12 (IL-12) is crucial in human immunodeficiency virus (HIV)-associated immune deficiency.1According to these authors, peripheral blood mononuclear cells (PBMC)from HIV-positive subjects produce lower levels of IL-12 in response to a wide range of stimuli, irrespective of the disease stage. Interferon-gamma (IFN-γ) production was decreased in response to IL-12, and upregulation of the IL-12 receptor β2 chain, critical for signal transmission, was impaired. The latter defect could be reversed by rIL-12 pretreatment. The authors logically conclude: “A primary IL-12 defect may lead to secondary deficiencies in expression of the genes for IL-12Rβ2 and IFN-γ, thus amplifying immune deficiency during HIV infection.” These data are in line with the “type-1 to type-2 shift” paradigm of HIV pathogenesis.2-4 Because addition of IL-12 to PBMC cultures from HIV-positive subjects was shown to partly restore responses to antigen stimulation,5 rIL-12 seems a good candidate for immunotherapy in acquired immunodeficiency syndrome (AIDS).
Other authors, including our group, observed HIV-associated cytokine imbalances, which are partly inconsistent with a type-1 to -2 shift.6-8 Increased levels of various cytokines, including IFN-γ and IL-12, were measured in serum of HIV-positive subjects,even in advanced disease.9-11 PBMC stimulation resulted in increased IFN-γ production in early HIV stages and lower IFN-γlevels in AIDS patients, as compared to controls.12,13Marshall's claim about a stimulus- and stage-independent impaired IL-12 production has also been challenged. One group demonstrated that IL-12 p40 production, induced by Staphylococcus aureus (SAC),inversely correlated with the patients' CD4 T count, and 2 studies indicated that IL-12 induced by lipopolysaccharide (LPS) andCandida, was not consistently decreased in HIV-positive subjects.14,15 Moreover, in Marshall's study, induction by stimuli other than SAC, resulted in lowered production of IL-12 p40,but not of IL-12 p70 (bioactive), in HIV-positive PBMC cultures. We recently studied the physiological pathway of IL-12 production through interaction between CD40-ligand (CD40L) and CD40. Impaired CD3/TCR-mediated induction of CD40L on CD4 T cells was observed in patients with advanced disease. On the contrary, stimulation of PBMC with CD40L and IFN-γ resulted in higher IL-12 p70 in early stages and only slightly decreased IL-12 production in cultures from AIDS patients. We concluded therefore that the main dysfunction was impaired upregulation of CD40L on T cells and not deficient IL-12 production capacity per se.8
In view of the discrepancies between various studies, we decided to compare IL-12 p70 production in blood cells from HIV-negative and HIV-positive patients, using the following conditions: (1) RPMI only,(2) A broadly mitogenic combination of phytohemagglutinin (PHA) and LPS, (3) T-cell receptor Vβ-stimulating Staphylococcusenterotoxin B (SEB), and (4) monocyte-stimulating SAC. All stimulations were done in whole blood, because it represents most closely the in vivo milieu. Samples were obtained from 18 HIV-negative lab workers and from 38 HIV-positive subjects, recruited at our outpatient clinic. Patients with active opportunistic diseases were excluded, but all Centers for Disease Control classes were represented: 12 patients had more than 500 CD4 T cells/μL, 16, between 200 and 500 CD4 T cells,and 10 had fewer than 200 CD4 T cells/μL (= AIDS). Most patients with CD4 T-cell counts lower than 500 were treated with a variety of highly active antiretroviral therapies (HAART) and all were monitored for viral load (VL) using the Cobas Amplicor HIV-1 Test Version 1.5(Roche Diagnostics, Brussels, Belgium). VL was below the detection limit of 400 copies/mL (2.6 log) in 22 patients. The VL median ± 95% confidence interval (CI) in the remaining 16 patients was 4.62 ± 0.69 log (range: 3.25 to >5.9 logs).
One hundred microliters of heparinized blood, diluted 1:10 in RPMI(GIBCO BRL, Paisley, Scotland), were cultured 4-fold in round-bottom polystyrene tubes (Falcon 2054, Becton Dickinson, Erembodegem,Belgium). Optimum concentrations of stimuli were determined in preliminary experiments: PHA (Difco, Detroit, IL) was used at 5 μg/mL plus LPS (Difco) at 25 μg/mL; SEB (Sigma, St Louis, Missouri) at 1μg/mL and SAC (Pansorbin, Calbiochem, La Jolla, CA) at 0.016 vol percent. After an incubation of 24 hours at 37°C in a 5% CO2 incubator, the supernatants were harvested and stored at −70°C. IL-12 concentrations were measured using the p70 ELISA (R&D Systems,Abingdon, UK).
