Abstract
Ephrin-A4 is a ligand for the erythropoietin-producing hepatocellular (Eph) receptor family of tyrosine kinases. We have identified a secreted form of ephrin-A4, denoted ephrin-A4 (s), which is encoded by an alternatively spliced mRNA and is produced by in vivo activated B cells in tonsils. Blood B cells secrete ephrin-A4 (s) upon stimulation via the B-cell antigen receptor. A subpopulation of tonsil cells in the crypts with a dendritic cell phenotype was shown to express EphA2, an Eph receptor tyrosine kinase that was found to be capable of binding an ephrin-A4 immunoglobulin chimeric protein. We conclude that ephrin-A4 (s) may play a role in the interaction between activated B lymphocytes and dendritic cells in human tonsils. (Blood. 2000;95:221-230)
The development of hematopoietic cells involves the commitment and differentiation of self-renewing pluripotent stem cells into mature cells of various lineages including B lymphocytes. In the human bone marrow, discrete stages of B lymphopoiesis can be discerned based on the ordered loss and acquisition of B–lineage-specific proteins and the state of rearrangement and expression of immunoglobulin (Ig) genes. Mature B cells that express a membrane-bound IgM receptor leave the bone marrow and migrate to peripheral lymphoid organs. Here, upon contact with an antigen, B cells may enter a second round of clonal expansion and differentiation, resulting in the formation of antibody-secreting plasma cells or memory B cells. These processes are controlled by interactions between differentiating B lymphocytes, B cells, and soluble molecules in the microenvironment. Although a number of key membrane-bound and soluble molecules have been identified in recent years, it has also become apparent that additional and as yet unknown ligands and receptors play a role in early and late B-cell differentiation processes.1-6
Receptor tyrosine kinases and their ligands play a critical role in regulating cellular survival, proliferation, and differentiation.7 On the basis of predicted structural homologies, sequence conservation, and similarity of ligands, receptor tyrosine kinases have been assigned to several subclasses.8One subclass, the erythropoietin-producing hepatocellular (Eph) carcinoma family of receptors, constitutes the largest known family of receptor tyrosine kinases. The Eph family of receptors comprises at least 14 distinct members,9,10 from Xenopus to man,9,11-16 that are highly conserved. Recently, a family of at least 8 membrane-bound ligands for Eph receptors, termed ephrins, has been identified.17 Members of this family share between 23% and 56% identity at the amino acid level and display promiscuous binding to different Eph receptors.18
Efficient activation of Eph receptors by ephrins requires anchoring the ligands to the cell membrane, either through a hydrophobic transmembrane region or a glycosyl phosphatidylinositol (GPI) group.19 Interestingly, membrane-bound ephrins may transduce signals upon interaction with their cognate receptor.20 Signaling via Eph receptors and their ligands has been implicated in axon guidance and fasciculation, regulation of cell migration, and definition of compartments in the developing embryo.17,21 In addition, Eph receptors appear to play a role in angiogenesis,22 fetal human B lymphopoiesis,23 and erythropoiesis.24
The recent notion that Eph receptors and their ligands may be selectively expressed in subpopulations of hematopoietic cells prompted us to search for expression of members of the ephrin family of ligands in human B-lineage cells. Here we report the identification of a splice variant of the ephrin-A4 gene, ephrin-A4 (s), which encodes a secreted form of this ligand. Soluble ephrin-A4 is produced by mature B cells in the tonsil and by blood B cells that are activated in vitro via their B-cell antigen receptor. An ephrin-A4 binding tonsillar cell subpopulation with a dendritic cell phenotype was shown to express the Eph-A2 receptor, 1 of 3 Eph receptors known to autophosphorylate upon ephrin-A4 binding.18 These results suggest that Eph receptors and their ligands play a role in interactions between activated B lymphocytes and other cell types in the microenvironment.
Materials and methods
Cell separation procedures
Tonsils, obtained from children undergoing routine tonsillectomy, were minced, and mononuclear cells (MNC) were purified by Lymphoprep (Nycomed Pharma, Oslo, Norway) density gradient centrifugation. In some experiments, tonsillar MNC were depleted of T cells by 2 rounds of rosetting with 2-aminoethyl-isothiouronium-bromide-treated sheep red blood cells.25 In other experiments, B cells and T cells were isolated with anti-CD19–coated or CD4-coated beads26 (Dynabeads; Dynal, Oslo, Norway). Detachment of beads from the cells was performed (DETACHaBEAD, Dynal) at ambient temperature for 45 minutes.27 The cells were washed twice in RPMI 1640 with 1% FCS before immunofluorescent staining.
