Rosetting, the binding of parasitized erythrocytes to 2 or more uninfected erythrocytes, is an in vitro correlate of disease severity in Plasmodium falciparum malaria. Although cell ligands and receptors have been identified and a role for immunoglobulin M has been suggested, the molecular mechanisms of rosette formation are unknown. The authors demonstrate unequivocally that rosette formation by P falciparum-infected erythrocytes is specifically dependent on human serum, and they propose that serum components act as bridging molecules between the cell populations. Using heparin treatment and Percoll density gradient centrifugation, they have developed an assay in which parasitized erythrocytes grown in serum-containing medium and optimally forming rosettes are stripped of serum components. These infected cells were no longer able to form rosettes when mixed with erythrocytes and incubated in serum-free medium. Rosette formation was restored by the addition of serum or certain serum fractions obtained by concanavalin A (conA) affinity, anti-IgM affinity, anion exchange, and gel filtration chromatography. The authors clearly demonstrate that multiple serum components—IgM and at least 2 others—are involved in rosette formation. Those others consist of 1 or more acidic components of high-molecular mass that binds to conA (but that is not thrombospondin, fibronectin, or von Willebrand's factor) and of at least 1 more basic, smaller component that does not bind to conA. Data on the size and number of rosettes formed support the authors' hypothesis that multiple bridges are involved in this complex cellular interaction. These findings have important implications for the understanding of pathogenic adhesive interactions of P falciparum and host susceptibility to severe malaria.
Plasmodium falciparum malaria is responsible for approximately 2 million deaths annually, most of which occur among children in sub-Saharan Africa. The devastating pathologic conditions that result in severe malaria are caused by complex cellular and molecular interactions between the human host and the parasite, after parasite invasion of erythrocytes. Serious clinical complications occur when these parasitized red blood cells (PRBC) sequester in the microvasculature of a variety of organs1 as a result of cytoadhesion and rosette formation. Cytoadhesion is a receptor-ligand interaction between the parasite protein P falciparumerythrocyte membrane protein 1 (PfEMP-1) and receptors on the surface of endothelial cells,2 whereas rosetting is the binding of PRBC to 2 or more uninfected red blood cells (RBC). Rosetting, occurring in conjunction with cytoadhesion, is believed to contribute to the development of cerebral malaria.3 4
Investigations to elucidate the molecular basis of rosette formation have been fueled by a number of studies demonstrating a correlation between rosette formation and disease severity in Africa,5-7 though studies carried out in Thailand8 and Papua New Guinea9 failed to show such a correlation. The parasite molecules first implicated as rosetting ligands were the low-molecular-mass polypeptides known as rosettins.10,11 Proteins of approximately 22 kd were found at the surface of 2 strains of rosetting parasites, whereas their nonrosetting counterparts lacked these molecules, and antibodies to these antigens were able to disrupt rosettes. However, the genes for these proteins have not yet been cloned. Recently, 2 separate studies using parasite clones R2912 and FCR3S1.213 have identified PfEMP-1 as a parasite ligand involved in rosetting.
Various molecules on uninfected erythrocytes have also been implicated as rosetting receptors. One study, using the Malayan Camp parasite, showed that CD36 was the erythrocyte receptor for this parasite isolate.14 Other studies using different parasites have described the involvement of the ABH blood group determinants.15 Recently, complement receptor 1 (CR1) has been shown to be involved in the rosetting of a number of parasite lines and clones.12 Another study, using parasite clone FCR3S1.2, suggested that heparan sulfate is a rosetting receptor,13 though its presence on the RBC surface is unconfirmed. Overall, little is known about the precise molecular mechanisms of interaction between infected and uninfected erythrocytes of a rosette. The role that serum components could play, as bridging molecules between the 2 cell populations (ie, as promoters of the cell-to-cell interaction) has not been evaluated in the previous studies that aimed to identify the parasite ligands and red cell receptors involved in rosetting.
There is now accumulating, but indirect, evidence that serum IgM and possibly IgG are involved in rosette formation by some parasite clones and isolates.4,16 In the first of these studies, Scholander et al4 examined transmission electron micrographs of a brain autopsy specimen from a patient who died of cerebral malaria and observed the presence of fibrillar strands on the PRBC surface, apparently forming bridges to adherent RBC. Fibrils were subsequently found on a number of PRBC from parasite isolates and clones propagated in vitro, and antibodies to human immunoglobulins were found by immunogold transmission electron microscopy to bind to the fibrillar strands. Clough et al16 established the requirement for serum to enable the formation of rosettes in culture and demonstrated that IgM may play a role in rosetting by P falciparum clone Palo Alto 1 (PA1). In this study the parasites were grown in the presence or absence of serum, to which IgM or antibody to IgM was added to the culture. However, the requirement that serum components act as bridging molecules between the PRBC and the RBC of a rosette was not demonstrated. The reason was that it was possible the lack of parasite rosettes grown in the absence of serum was the result of a failure of the PRBCs to express the rosetting phenotype under these conditions.
The purpose of the current study was to determine whether there is an absolute requirement for human serum components to act as bridging molecules between P falciparum-infected and P falciparum-uninfected RBC in rosettes. We describe a serum-free assay system that used parasites grown in serum-containing medium and capable of rosetting to enable us to establish the absolute requirement for human serum and to test the ability of serum fractions produced by various chromatographic techniques to support rosetting. We establish that multiple serum molecules in addition to IgM are involved in rosette formation, and we define some of the biochemical characteristics of the bridging molecules involved.
Materials and methods
Chemicals and reagents
RPMI 1640, gentamicin, glutamine, hypoxanthine, and Albumax I (a lipid-rich bovine serum albumin) were obtained from GibcoBRL (Life Technologies, Paisley, UK). Pefabloc SC was obtained from S. Black (Import and Export, Hertford, UK). Antihuman IgM (μ-chain specific) agarose was obtained from Sigma Chemical (Dorset, UK). Thrombospondin, fibronectin, and von Willebrand's factor were obtained from Calbiochem (Nottingham, UK). All other chromatography matrices and Percoll were obtained from Amersham Pharmacia Biotech (St Albans, UK), and all other reagents were obtained from Sigma Chemical.
Serum and erythrocytes
Nonimmune human AB serum (NHS) and O Rh-positive erythrocytes were obtained from the North London Blood Transfusion Service (Colindale, London, UK). NHS was used from a single donor for each experiment. Animal sera were obtained from Sigma. C1q-deficient serum17and C3-deficient serum18 were kindly provided by Dr M. Botto (Hammersmith Hospital, Imperial College School of Medicine, London, UK). All sera were kept frozen at −20°C before use. Heat-inactivated NHS was obtained by heating the serum to 56°C for 30 minutes. All sera were used at a final concentration of 10% vol/vol.