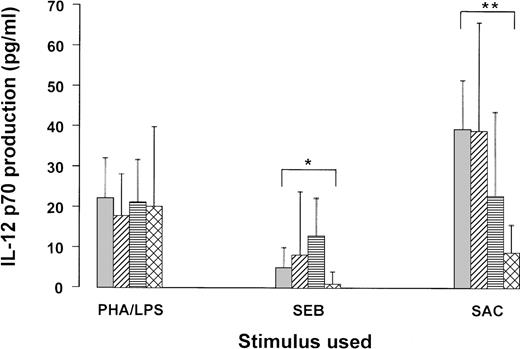
IL-12 could not be measured in any supernatant of medium control cultures (not shown). Stimulated IL-12 levels (median and CI) are depicted in the figure. The Mann-Whitney U test was used to calculate the significance of differences between the controls and the patient groups, stratified according CD4 T count. In general, IL-12 production was highly variable, but several tendencies are obvious. In blood cultures of HIV-negative subjects, SAC was the strongest IL-12 inducer, followed by PHA/LPS, whereas SEB was relatively weak. The IL-12 response to LPS/PHA was not statistically different between patients and controls. The response to SAC was unaltered in patients with more than 500 CD4 T cells, slightly (not significant) decreased in patients with intermediate CD4 T counts, and significantly (p < .01) reduced in AIDS patients. Interestingly, SEB induced slightly (but n.s.) more IL-12 in the cultures from patients with more than 200 CD4 T cells/μL, whereas in AIDS patients SEB-stimulated IL-12 production was significantly (p < .05) decreased. The latter results are similar to those we previously obtained with CD40L in peripheral blood mononuclear cells (PBMC) cultures.8
Figure. IL-12 production in whole blood cultures from HIV-negative and HIV-positive subjects.
Blood was obtained from 18 HIV-negative controls ( ), 12 HIV-positive subjects with CD4 T-cell counts higher than 500/μL (
), 12 HIV-positive subjects with CD4 T-cell counts higher than 500/μL ( ), 16 subjects with CD4 T counts 200-500/μL (□), and 10 subjects with CD4 T counts lower than 200/μL (
), 16 subjects with CD4 T counts 200-500/μL (□), and 10 subjects with CD4 T counts lower than 200/μL ( ). The blood was diluted 1/10 in medium and incubated with the indicated stimuli.
). The blood was diluted 1/10 in medium and incubated with the indicated stimuli.
Figure. IL-12 production in whole blood cultures from HIV-negative and HIV-positive subjects.
Blood was obtained from 18 HIV-negative controls ( ), 12 HIV-positive subjects with CD4 T-cell counts higher than 500/μL (
), 12 HIV-positive subjects with CD4 T-cell counts higher than 500/μL ( ), 16 subjects with CD4 T counts 200-500/μL (□), and 10 subjects with CD4 T counts lower than 200/μL (
), 16 subjects with CD4 T counts 200-500/μL (□), and 10 subjects with CD4 T counts lower than 200/μL ( ). The blood was diluted 1/10 in medium and incubated with the indicated stimuli.
). The blood was diluted 1/10 in medium and incubated with the indicated stimuli.
The same data were reanalyzed after stratifying the patients according to VL (fewer than and more than 400 copies, respectively). This analysis showed IL-12 concentrations of 21.1 ± 9.9 and 9.4 ± 10.6 pg/mL (p = .07) after PHA/LPS stimulation, 9.3 ± 6.4 and 4.6 ±5.6 pg/mL (n.s.) after SEB stimulation, and 18 ± 14.5 and 13.3 ±19.4 pg/ml (n.s.) after SAC. Clearly, there is a tendency of lowered IL-12 production in subjects with a measurable VL, regardless of the stimulus used. Nevertheless, among the latter patients no correlation was found between the IL-12 production and VL (Spearman rank test).
In summary, our present data indicate that the influence of in vivo HIV infection on in vitro IL-12 production is more complex than Marshall et al suggested. Both the nature of the stimulus and the stage of the disease determine the IL-12 p70 output in culture. The IL-12 response to SAC decreased according to CD4 T counts. Other stimuli, including SEB and CD40L, had a double-edged effect: a relative overproduction of IL-12 in non-AIDS patients and a defective IL-12 production in AIDS patients. Unfortunately, the effects of viral load itself and of viral load reduction by highly active antiretroviral therapy (HAART) on IL-12 production remain unclear, even in our present study.
Together with earlier observations, the present data indicate that both immune overactivation and immune deficiency are reflected in IL-12 production during HIV infection. Although IL-12 could be useful to enhance deficient cell-mediated immunity, it also has potential proinflammatory and HIV-enhancing effects. Therefore, more studies are needed to unravel the role of dysregulated IL-12 production in HIV pathogenesis before immunotherapy with IL-12 itself or IL-12 inducers can be considered.
This work was supported by a grant from the Janssen Research Foundation.