For isolation of adherent cells, tonsils were minced and washed before adding a collagenase solution (Collagenase/Dispase/DnaseI; Boehringer Mannheim, Mannheim, Germany) followed by incubation for 15 minutes at 37°C. The solution was discarded, and fresh solution was added for an additional 2 hours at 37°C. The cells were washed twice in phosphate-buffered saline (PBS), resuspended in RPMI 1640 with 5% FCS, and seeded in tissue culture flasks coated with bovine collagen (Vitrogen; Collagen Biomaterials, Palo Alto, CA). The cells were incubated overnight and detached from the flasks with PBS/1 mmol/L EDTA.
Venous blood was obtained from healthy volunteers, and MNC were obtained by Ficoll-Paque density centrifugation. CD4+ T cells or CD19+ B cells were isolated by magnetic bead separation, as described for the tonsil cells.
Cell culture
Blood B cells were stimulated in RPMI 1640 medium containing 10% FCS with anti-μ antibodies (F[ab′]2fragment) (Dako, Glostrup, Denmark) at a final concentration of 37.5 μg/mL fixed Staphylococcus aureus bacteria (SAC 1/20 000; Calbiochem-Behring, Cambridge, England), 5 × 10-8mol/L of the phorbol ester 12-O-tetradecanoyl-phorbol 13-acetate (TPA), or 5% T-cell supernatant. T-cell supernatant is collected from a 24-hour PHA stimulation of MNC from 5 different donors.28 CD4+ blood T cells were stimulated with TPA as described for the B cells. CD4+ and CD8+ T cells were stimulated with anti-CD3 coated beads (Dynal) at a concentration of 2 beads per cell for the indicated times.
Cell lines
The following human cell lines were used in this study: pre-B cell line Reh (ATCC CRL 8286) and Nalm 6;29mature-B cell lines Bjab (Dr G. Moldenhauer, University of Heidelberg, Heidelberg, Germany) and Daudi (ATCC CCL 213); plasmacytoid cell lines U266 (ATCC TIB 196); T cell lines JM, Jurkat (ATCC TIB 152), and HPB ALL; myeloid cell lines KG1-A (ATCC CCL 246); HL-60 (ATCC CCL 240) and U937 (ATCC CRL-1596); erythroid precursor cell line K562 (ATCC CCL-243); and cervical carcinoma cell line HeLa (ATCC CCL 2). All cell lines were grown in RPMI 1640 medium supplemented with 5% FCS at 37°C in a humidified atmosphere with 5% CO2.
RNA isolation, Northern blot analysis, and first strand cDNA synthesis
Total RNA was extracted from cells or tissues by standard methods, and 10 μg was size-fractionated on a 1% agarose formaldehyde denaturing gel, transferred to nitrocellulose membranes, and cross-linked by baking for 2 hours at 80°C. We used a commercially available multiple tissue Northern blot (Immune blot 1; Clontech, Palo Alto, CA). Prehybridization (1 hour) and hybridization were performed in hybridization buffer (5 × SSPE, 10% dextran sulfate, 0.1% SDS, 50% formamide, 100 μg/mL sheared salmon sperm DNA) at 42°C. The membranes were hybridized overnight with either a32P-dCTP–labeled ephrin-A4 cDNA probe or a control β-actin probe. After hybridization, the membranes were washed under high stringency in 0.2 SSC/0.1% SDS at 65°C. PolyA+ mRNA was isolated from cells or tissues using oligo-dT beads,30 and first-strand cDNA was synthesized directly on mRNA bound to oligo-dT beads,30 as previously described. Finally the first-strand cDNA beads were washed twice in 100 μL TE buffer, solved in 25 μL TE, and stored at -20°C.