Parasite culture
The cloned P falciparum parasites PA1(from the Palo Alto line, Uganda), previously well characterized in terms of its rosetting properties19 and also referred to as FCR3S by some authors,13 was used for all experiments. Parasites were cultured continuously in RPMI 1640 supplemented with 11 mmol/L glucose, 2 mmol/L glutamine, 200 μmol/L hypoxanthine, and 20 μg/mL gentamicin (complete medium, CM) containing NHS (10% vol/vol) (CM-NHS). Washed erythrocytes were added to the culture medium at a hematocrit level of 2%. Parasites were maintained tightly synchronized according to the methods of Lambros and Vanderberg20 and Holder and Freeman,21 so that the age span of the parasites was less than 6 hours for all experiments. Maintenance of the rosetting phenotype (R+) above 50% of the parasitized erythrocytes in rosettes, and typically above 65%, was achieved by Percoll gradient centrifugation using 63% Percoll.22 Rosetting levels were determined as described below.
Assay for assessment of the role of serum and serum fractions in rosette formation
Cultures (25-50 mL) of late-stage parasites (early schizonts) grown in CM-NHS and containing between 5% and 10% parasitemia were harvested and PRBC purified using a modification of the method of Handunnetti et al.22 Briefly, the cells were treated with 13.4 μg/mL heparin (sodium salt, grade 1A from porcine intestinal mucosa) to disrupt the rosettes, and they were incubated for 15 minutes at 37°C. The completeness of rosette disruption was assessed by fluorescence microscopy with an MC100 spot microscope (Axioshop; Zeiss, Oberkoden, Germany) × 1000 objective, in incident ultraviolet light, after the addition of ethidium bromide (50 μg/mL) to stain the parasite nucleic acid. The heparin-treated cells (0.5-1 mL) were layered onto a cushion of 70% Percoll (1.5-3 mL) containing 13.4 μg/mL heparin and prepared as follows: 9 parts undiluted Percoll were diluted with 1 part of ×10 concentrated, phosphate-buffered saline (PBS) (137 mmol/L NaCl, 3 mmol/L KCl, 10 mmol/L Na2HPO4, 1.76 mmol/L KH2PO4, pH 7.2). To provide a 70% Percoll solution, 78 mL of this stock Percoll solution was mixed with 22 mL RPMI 1640 containing 20 μg/mL gentamicin (RPMI–gentamicin), and heparin (13.4 μg/mL) was added. The sample was spun at 1900g for 10 minutes at 20°C, and the interface containing the mature PRBC was removed and washed twice with RPMI–gentamicin. After assessing the percentage parasitemia by Giemsa staining of an aliquot of the pelleted cells (the parasitemia varied between 55% and 95%), the pellet was diluted in RPMI–gentamicin, and 2-μL aliquots were added to Eppendorf tubes containing 30 μL complete medium with Albumax I (0.5% wt/vol) in place of the NHS (CM-Albumax I). This CM-Albumax I was either unsupplemented (negative control), supplemented with NHS (10% vol/vol; positive control), or supplemented with varying amounts of serum fractions obtained after chromatographic separation procedures (see below). Eppendorf tubes containing parasitized cells in the various media were then incubated at 37°C for 30 minutes to allow the parasites to recover before they were transferred to a 96-well, flat-bottomed plate, to which was added equal volumes (30 μL) of the same supplemented CM-Albumax medium containing erythrocytes (2.2 μL) that had been previously washed in RPMI–gentamicin. Cell densities were determined: hematocrit levels of the cultures were between 4% and 5%, and parasitemia levels were between 5% and 10% for all assays. Ethidium bromide (50 μg/mL) was added to all samples, the plate was placed into a gas box with a mixture of 5% O2, 7% CO2, and 88% N2, and samples were incubated at 37°C for 45 minutes. For each aliquot, rosetting was assessed by placing duplicate 10 μL samples on a slide under coverslips and counting 200 parasitized cells per coverslip using a fluorescence microscope ( × 1000 objective, studied in incident ultraviolet light). The rosetting rate was expressed as the percentage of parasitized cells that bound 2 or more uninfected erythrocytes to their surfaces. To assess the average size of rosettes formed, the number of uninfected RBC in at least 100 rosettes was counted per sample, and the mean was calculated. The significance of differences between 2 mean rosette sizes was assessed at a confidence limit of P = .05.
Chromatographic techniques
NHS (5-10 mL) was spun at 10,000g for 10 minutes to remove any particulate matter, filtered through a 1.2 μmol/L filter, and loaded onto the various columns as described below. All columns were packed and run according to the manufacturer's instructions. Soybean trypsin inhibitor (10 μg/mL) was added to each of the eluted fractions from all columns. The fractionation of serum on each column was repeated using at least 2 different batches of serum.
Concanavalin A–Sepharose affinity chromatography
An equal volume of binding buffer (containing 20 mmol/L Tris-HCl, 0.5 mol/L NaCl, pH 7.4) was added to the spun and filtered serum, and the sample was loaded onto a 5-mL conA–Sepharose affinity column (XK 1.6 cm × 20 cm; Pharmacia, Uppsala, Sweden) equilibrated in the same binding buffer. After the column was washed with 10 vol binding buffer, the bound fraction was eluted with 0.2 mol/L methyl α-D-mannopyranoside.
Antihuman IgM-agarose affinity chromatography
An equal volume of binding buffer (containing 10 mmol/L phosphate, 0.5 mol/L NaCl, 1 mmol/L EDTA, pH 7.2) was added to the spun and filtered serum, and the sample was loaded onto a 5-mL antihuman IgM agarose (IgM affinity) column (econo-column, 1 cm×10 cm; Bio-Rad, Hemel Hempstead, UK) equilibrated in the same binding buffer. After the column was washed with 5 to10 vol binding buffer, the bound fraction was eluted with 100 mmol/L glycine-HCl, 150 mmol/L NaCl, pH 2.4. After the addition of 1 mol/L Tris-HCl, pH 9 (100 μL/mL of eluted fraction), pH neutrality was immediately restored.
Ion exchange chromatography on Sepharose Q
An equal volume of binding buffer (containing 20 mmol/L Bis-Tris propane, 35 mmol/L Na2SO4, pH 7.2) was added to the spun and filtered serum, and the sample loaded onto a 5-mL Sepharose Q, quaternary ammonium anion exchange column (1.6 cm × 20 cm; Pharmacia XK) equilibrated in the same binding buffer. After the column was washed in 5 vol binding buffer, the bound fraction was eluted with a 200-mL linear salt gradient between 0 and 0.5 mol/L NaCl in the same buffer, and 4-mL fractions were collected.
Gel filtration chromatography on Sephacryl S-200 HOURs
Pefabloc (1 mmol/L) and EDTA (0.5 mmol/L) were added to the spun and filtered serum, and the sample was loaded onto a 500-mL Sephacryl S-200 HOURs column (Pharmacia XK 2.6 cm × 100 cm) equilibrated and eluted with phosphate-buffered saline. Fractions (6 mL) were collected.