Isolation and nucleotide sequence analysis of ephrin-A4 cDNAs
An inventory of ephrin sequences was performed on first-strand cDNA generated from mRNA isolated from the pro-B cell line Reh. The primers used were based on the conserved amino acid sequences LY(L/M)V (primer ephlig5′: CGG ATC CGT (C/T/A/G)TA TA(T/C) ATG GT) and (D/Y)YYY(S/T) (primer ephlig3′: CGA ATT C(A/G)(A/T) (G/T/A)AT (G/A)TC (G/A)(A/T)A (A/T/C/G)T(A/C)) present in ephrin-A1, ephrin-A3, ephrin-A4, and ephrin-B1 sequences.31 32 PCR amplification was performed on oligo-dT–immobilized cDNA reverse transcribed from 500 ng of mRNA, using 0.75 μg of each primer and 40 cycles of 1 minute at 94°C, 2 minutes at 37°C, and 3 minutes at 63°C. Amplified products with the expected size (approximately 180 base pair [bp]) were isolated from agarose gels, ligated into T-vector (Promega, Madison, WI), and used for dideoxy sequencing with the sequenase system (Stratagene, La Jolla, CA).
The ephrin-A4 PCR fragment, obtained after cloning and sequence analysis, was labeled with 32P-dCTP in a PCR reaction using the degenerate primers ephlig3′ and ephlig5′. Fifty ng of ephrin-A4 PCR fragment was used as template in a 50-μL reaction containing 0.05 mmol/L dATP, dGTP, and dTTP; 5 μL32P-dCTP (300 Ci/mmol/L; Amersham Pharmacia Biotech, Uppsala, Sweden); and 250 ng of each degenerate primer. The PCR conditions were 12 cycles with 1 minute at 94°C, 1 minute at 45°C, and 1 minute at 72°C. The resulting probe was used to screen an Reh cell line cDNA library constructed in the expression vector pCDM8.34 Four cDNA clones were isolated. All clones were sequenced from both ends using the T7 primer and a pCDM8 specific reverse primer. Homology to known sequences was assessed (Blast program; National Center for Biotechnology Information, Bethesda, MD).
Amplification of ephrin-A4 transcripts
To detect both ephrin-A4 transcripts, we employed a semiquantitative PCR approach using a forward primer common to both variants and reverse primers specific for each splice variant. Primers to amplify ephrin-A4 (s) were forward primer 1A: 5′-GTG GAG CTG GGC CTC AAC GAT TAC C-3′ (nucleotides 169-186) and reverse primer 2: 5′-GGA GAG GAA CCT TCC CTC-3′ (nucleotides 489-506 in ephrin-A4 (s) sequence) yielding a PCR product of 337 bp. Primers to amplify ephrin-A4 (m) were forward primer 1A (same as for ephrin-A4(s)) and reverse primer 3: 5′ GAG TCA GGC CAT CCT GTT G (nucleotides 500-520 in ephrin-A4 (m) sequence), yielding a PCR product of 351 bp. The PCR conditions were 2 minutes at 94°C followed by 40-second cycles at 94°C, 56°C, and 72°C. As previously described, semiquantitative PCR and control β-actin PCR using primers23 were performed.
The samples were separated on a 1.5% agarose gel, blotted to nitrocellulose filters, and probed with 32P-dCTP labeled full-length ephrin-A4 cDNA probe or β-actin. Both ephrin-A4 (s) and ephrin-A4 (m) amplified fragments were cut out of the gel, cloned into T-vector (Promega), and subsequently sequenced to confirm the identity of the sequences.
Nucleotide sequence analysis
Two full-length cDNA clones (ephrin-A4 [m] and ephrin-A4 [s]) and a genomic ephrin-A4 clone were sequenced in both directions after restriction fragment subcloning (pBluescript SK, Stratagene) with either T3, T7, or ephrin-A4 specific primers. Double-stranded sequencing was performed on plasmid DNA using T7 DNA polymerase (Amersham Pharmacia Biotech), dideoxy nucleotides (USB, Cleveland, OH), and 35S-dATP (NEN, Boston, MA). Database searches were performed using a network service (NCBI).
In situ hybridization
A dioxygenin-(Dig)-11-dUTP labeled probe for in situ hybridization was synthesized by PCR34 using the forward primer 1B 5′-GGG CGA TGC GGC TGC TGC, nucleotides 23-40 and reverse primer 3 (described above) and the cloned ephrin-A4 (m) cDNA as a template. A negative control probe was prepared by amplification of a fragment of the Echerichia coli neomycin-resistant gene using specific primers and Dig-11-dUTP.
Frozen tissue sections of 5-6 μm were fixed in 4% formaldehyde, washed, dehydrated, and blocked for endogenous peroxidase with 1.5% H2O2 in methanol. mRNA was made accessible for the probes by treatment with proteinase K (1 μg/mL) and Triton- × 100 (0.005% in PBS). Sections were preincubated for 10 minutes at 42°C with a 25-μL hybridization mixture (30% formamide, Tris/EDTA buffer, 4 × standard saline citrate, 1 μg/μL yeast tRNA, 1 μg/μL herring sperm DNA) (Boehringer Mannheim) before the denatured probe was added for 18 hours at 37°C. After hybridization, the slides were washed, and the probe was detected with a monoclonal anti-Dig antibody (1:50 in PBS/1% BSA; Boehringer Mannheim). The signal was detected with horseradish-peroxidase-conjugated swine antirabbit antibody (1:100 in PBS/1% BSA; DAKO, Glostrup, Denmark) and visualized with diaminobenzidine with nickel intensification.
Isolation of genomic ephrin-A4 clones
We screened 106 plaque-forming units from a human lymphocyte genomic library in λ-DASH (Stratagene) with32P-dCTP labeled ephrin-A4 cDNA insert. Hybridizing clones were plaque purified in subsequent screening rounds. BamHI fragments were subcloned from selected phages (pBluescript SK, Stratagene) for nucleotide sequence analysis.
Preparation of ephrin-A4 specific antibodies
Antisera were made against a synthetic peptide corresponding to peptide sequence SHPKEPESSQDPLEE (amino acid number 160-175), in a specific part of ephrin-A4 (s) (Figure 1A), thus only recognizing ephrin-A4 (s). Rabbits were initially immunized in the scruff with 300 μg KLH-coupled peptide mixed with Freunds complete adjuvant. Animals were boosted once a month with the antigen in Freunds incomplete adjuvant. Serum was collected before each new booster round and screened for specific antibodies in an enzyme-linked immunosorbent assay (ELISA). Affinity-purified antibodies were obtained by running high-titer rabbit antiserum through a column of ephrin-A4 specific peptide coupled to 4 NHS-Sepharose beads (Amersham Pharmacia Biotech, Uppsala, Sweden). Specific antibodies were eluted with 0.1 mol/L glycin/HCl pH 2.5 and dialyzed against PBS.
Ephrin-A4 cDNA clones.
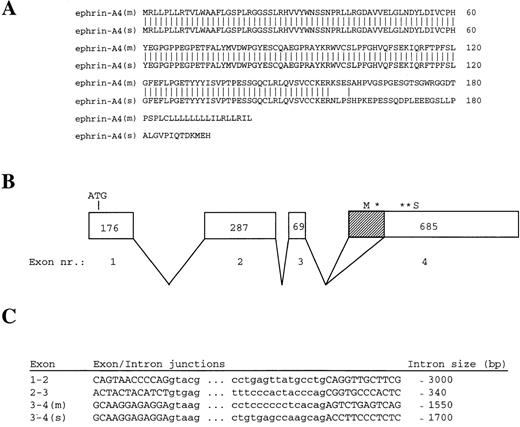
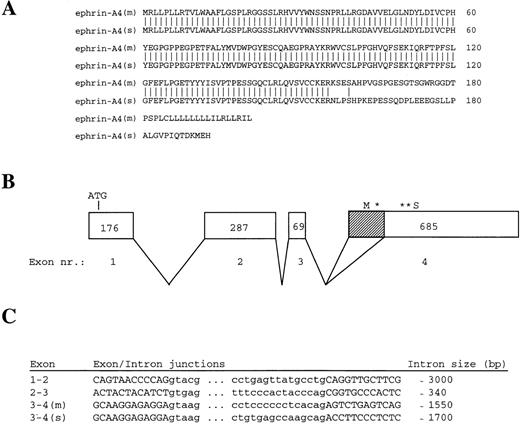
Amino acid sequences of ephrin-A4 (m) and ephrin-A4 (s) and ephrin-A4 gene structure. (A) Comparison of the amino acid sequences of ephrin-A4 (m) and ephrin-A4 (s). Amino acid numbering is depicted on the right. (B) Schematic presentation of the exon-intron organization of the ephrin-A4 gene. Exons are boxed. The size of the exons in bp are indicated by numbers in the boxes. Shaded sub-box in exon IV denotes the part of the mRNA that is spliced out in the ephrin-A4 (s) variant. *Denotes translation stop codon in the ephrin-A4 (m) sequence. **Denotes translation stop codon in the ephrin-A4 (s) sequence. (C) Sequences of exon-intron junction and the size of the introns. Capital letters denote the exons, and small letters denote the introns.