Analysis and storage of serum fractions obtained by chromatography
Absorbance at 280 nm (A280) of the fractions from each of the columns was determined, and fractions were pooled and concentrated to approximately the original loading volume. Gradient fractions from the ion exchange column, however, were concentrated to 2.5 times less than the loading volume, using an Amicon stir cell (YM 10 filters; Amicon, Beverly, MA). To prevent interference in the rosetting assay, NaCl, glycine, and methyl α-D-mannopyranoside were diluted to low levels (<100 mmol/L, 5 mmol/L, and 5 mmol/L, respectively) in eluted fractions. Aliquots (up to 20 μg protein) of the fractions before and after pooling and concentration, in the absence (nonreduced) or presence (reduced) of 0.1 mol/L dithiothreitol, were resolved by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) (10% acrylamide) according to the method of Laemmli.23 Gels were stained with Coomassie brilliant blue. Aliquots of the pooled and concentrated fractions were fast frozen as soon as possible after preparation (within 2 days of running the column) using liquid nitrogen, and they were stored at −70°C until they were used in the rosetting assay when they were fast thawed at 37°C. Fractions were used in the rosetting assay at concentrations between 10% and 15% vol/vol.
Results
Rosette formation is serum dependent
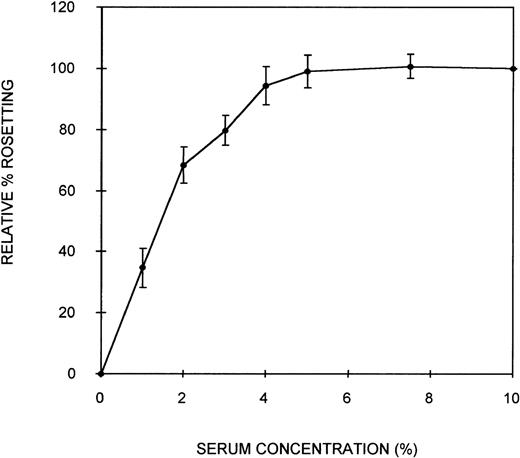
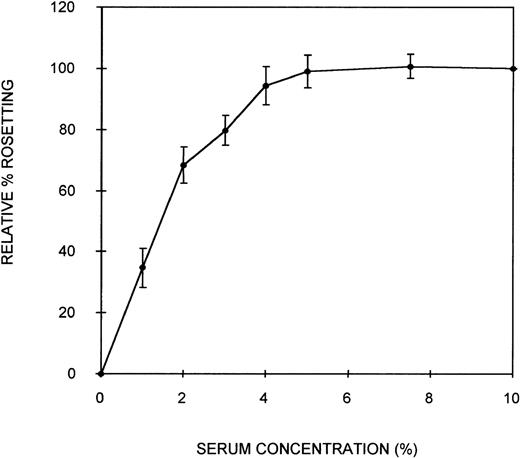
Initially, an assay was developed to assess the ability of serum components to act as bridging molecules between the PRBC and uninfected erythrocytes of PA1 rosettes. Parasites were grown in culture in complete medium containing NHS (10% vol/vol) to ensure that the parasites were capable of expressing the rosetting phenotype. Preliminary experiments demonstrated that the rosetting rate of these cultures was greatest at the early schizont stage (∼30 hours after the invasion of RBC). Therefore, all subsequent assays were conducted with early schizonts. To remove the serum components effectively from the cells and to separate the PRBC from the uninfected erythrocytes, a combination of heparin treatment and Percoll density gradient centrifugation was used. Heparin completely disrupts the rosettes of PA1 at a concentration of approximately 10 μg/mL, allowing serum components that could act as bridging molecules to be released from physical entrapment within the rosette. Subsequent density gradient treatment partially separated PRBC from the uninfected cells. A typical parasitemia of the PRBC-enriched layer was 65%. Mixing these washed PRBC with freshly washed erythrocytes in complete medium without serum resulted in crenation of the cells, which prevented the visualization of rosettes. Therefore, Albumax-I, which prevents crenation, was added to the complete medium. CM-Albumax I could not support rosetting of the PRBC in the absence of serum, and typically no rosettes were observed (the rosetting rate was always less than 5%). This suggested that either there was an absolute requirement for serum component(s) to act as bridging molecules and to promote the interaction between the 2-cell populations of a rosette, that the PRBC had been damaged by the treatment, or that Albumax I inhibited rosette formation. PRBC-RBC mixes were incubated in CM-Albumax I medium containing NHS (10% vol/vol). The rosetting rate in these samples was similar to that observed before heparin and Percoll treatment, which typically consisted of more than 60% rosetting (rosetting rates of the culture before and after treatment were within 10% of each other). This indicated that there was an absolute requirement for serum component(s) to act as bridging molecules and to promote interaction between the cell populations. In studies in which the culture was treated with heparin, washed thoroughly instead of undergoing Percoll treatment, and placed in the CM-Albumax I medium, a dramatic reduction in rosetting to approximately 10% to 15% also resulted. All the studies presented here used the Percoll-based assay method because it appeared to be a more effective treatment for the removal of serum (background rosetting rate of 0%-5% for the heparin-Percoll method versus 10%-15% for the heparin-washing method), and it enabled the addition of uninfected RBC not present in the original culture. Time-course studies indicated that rosette reformation in the presence of serum was rapid (within 10 minutes). However, the incubation time was increased to 45 minutes to ensure optimum rosette formation in all assays. A serum titration curve showing that at least 5% (vol/vol) serum was required for optimum rosette formation is presented in Figure 1. At low serum concentrations (1%), the rosettes formed were small and easily disrupted, but at 10% serum concentrations, the rosettes were large and robust (ie, the cells appeared firmly adhered to each other) (average number of uninfected RBC in the rosettes, 4.3). Plasma was no more effective at promoting rosette formation than serum (data not shown). A parasite isolate obtained from a patient from Uganda also demonstrated serum-dependent rosetting at high levels (approximately 80%), and a serum titration curve similar to that shown in Figure 1was obtained (data not shown). The negative control without serum (CM–Albumax I) and the positive control with 10% vol/vol serum (CM–Albumax I–NHS) were included in all subsequent experiments designed to assess the effect of serum and serum fractions as bridging molecules in rosette formation.
Effect of serum concentration on rosette formation byP falciparum PA1-infected RBC.
PRBC cultured in CM-NHS and rosetting at high levels (more than 60%) were stripped of serum components using combination heparin treatment to disrupt the rosettes and Percoll density gradient centrifugation to separate the PRBC from uninfected cells and medium components. The PRBC were mixed with freshly washed, uninfected erythrocytes in a serum-free medium (CM-Albumax I) in the absence or presence of increasing concentrations of serum, and rosetting was assessed by fluorescence microscopy (×1000 objective in incident ultraviolet light). The relative percentage rosetting is defined as ×100 the rate of rosetting in a culture in which the CM-Albumax I medium is supplemented with the indicated amount of serum/the rate of rosetting in a culture in which the CM-Albumax I medium is supplemented with 10% (vol/vol) NHS. Results are presented as the mean of 3 separate experiments, each using a different batch of human nonimmune AB serum ± SD.