Ephrin-A4 cDNA clones.
Amino acid sequences of ephrin-A4 (m) and ephrin-A4 (s) and ephrin-A4 gene structure. (A) Comparison of the amino acid sequences of ephrin-A4 (m) and ephrin-A4 (s). Amino acid numbering is depicted on the right. (B) Schematic presentation of the exon-intron organization of the ephrin-A4 gene. Exons are boxed. The size of the exons in bp are indicated by numbers in the boxes. Shaded sub-box in exon IV denotes the part of the mRNA that is spliced out in the ephrin-A4 (s) variant. *Denotes translation stop codon in the ephrin-A4 (m) sequence. **Denotes translation stop codon in the ephrin-A4 (s) sequence. (C) Sequences of exon-intron junction and the size of the introns. Capital letters denote the exons, and small letters denote the introns.
Construction of chimeric proteins and binding to cells
An expression vector with the mouse IgG2b heavy chain constant region was constructed. The IgG2b sequence, encompassing the hinge and CH2 and CH3 regions, was amplified by PCR using a genomic IgG2b fragment (accession number v00 763.em_ro) as the template. BamHI and XhoI restriction sites were included in the forward and the reverse primer respectively (2bfor: 5′ CCG GGA TCC GAG CCC AGC GGG CCC ATT TC and 2brev: 5′ GGC TCT AGA TGC AGG CAG AAA CCT CAT TC). PCR products were digested with BamHI and XhoI and ligated into the pCDNA1 vector (InVitrogen, Carlsbad, CA). Two chimeric proteins were generated with this vector, ephrin-A4-Fc and CD19short-Fc. To generate ephrin-A4-Fc, the common part of the 2 ephrin-A4 variants was amplified (excluding the GPI-signal sequence or 3′ end of ephrin-A4 (s)), using a vector-specific primer (T7) and a reverse ephrin-A4 primer (Ephrinrev: 5′ CCG GGA TCC AAC AGG GAT GGG CTG ACT) including a BamHI site. The resulting product was cleaved with HindIII and BamHI and ligated in pCDNA1. A construct for production of a control Fc-chimeric protein was generated with the signal sequence and the first 30 amino acids of the CD19 molecule. This fragment of CD19 was amplified from CD19 cDNA in πiH3 vector35 using a vector-specific primer and a reverse CD19 primer (CD19rev: 5′ CCG CGG ATC CGG TCA GCT GCT GAG TGG G) including a BamHI site. The resulting product was cleaved with HindIII and BamHI and ligated into pCDNA1γ 2b.
Plasmids encoding ephrin-A4-Fc or CD19short-Fc were transfected into COS cells,33 and the chimeric proteins were purified from culture supernatant by affinity chromatography on a protein-G column (Pharmacia). Integrity of the fusion proteins was confirmed by labeling transfected COS cells overnight with 35S-methionine, purifying the fusion proteins from the culture medium, and separating purified products on a 12% acryl amide gel (data not shown).
Cells in solution (PBS/0.1% BSA/0.1% Na-azide) were preincubated for 10 minutes with human aggregated IgG (Pharmacia) and then incubated with 25 μg/mL fusion protein for 1 hour at 4°C. The cells were washed and stained with a PE-labeled anti-mouse Ig polyclonal antibody (Ig-RPE; Southern Biotechnology Associates, Birmingham, AL) directed to the Ig tail of the fusion proteins. Double staining of the cells was performed with the following FITC-labeled antibodies: anti-CD4, anti-CD11c, anti-CD13, anti-CD21, anti-CD45, and anti HLA-DR (all from DAKO); anti-CD19 (Becton Dickinson, San Jose, CA); anti-CD31 and anti-CD86 (Pharmingen, San Diego, CA); anti-CD38 and anti-CD34 (Coulter Immunotech, Pittsburgh, PA); and anti-CD40 (Caltag, Burlingham, CA). We also used biotin-labeled anti-CD123 (Pharmingen).
Western blotting
Whole cell lysates were made from different fractions of tonsil adherent cells and tonsil B and T cells. Ephrin-A4 binding cells in the tonsil adherent fraction were isolated by first binding ephrin-A4-Fc fusion protein to rabbit anti-mouse IgG2b-coated beads (Dynabeads, Dynal) followed by incubation of the tonsil adherent cells with these beads for 1 hour at 4°C before washing the beads twice in PBS. CD34+ endothelial cells were isolated from the ephrin-A4 depleted cells using anti-CD34 coated beads (Dynal) as described for the ephrin-A4 binding cells. Cells depleted for ephrin-A4 binding cells and CD34+ cells are denoted rest adherent.
Ten μg of cell lysate were separated by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and blotted to nitrocellulose membranes. The membranes were hybridized with a polyclonal anti–EphA-2 antiserum (Santa Cruz Biotechnology, Santa Cruz, CA) and visualized (ECL system; Amersham).
COS cells were either transfected with plasmids encoding ephrin-A4 (m) or ephrin-A4(s) or mock-transfected.33 Supernatants and cells were collected 4 days after transfection. The cells were lysed (PBS/1% NP-40), and 10 μg of total cellular protein or 10 μL of culture supernatants was separated (SDS-PAGE). Tonsil B and T cells were lysed, and 10 μg of protein was separated (SDS-PAGE). All samples were blotted to nitrocellulose and hybridized with anti–ephrin-A4(s) antiserum and visualized (Amersham).
B cells (1 × 105 cells in 200 μL RPMI 1640 with 10% FCS per well of a 96-well plate) were stimulated with anti-μ, SAC, and T-cell supernatants in different combinations.
After 6 days of stimulation, culture supernatants were harvested, and 10 μL of supernatant were separated (SDS-PAGE) and blotted to nitrocellulose filter. The filters were hybridized with biotinylated anti–ephrin-A4 (s) antiserum and developed as described above.
Immunohistochemistry
Frozen sections of tonsil were fixed with methanol and pretreated with H2O2 to block endogenous peroxidase activity. The sections were incubated with anti-Eck (Eph-A2) antiserum (1/50, Santa Cruz Biotechnology) or normal rabbit antiserum with the same concentration. After washing, the cells were incubated with biotinylated goat anti-rabbit Ig (Vector Laboratories, Burlingham, CA). The signal was developed with avidin-peroxidase (Vector Laboratories) and diaminobenzidine. The tissues were counterstained with hematoxylin.
Results
Isolation and characterization of ephrin-A4 cDNA clones
The recent notion that Eph receptors may be selectively expressed in subpopulations of human B lymphocytes prompted us to search for expression of members of the ephrin family of ligands for Eph receptors in human B-lineage cells. RNA extracted from the human pro-B cell line Reh was analyzed for the presence of ephrin-A transcripts in PCR using degenerate primers hybridizing to conserved stretches of nucleotides in the ephrin-A1, ephrin-A3, ephrin-A4, and ephrin-B1 sequences.31 32 Nucleotide sequence analysis of 12 cloned PCR fragments unveiled the presence of the ephrin-A4 sequence.
A 32P-labeled ephrin-A4 PCR fragment was prepared and used to probe an Reh pro-B cell plasmid cDNA library. The cDNA insert of 2 hybridizing clones, 5.1 and 2.1, was sequenced from the 5′ and 3′ end, and the partial sequences were found to represent the published ephrin-A4 sequence. In contrast to the published ephrin-A4 sequence,32 both clones contained the 3′ untranslated region, including a poly-A tail. In agarose gel electrophoresis, a slight difference in size was observed between clones 5.1 and 2.1. The complete nucleotide sequences, which are identical to the published ephrin-A4 sequence, showed that clone 5.1 is 1182 nucleotides long and encodes a protein of 201 amino acid residues. Clone 2.1 is 1036 nucleotides long and lacks a 146 bp stretch (position 498-643) at the 3′ end of the open reading frame. Clone 2.1 encodes a protein of 193 amino acid residues. As a result of the frame shift incurred by the missing 146 bp, clone 2.1 differs by 37 amino acids from clone 5.1 at the carboxy terminus (Figure 1A). This altered carboxy terminus does not contain a typical transmembrane region nor does it harbor the GPI-signal sequence present in membrane-bound ephrin-A4.32This suggests that clone 2.1 may encode a secreted molecule. The mRNA corresponding to cDNA clone 5.1 was named ephrin-A4 (m), and the mRNA corresponding to cDNA clone 2.1 was named ephrin-A4 (s).