Effect of serum concentration on rosette formation byP falciparum PA1-infected RBC.
PRBC cultured in CM-NHS and rosetting at high levels (more than 60%) were stripped of serum components using combination heparin treatment to disrupt the rosettes and Percoll density gradient centrifugation to separate the PRBC from uninfected cells and medium components. The PRBC were mixed with freshly washed, uninfected erythrocytes in a serum-free medium (CM-Albumax I) in the absence or presence of increasing concentrations of serum, and rosetting was assessed by fluorescence microscopy (×1000 objective in incident ultraviolet light). The relative percentage rosetting is defined as ×100 the rate of rosetting in a culture in which the CM-Albumax I medium is supplemented with the indicated amount of serum/the rate of rosetting in a culture in which the CM-Albumax I medium is supplemented with 10% (vol/vol) NHS. Results are presented as the mean of 3 separate experiments, each using a different batch of human nonimmune AB serum ± SD.
Heat-inactivated NHS and human serum from an individual deficient in either C1q17 or C318 supported the formation of rosettes similarly to that of NHS. Various animal sera, including adult bovine, fetal calf, chicken, sheep, and guinea pig, were tested for their ability to support rosette formation by PA1 in the assay system (all used at a final concentration of 10% vol/vol). However, none of those tested could support rosette formation to more than 15% of that of NHS.
After showing the requirement for human serum components as bridging molecules between the PRBC and uninfected erythrocytes of PA1 rosettes, we sought to characterize the molecule(s) involved. Nonimmune human AB serum was fractionated using a variety of chromatographic techniques. Molecules were separated based on differences in affinity, size, and charge, and the fractions obtained were tested in the biologic assay system for their ability to support rosette formation.
Multiple serum components are involved in bridging parasite-infected erythrocytes to uninfected erythrocytes
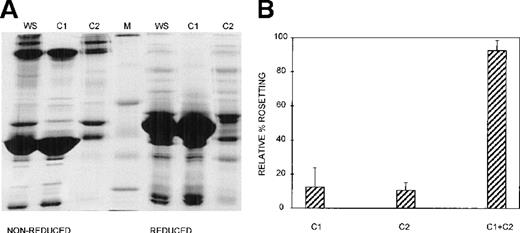
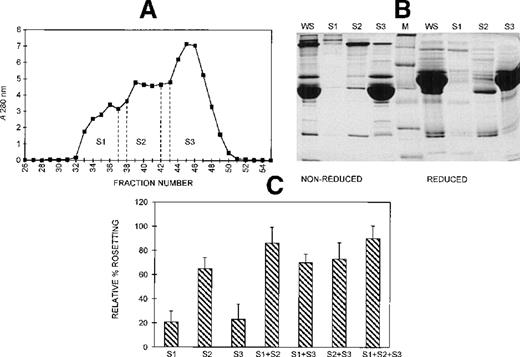
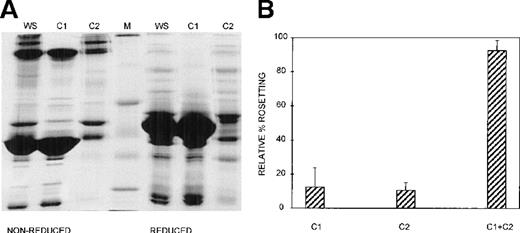
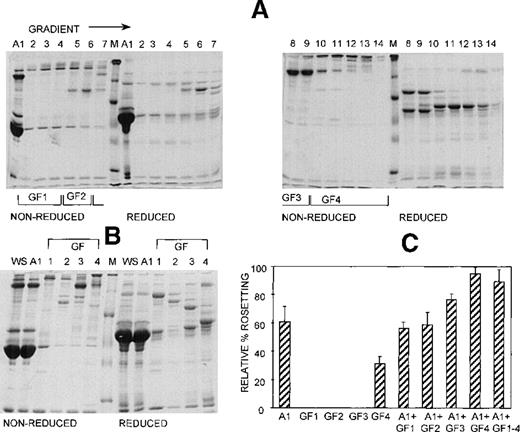
Three different techniques—conA affinity, anion exchange, and gel filtration chromatography—independently demonstrated that multiple components are involved in PA1 rosette formation. ConA is a lectin with affinity for glucose and mannose residues; therefore, it binds to many glycoproteins and glycolipids in serum, including IgM. The unbound material contains nonglycosylated proteins (eg, albumin) and glycosylated molecules (eg, IgG). The SDS-PAGE profile of the 2 fractions is shown in Figure 2A, and it demonstrates the mutual exclusion of components into either the conA unbound (C1) or bound (C2) fractions. As shown in Figure 2B, neither the unbound nor the bound fractions alone were able to support PA1 rosette formation to more than 15% of the levels in whole serum. However, when the 2 fractions were combined, the rosette formation rate was restored to levels similar to those of 10% whole serum. Furthermore, as shown in Figure 3 and Table1, in the presence of either fraction alone, the rosettes were small and fragile, and the average size was significantly smaller than the size of rosettes in 10% whole serum (2.7 uninfected RBC/rosette in C1 and 2.3 uninfected RBC/rosette in C2 versus 4.3 uninfected RBC/rosette in 10% whole serum) (P = .05). However, when the 2 fractions were combined, the rosette size was similar to that in 10% whole serum.
At least 2 serum components are required for rosette formation, as demonstrated using conA affinity chromatography.
(A) SDS-PAGE profile of fractions eluted from a conA–Sepharose affinity column. Aliquots of whole serum (WS) and conA-unbound (C1) and conA-bound, eluted (C2) fractions (up to 20 μg protein, corresponding to the same relative amount of each fraction) were resolved by SDS-PAGE (10% acrylamide) in the absence (nonreduced) or presence (reduced) of 0.1 mol/L dithiothreitol. The gel was stained with Coomassie brilliant blue. Molecular mass markers (M) are 30, 46, 66, 97, and 220 kd from bottom to top, respectively. (B) Effect of the conA fractions on rosette formation. The rosetting assay was conducted as described in the legend to Figure 1, and the fractions were used at 10% (vol/vol) each, for all experiments. Two different batches of serum were fractionated, and the assay was conducted in duplicate for each batch (mean ± SD for 4 experiments).
At least 2 serum components are required for rosette formation, as demonstrated using conA affinity chromatography.