The ephrin-A4 (s) cDNA results from an alternative splice in exon IV
To determine the molecular basis for the differences in ephrin-A4 (m) and ephrin-A4(s), a human genomic library in λ-DASH was screened with a 32P-labeled ephrin-A4 probe. A hybridizing clone covering the entire ephrin-A4 gene of approximately 7.5 kilobases was subjected to restriction mapping, subcloning, and partial nucleotide sequence analysis. Exon sequences and exon-intron boundaries were determined using vector and exon-specific primers. The ephrin-A4 gene consists of 4 exons; the translation start codon is in the first exon, and the GPI-linkage signal sequence, the stop codons, and the 3′ untranslated sequence are in the fourth exon (Figure 1B). The 146 bp stretch missing in the ephrin-A4 (s) sequence was spliced out of exon IV using the internal consensus splice donor site AG (Figure 1C).
Ephrin-A4 gene expression in tissues and hematopoietic cells
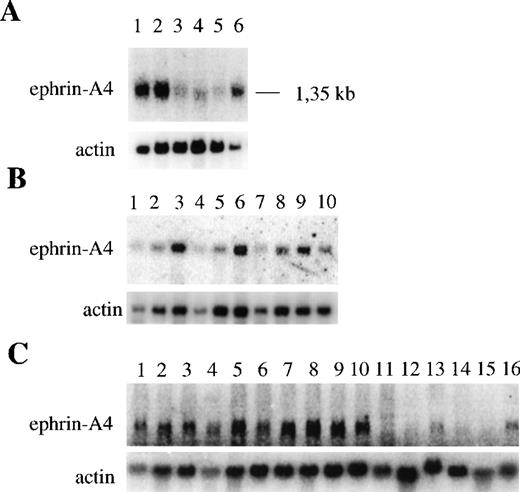
It has previously been reported that the ephrin-A4 gene is expressed in the adult human spleen, prostate, ovary, small intestine, and colon and in the fetal heart, lung, and kidney.32 We confirmed and extended these findings by showing, using Northern blot analysis, that the ephrin-A4 gene is abundantly expressed in adult spleen and lymph node and in fetal liver. It is weakly expressed in adult peripheral blood leukocytes, thymus, and bone marrow (Figure2A). Northern blot analysis of purified hematopoietic cells showed high levels of ephrin-A4 expression in tonsil B cells, intermediate levels of expression in tonsil T cells, and low levels of expression in blood B cells and CD4+ and CD8+ T cells (Figure 2B). In contrast to blood, human tonsils contain many activated B cells, raising the possibility that ephrin-A4 expression is induced during B-cell activation. Indeed, ephrin-A4 mRNA levels were upregulated by stimulation of blood B cells through their B cell receptor with anti-μ antibodies to a level comparable to that observed in freshly isolated tonsil B cells. In addition, ephrin-A4 expression is upregulated in anti-CD3 stimulated CD4+ and CD8+ blood T cells. Stimulation with the phorbol ester TPA did not induce the expression of ephrin-A4 in either blood B cells or T cells (Figure 2B).
Expression of ephrin-A4 mRNA in different human tissues and cell types.
Upper panels show ephrin-A4 hybridization; lower panels, β-actin hybridization. (A) Expression in different hematopoietic tissues: (1) spleen, (2) lymph node, (3) thymus, (4) peripheral blood leukocytes, (5) bone marrow, and (6) fetal liver. (B) Expression in freshly isolated, cultured B and T lymphocytes: (1) peripheral blood B cells, (2) 24-hour TPA-stimulated blood B cells, (3) 24-hour anti-μ–stimulated blood B cells, (4) peripheral blood CD4+ T cells, (5) 24-hour TPA-stimulated blood CD4+ T cells, (6) 24-hour anti-CD3–stimulated blood CD4+ T cells, (7) peripheral blood CD8+ T cells, (8) 24-hour anti-CD3–stimulated blood CD8+ T cells, (9) tonsil B cells, and (10) tonsil T cells. (C) Expression in hematopoietic cell lines: (1) Tom-1, (2) BV173, (3) Reh, (4) Nalm-6, (5) Daudi, (6) Bjab, (7) U266, (8) U698, (9) JM, (10) Jurkat, (11) HPB ALL, (12) JY, (13) KG1-A, 914) HL60, (15) U937, and (16) K562.