(A) SDS-PAGE profile of fractions eluted from a conA–Sepharose affinity column. Aliquots of whole serum (WS) and conA-unbound (C1) and conA-bound, eluted (C2) fractions (up to 20 μg protein, corresponding to the same relative amount of each fraction) were resolved by SDS-PAGE (10% acrylamide) in the absence (nonreduced) or presence (reduced) of 0.1 mol/L dithiothreitol. The gel was stained with Coomassie brilliant blue. Molecular mass markers (M) are 30, 46, 66, 97, and 220 kd from bottom to top, respectively. (B) Effect of the conA fractions on rosette formation. The rosetting assay was conducted as described in the legend to Figure 1, and the fractions were used at 10% (vol/vol) each, for all experiments. Two different batches of serum were fractionated, and the assay was conducted in duplicate for each batch (mean ± SD for 4 experiments).
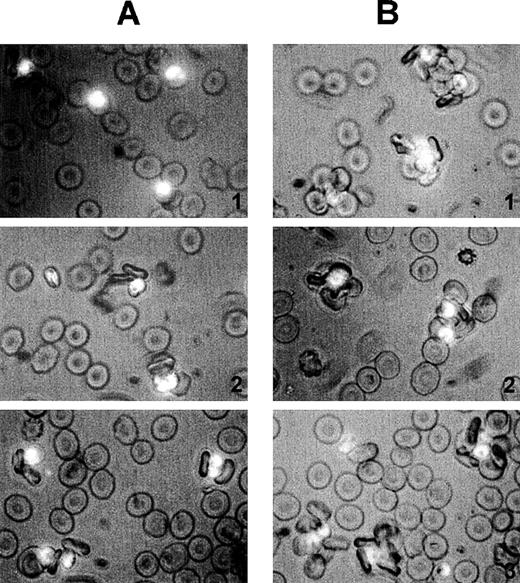
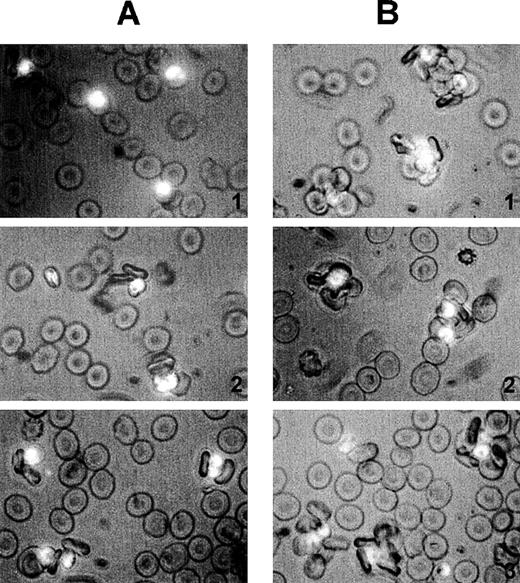
Size and quality of rosettes are determined by multiple serum components.
Rosettes visualized by immunofluorescence in the presence of ethidium bromide to stain parasite nucleic acid (× 1000 objective in incident ultraviolet light) showing the difference between the effect of individual and combined serum fractions. (A) Small rosettes formed in the presence of individual serum fractions. The adhesion between the cells was weak, and the rosettes sometimes fell apart. Panel 1, conA-unbound fraction, C1; panel 2, anion exchange-unbound fraction, A1; panel 3, IgM affinity-unbound fraction, M1. (B) Large rosettes formed in the presence of whole serum or combined fractions. These rosettes were robust and stable, the interaction between the cells was strong, and the rosettes did not fall apart. Panel 1, conA-unbound fraction C1 + bound fraction C2; panel 2, anion exchange-unbound fraction A1 + gradient fraction GF4; panel 3, IgM affinity-unbound fraction M1 + bound fraction M2 (ie, IgM).
Size and quality of rosettes are determined by multiple serum components.
Rosettes visualized by immunofluorescence in the presence of ethidium bromide to stain parasite nucleic acid (× 1000 objective in incident ultraviolet light) showing the difference between the effect of individual and combined serum fractions. (A) Small rosettes formed in the presence of individual serum fractions. The adhesion between the cells was weak, and the rosettes sometimes fell apart. Panel 1, conA-unbound fraction, C1; panel 2, anion exchange-unbound fraction, A1; panel 3, IgM affinity-unbound fraction, M1. (B) Large rosettes formed in the presence of whole serum or combined fractions. These rosettes were robust and stable, the interaction between the cells was strong, and the rosettes did not fall apart. Panel 1, conA-unbound fraction C1 + bound fraction C2; panel 2, anion exchange-unbound fraction A1 + gradient fraction GF4; panel 3, IgM affinity-unbound fraction M1 + bound fraction M2 (ie, IgM).
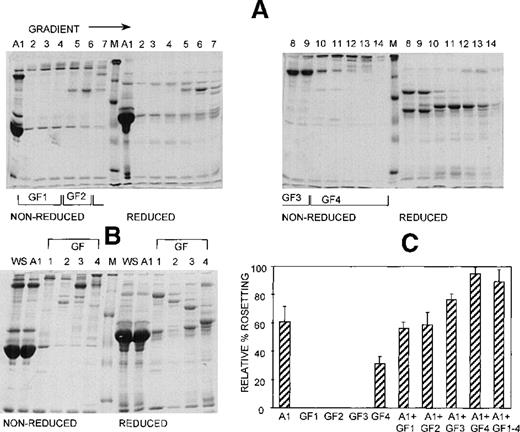
Anion exchange chromatography, which separates molecules based on differences in isoelectric point (PI), was initially carried out at pH 8, and the bound fraction was eluted with a 0 to 0.5 mol/L salt gradient. Under these conditions, most serum components bind to the column, and only the most basic components (eg, IgG) do not bind. Rosetting activity was mediated by the bound material only in fractions that were eluted after albumin, suggesting that the PIs of the components involved in rosetting were lower than those of albumin (PI = 4.8) (data not shown). We then sought to provide additional evidence for the requirement of multiple components in rosette formation by using a pH for the chromatography that would allow separation of the rosetting components into an unbound, more basic fraction containing albumin (higher PI) and a bound, more acidic fraction (lower PI). Chromatography was carried out at pH 7.2, and the bound material was eluted with a 0 to 0.5 mol/L NaCl gradient. Gradient fractions with similar protein profiles, as determined by SDS-PAGE (Figure 4A) were pooled into 4 gradient fractions (G1 to G4) (Figure 4B). As shown in Figure 4C, the unbound fraction (A1) alone supported a high level of rosette formation (61% relative rate). However, as shown in Table 1 and Figure 3, the average sizes of the rosettes were significantly smaller than they were in 10% whole serum (2.6 versus 4.4 uninfected RBC) (P = .05). Both cell populations appeared to be less strongly bound together than they were with whole serum (Figure 3, panel A2). Of the bound fractions, only GF4 was able to support rosetting when tested alone, albeit at much lower levels than with A1 (31% relative rate), and these rosettes were also small and fragile (Table 1). However, when combined, the rosetting rate and the rosette size with A1+GF4 were similar to those of whole serum, indicating that components in both A1 and the gradient fraction GF4 were involved in rosette formation (Figures 3 [panel B2], 4; Table 1). Gradient fraction GF3 also markedly increased the rosetting rate when combined with A1, but to a lower level than GF4; this probably results from the spanning of serum components between adjacent fractions.
At least 2 serum components are involved in rosette formation, as demonstrated using anion exchange chromatography.
SDS-PAGE profile of fractions eluted from a Sepharose Q anion exchange column. Aliquots of the unbound fraction (A1) and gradient fractions (GF) before (A) and after (B) pooling and concentration (aliquots of up to 10 μg protein, corresponding to 10 times more gradient fraction than the WS and A1 fractions), were resolved by SDS-PAGE (10% acrylamide) as described in the legend to Figure 2. (C) Effect of the fractions on rosette formation. The rosetting assay was conducted as described in the legend to Figure 1, and the fractions were used at 15% (vol/vol) of A1 and 10% (vol/vol) of GF1-4 for all experiments. Two different batches of serum were fractionated, and the assay was conducted in duplicate for each batch (mean ± SD for 4 experiments).
At least 2 serum components are involved in rosette formation, as demonstrated using anion exchange chromatography.
SDS-PAGE profile of fractions eluted from a Sepharose Q anion exchange column. Aliquots of the unbound fraction (A1) and gradient fractions (GF) before (A) and after (B) pooling and concentration (aliquots of up to 10 μg protein, corresponding to 10 times more gradient fraction than the WS and A1 fractions), were resolved by SDS-PAGE (10% acrylamide) as described in the legend to Figure 2. (C) Effect of the fractions on rosette formation. The rosetting assay was conducted as described in the legend to Figure 1, and the fractions were used at 15% (vol/vol) of A1 and 10% (vol/vol) of GF1-4 for all experiments. Two different batches of serum were fractionated, and the assay was conducted in duplicate for each batch (mean ± SD for 4 experiments).
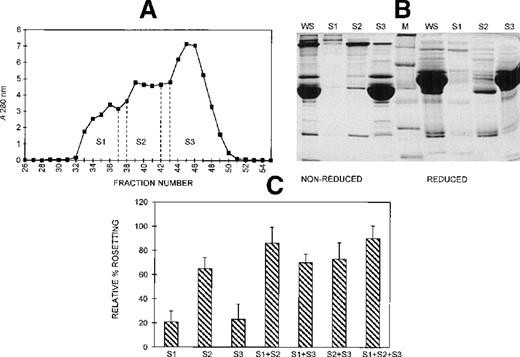
Gel filtration chromatography of serum on a Sephacryl S-200 column was performed to provide information on the size range of the components involved in rosette formation. The elution profile of the serum from this column is shown in Figure 5A. The 3 peaks of absorbance correspond with the presence of IgM (S1, higher molecular mass), IgG (S2, intermediate molecular mass), and albumin (S3, lower molecular mass). The SDS-PAGE profile of the 3 fractions of pooled eluate is provided in Figure 5B. Although the size differential is clear, it is also apparent that some components spanned more than 1 fraction. The activity of each of these fractions in the assay, tested individually and in combination, is shown in Figure 5C. When all 3 fractions were added, rosette formation was restored to 85% of the rate obtained with 10% whole serum, and the average size of rosettes was not significantly different from the size of rosettes in whole serum (4.0 versus 4.3 uninfected RBC) (Figure 3). Fraction 2 alone supported rosette formation at high levels (65% relative rate), as did any combination containing fraction 2. In contrast, fractions 1 and 3 individually provided only 20% of the activity of whole serum. However, when fractions 1 and 3 were combined, they also supported rosette formation at high levels (70% relative rate). It should be noted, however, that each of the individual fractions and each of the paired fractions tested resulted in rosettes that were significantly smaller than rosettes formed with whole serum (Table 1).
Different-sized serum components are required for rosette formation.
(A) Sephacryl S-200 elution profile. Six-microliter fractions were collected, and the A280nm was determined for each. Fractions were pooled (S1-S3) and concentrated to the original volume of serum loaded. (B) SDS-PAGE profile of proteins in pooled fractions eluted from Sephacryl S-200. Aliquots of the whole serum (WS) and the pooled fractions (S1-S3) (up to 20 μg protein loaded, corresponding to the same relative volumes of each) were resolved by SDS-PAGE (10% acrylamide) as described in the legend to Figure 2. Molecular mass markers (M) are 19, 30, 46, 66, 97, and 220 kd from bottom to top, respectively. (C) Effect of the individual and recombined S-200 fractions on rosette formation. The rosetting assay was conducted as described in the legend to Figure 1, and the fractions were used at 15% (vol/vol) each for all experiments. The column was run using 2 different batches of serum, and the assay was conducted in duplicate for each batch (mean ± SD for 4 experiments).
Different-sized serum components are required for rosette formation.
(A) Sephacryl S-200 elution profile. Six-microliter fractions were collected, and the A280nm was determined for each. Fractions were pooled (S1-S3) and concentrated to the original volume of serum loaded. (B) SDS-PAGE profile of proteins in pooled fractions eluted from Sephacryl S-200. Aliquots of the whole serum (WS) and the pooled fractions (S1-S3) (up to 20 μg protein loaded, corresponding to the same relative volumes of each) were resolved by SDS-PAGE (10% acrylamide) as described in the legend to Figure 2. Molecular mass markers (M) are 19, 30, 46, 66, 97, and 220 kd from bottom to top, respectively. (C) Effect of the individual and recombined S-200 fractions on rosette formation. The rosetting assay was conducted as described in the legend to Figure 1, and the fractions were used at 15% (vol/vol) each for all experiments. The column was run using 2 different batches of serum, and the assay was conducted in duplicate for each batch (mean ± SD for 4 experiments).
Because we had established that there were multiple serum components involved in rosette formation of PA1 using affinity for conA, PI, and size as criteria, we then sought to determine the relationship among these 3 criteria in the context of the various serum components involved in rosette formation. Fractions from the 3 columns were tested in combination, and the results are provided in Table2. Combinations of the conA fractions with the anion exchange fractions showed that the conA unbound fraction (C1) was able to combine with the anion exchange gradient fraction (GF4) to restore rosette formation to a similar relative rate, 10% whole serum. Rosette sizes were not significantly different than those formed in 10% whole serum. A similar result was obtained with the conA-bound fraction (C2) and the anion exchange-unbound fraction (A1). Conversely, the unbound fractions from the 2 columns (C1 + A1) were not active together (40% relative rate), and rosette sizes were significantly smaller than those of rosettes formed in whole serum were (2.8 vs 4.3;P = .05). A similar result was obtained with the bound fractions from the 2 columns. These data suggest that C1 and A1 contain similar rosetting components, as do C2 and GF4. Combination of the conA fractions with the S-200 higher and lower molecular mass fractions provides some idea of the relative size of the rosetting components in the 2 fractions. C1 complements S1 (higher molecular mass fraction), suggesting that because higher and lower molecular mass components are important for stable rosette formation, the C1 predominantly contains the lower molecular mass activity. Similarly, C2 complements S3 (lower molecular mass fraction), suggesting that C2 predominantly contains the higher molecular mass component(s) involved in rosetting. Combination of the anion exchange fractions with the S-200 fractions supports this proposition because A1 complements S1 relative to S3 and GF4 complements S3 relative to S1.
IgM is one of the serum components involved in PA1 rosette formation
IgM was purified from nonimmune human AB serum by affinity chromatography. This provided 2 fractions, an unbound fraction (M1) with a profile identical to that of whole serum that was depleted of IgM and a bound IgM fraction (M2) that was devoid of other proteins, as shown by SDS-PAGE and immunoblotting (data not shown). The unbound, IgM-depleted fraction supported rosette formation between 50% and 65% of the level achieved with whole serum, whereas the IgM fraction alone had a low level of assay activity (less than 10%), even when it was tested at a high concentration (30% vol/vol) (data not shown). Furthermore, the rosettes formed in the IgM-depleted serum were significantly smaller than those formed in whole serum (average number of uninfected RBC per rosette, 2.7 versus 4.3, respectively) (P = .05) (Figure 3, panel A3; Table 1). Rosette formation increased to a rate similar to that of 10% whole serum when the 2 fractions were combined. IgM levels were similar to those in the 10% whole serum control, and the average size of the rosettes increased to 3.9 uninfected RBC per rosette, which is not significantly different than that in the 10% whole serum control (Figure 3, panel B3; Table1).
To provide additional data on the involvement of IgM in rosette formation, the fractions from the conA, anion exchange and S-200 columns that contained IgM were replaced with affinity-purified IgM (M2). Western blot experiments confirmed the presence of IgM exclusively in the conA-bound fraction, predominantly in the anion exchange GF4 (with a much lower but detectable level in GF3), and predominantly in S1 with a just detectable amount in S2 (data not shown). When the IgM (M2) was combined with C1, A1, or S2 + S3, the rosette formation rate did not increase significantly compared with the level achieved with these fractions alone. In addition, as shown in Table 1, the combination of C1 + M2 and S2 + S3 + M2 did not increase the size of rosettes over those obtained with C1 or S2 + S3, which remained significantly smaller than the size of rosettes in whole serum (P = .05). Other components than IgM—high molecular mass, glycosylated component(s), IgM—in the S200 S1 and the conA C2 fractions must therefore be essential for IgM to play its role in rosette formation. In contrast, the addition of IgM to A1 increased the average size of rosettes from 2.6 uninfected RBC (significantly less than whole serum) to 4.2 uninfected RBC (not significantly different to 10% whole serum). These data suggest that a major contributor to rosette formation in GF4 is IgM.
To explore the possibility that the other high molecular mass glycosylated components important in rosette formation in the conA C2 fraction were adhesive glycoproteins involved in cell-cell or cell-matrix adherence systems, von Willebrand's factor, fibronectin, and thrombospondin were each added to conA C1 + M2 (IgM) individually or in combination. Each adhesive glycoprotein was tested on 2 separate occasions, at a range of concentrations similar to those in medium containing 10% serum or plasma. von Willebrand's factor (0.5-10 μg/mL), fibronectin (3-100 μg/mL), and thrombospondin (0.2-4 μg/mL) did not support rosette formation; the number and size of rosettes formed was similar to those formed with conA M2 alone. For each adhesive glycoprotein and at all concentrations tested, the relative rosette formation rate was within 10% of the relative rosette formation rate of conA M2 alone (25%; mean of 3 separate experiments). The average size of rosettes formed was also similar to conA M2 alone and was significantly less than the average size of rosettes formed in whole serum in all experiments.
Discussion
We have shown unequivocally that rosette formation of P. falciparum is dependent on the presence of specific serum components that appear to act as bridging molecules between infected and uninfected cells of a rosette. Serum fractionation using a variety of chromatographic techniques indicated that multiple components are involved in the adhesion interaction, including IgM and at least 2 other molecules. The current study was conducted with a parasite clone. A parasite isolate rosetting at high levels and obtained from a patient from Uganda was shown to be serum dependent. Furthermore, most rosetting parasites obtained from children with P. falciparummalaria in Malawi, East Africa have been found to be dependent on serum for rosette formation (Rogerson personal communication). This indicates that the serum requirement for rosetting is a widespread phenomenon inP. falciparum malaria.
It is interesting that rosette formation of P. falciparum-infected erythrocytes is dependent on human serum. Rosette formation was not supported to any extent by serum obtained from adult bovine, fetal calf, chicken, sheep, or guinea pig. We observed a similar ability to support rosette formation between batches of individual human sera (Figure 1). These batches were all from non-malaria immune persons in the UK. Sera obtained from malaria-endemic regions might be variable in their ability to support rosette formation. Because rosetting has been found to correlate with disease severity,5-7 polymorphisms in the serum molecules involved in rosetting are possible. In addition, sera from persons in a malaria-endemic region are likely to contain antibodies to parasite proteins on the surface of the infected cell, and these may inhibit rosette formation.
The severe pathologic conditions of P. falciparum malaria are associated with the sequestration of parasites in the microvasculature of a variety of organs, a process believed to be the result of cytoadherence and rosetting. The finding that serum components are critical for rosette formation has important implications in the understanding of these adhesive interactions. PfEMP-1, an antigenically variant protein product of the var gene family, appears to be the parasite ligand involved in cytoadherence24 and rosetting.12 13 It is possible that a population of these proteins adheres to endothelial cells and uninfected erythrocytes with serum components as bridging molecules, which emphasizes the importance of identifying the serum molecules involved in bridge formation and the molecular basis for this adhesion interaction.
A number of adhesive interactions involve plasma components as bridging molecules between cell populations and provide precedents for the rosetting process. These interactions occur under physiological or pathologic circumstances and include the adhesion of leukocytes to endothelium through fibrinogen, a process involved in leukocyte trafficking and recirculation,25 the adhesion of apoptotic neutrophils to macrophages through thrombospondin,26 and the invasion mechanism of pathogenic mycobacteria in which the complement cleavage product C2a associates with the mycobacterial surface and cleaves C3, resulting in macrophage recognition.27 It has been suggested that P. falciparum-infected erythrocytes attach to endothelial cells through several receptor-ligand pairs. In addition to PfEMP-1, modified erythrocyte band 3 may play a role in cytoadherence; it has been proposed that this interaction with the endothelium occurs through thrombospondin.28 A number of adhesive glycoproteins involved in cell–cell and cell–matrix interactions, including thrombospondin, von Willebrand's factor, and fibronectin, present in the conA-bound fraction (C2) were tested for their ability to support rosette formation in combination with the conA-unbound fraction (C1) and IgM. However, each of these high-molecular-mass adhesive glycoproteins supported rosette formation similarly to C1+ IgM alone, suggesting either that they were not involved in the bridging interaction between the parasitized and the uninfected cells or that additional components in the bound fraction (C2) were required for bridge formation.
One study that implicated PfEMP-1 as the rosetting ligand on the parasitized cell also identified CR1 as a rosetting receptor on the uninfected cell.12 This may suggest that complement components are the bridging molecules. Both C3b and C4b, opsonins that are produced by the activation of the complement cascades, bind to CR1. However, our studies provide no evidence that complement activation is important in rosette formation of PA1. The use of heat-treated serum and of C1q- and C3-genetically deficient sera in the assay did not affect rosetting. Other workers have also reported that heat-treated serum supports rosetting just as effectively as fresh serum (Rowe JA, DPhil Thesis, University of Oxford, 1994). Because most of the CR1 studies were carried out with a different parasite clone, R29, it is possible that different mechanisms are responsible for rosette formation in the different parasites or that CR1 is able to bind to other serum factors that do not involve complement activation for their formation.
Multiple serum components are involved in rosette formation, highlighting the complexity of this interaction, as evidenced by the results of 3 different fractionation techniques—conA affinity, anion exchange, and gel filtration. It is therefore possible that in the presence of whole serum at optimum levels, there are individual, unrelated, parallel bridges involving single serum components that span the 2 cell populations. Alternatively, there may be serial bridges in which more than 1 molecule is involved or a combination of these 2 types of bridge. The conA data (Figure 2) show that both unbound (C1) and bound (C2) serum components are essential for rosette formation because only a very low level of rosetting is observed with either fraction alone. This may indicate that there are no parallel bridges, that all bridges involve more than 1 serum component and these are in different fractions, or that, perhaps more likely, multiple bridges are required to achieve great enough strength of interaction to allow stable rosette formation. The anion exchange data are somewhat different (Figure 4). The unbound material (A1) is able to support rosetting to a relatively high level, though the rosettes are small and fragile. Thus, although the combination data (Table 2) suggest that C1 and A1 are similar, A1 must contain additional component(s) that allow rosettes to form. It is possible that the components form individual parallel bridges so that the strength of interaction, though relatively weak, is strong enough to allow small rosettes to form. Alternatively, a serial bridge formed by the components in A1 may allow the cells to interact.
The S-200 gel filtration data also provide evidence for the involvement of multiple serum components in rosetting, and they provide additional understanding of the relative sizes of the molecules involved (Figure5). These data suggest that there is a rosetting component(s) of higher molecular mass, probably more than 150 kd, that spans fractions F1 and F2 and a rosetting component(s) of lower molecular mass, probably more than 60 kd, that spans fractions F2 and F3. Hence, F2 is active alone, and F1 and F3 combined support rosetting. The fact that components span fractions probably accounts for the small size of rosettes for all fraction combinations, except S1 + S2 + S3 because it is likely that 1 or more of the components responsible for rosette formation is limiting in all single and paired fraction samples.
We have provided clear evidence that IgM is 1 of the bridging molecules involved in rosette formation and that it is a major contributor to rosette size and stability. Removal of IgM from the serum resulted in the formation of rosettes that were significantly smaller than those in whole serum (Figure 3, Table 1). The addition of IgM to the depleted serum resulted in rosettes that were not significantly different in size to those in whole serum. It is possible that IgM forms independent parallel bridges that stabilize the rosettes and allow more uninfected cells to bind. Alternatively, IgM may contribute to the strength of an interaction. It is clear that IgM alone cannot support rosette formation, even when added at high concentration (30% vol/vol). This suggests that IgM cannot span the 2 cell populations and another molecule(s) is required for binding (ie, it is involved in a serial bridge) or that the strength of interaction brought about by IgM alone is insufficient to hold together the 2 cell populations. In the latter case, other bridges would be required to achieve the cumulative strength needed. When IgM was added back to the conA C1 fraction, it was unable to increase the percentage of rosette formation or the size of rosettes, and it was unable to replace the high molecular mass fraction of the gel filtration column. This supports the notion that something else is present in the C2 and S1 fractions that either forms a serial bridge with the IgM or contributes a parallel bridge to strengthen the interaction. When the IgM was added back to the anion exchange-unbound fraction (A1), it was able to increase the size of the rosettes significantly, but it did not restore the percentage rosetting to control levels. Thus, a major component in the anion exchange gradient fraction (GF4) that contributes to rosetting is IgM. However, it is possible that other component(s) in GF4 have a role in rosette formation because this fraction alone supports a low level of rosette formation, whereas IgM alone cannot. Whatever its bridging mechanism, it appears that the intact IgM molecule is required because IgM monomers do not increase the size of rosettes formed when they are added back to IgM-depleted serum (Black J, unpublished observation).
The data on the combination of the different column fractions (Table 2) provide initial understanding of the types of molecule involved in addition to IgM, though the identity of these molecules is unknown. One or more of the molecules is of lower molecular mass, does not bind to conA, and is acidic (with a lower PI than albumin). However, it is less acidic than the other molecule(s) involved, which are of higher molecular mass and glycosylated. Plasma and serum are extremely complex mixtures of molecules, and many of these have not been identified in terms of their sequence or function. This, together with the emerging understanding of the complexity of the rosetting interaction, makes identification of the molecules involved a difficult but achievable task. It should now be possible to combine the fractionation approaches presented here, to produce a purification strategy that enables further resolution of the components. Additional sophisticated methods of analysis are required, including mass spectroscopy, to identify the important molecules involved in rosetting. Polymorphisms in the serum molecules responsible for this cell interaction may result in individuals with differing abilities to support rosette formation. Because rosette formation in vitro has been shown to be correlated with disease severity in a number of studies carried out in Africa,5-7 such polymorphisms may dramatically affect the likelihood of developing severe P. falciparum malaria. The propensity of a particular parasite to form rosettes is therefore governed by the interplay of a number of different factors, including parasite genotype, host RBC receptor, and serum component polymorphisms.
Acknowledgments
The authors thank Dr A. A. Holder for helpful advice and for critical reading of the manuscript. They also thank Professor C. Green for support throughout this work.
Supported by Northwick Park Institute for Medical Research, The Wellcome Trust, and the British Infection Society.
Reprints:Geoffrey Pasvol, Department of Infection and Tropical Medicine, Imperial College School of Medicine, The Lister Unit, Northwick Park Hospital, Watford Road, Harrow, Middlesex, HA1 3UJ, United Kingdom; e-mail: g.pasvol@ic.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.