Expression of ephrin-A4 mRNA in different human tissues and cell types.
Upper panels show ephrin-A4 hybridization; lower panels, β-actin hybridization. (A) Expression in different hematopoietic tissues: (1) spleen, (2) lymph node, (3) thymus, (4) peripheral blood leukocytes, (5) bone marrow, and (6) fetal liver. (B) Expression in freshly isolated, cultured B and T lymphocytes: (1) peripheral blood B cells, (2) 24-hour TPA-stimulated blood B cells, (3) 24-hour anti-μ–stimulated blood B cells, (4) peripheral blood CD4+ T cells, (5) 24-hour TPA-stimulated blood CD4+ T cells, (6) 24-hour anti-CD3–stimulated blood CD4+ T cells, (7) peripheral blood CD8+ T cells, (8) 24-hour anti-CD3–stimulated blood CD8+ T cells, (9) tonsil B cells, and (10) tonsil T cells. (C) Expression in hematopoietic cell lines: (1) Tom-1, (2) BV173, (3) Reh, (4) Nalm-6, (5) Daudi, (6) Bjab, (7) U266, (8) U698, (9) JM, (10) Jurkat, (11) HPB ALL, (12) JY, (13) KG1-A, 914) HL60, (15) U937, and (16) K562.
High levels of ephrin-A4 expression were also detected in hematopoietic cell lines representing various lineages and differentiation stages (Figure 2C). The early B cell lines (Tom-1, BV173, Reh, Nalm-6), the mature B cell lines (Daudi, Bjab, U698) the plasmacytoid B cell line (U266), and the T cell lines (JM and Jurkat) all showed strong expression of ephrin-A4 mRNA, while a weaker expression was observed in the promyeloid cell line KG1-A and the erythroid cell line K562. No expression was observed in the T cell line HPB-ALL and JY and in the myeloid cell lines HL-60 and U937.
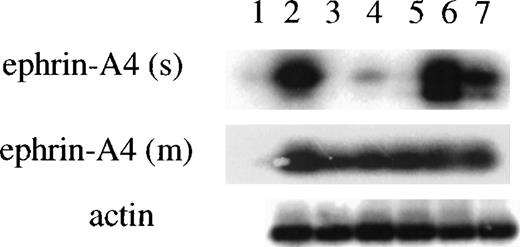
The Northern blot analysis did not discriminate between cells expressing the ephrin-A4 (m) or ephrin-A4 (s) variant. In subsequent experiments, a semiquantitative RT-PCR approach was employed with primer sets that discriminate between ephrin-A4 (s) and ephrin-A4 (m). In all PCR experiments, the quality and amount of cDNA were assessed by PCR with primers specific for β-actin. In all populations analyzed, high levels of expression of the ephrin-A4 (m) form were detectable. In the Reh cell line, freshly isolated tonsil B cells and 24-hour anti-μ–stimulated blood B cells, both high levels of expression of the ephrin-A4 (s) form, were detectable (Figure 3). In contrast, very low levels of expression of ephrin-A4 (s) were detectable in tonsil T cells and in anti-CD3– activated CD4+ or CD8+ T cells (Figure 3).
Semiquantitative PCR analysis of the expression of ephrin-A4 (m) and ephrin-A4 (s) mRNA in freshly isolated and stimulated B and T lymphocytes.
(1) water control, (2) Reh pro-B cells, (3) tonsil T lymphocytes, (4) 24-hour anti-CD3– stimulated CD8+ blood T lymphocytes, (5) 24-hour anti-CD3–stimulated CD4+ blood T lymphocytes, (6) tonsil B lymphocytes, and (7) 24-hour anti-μ–stimulated blood B cells. The upper panel shows ephrin-A4 (s) specific PCR (28 cycles); the middle panel, ephrin-A4 (m) specific PCR (30 cycles); and the lower panel, β-actin specific PCR (24 cycles).
Semiquantitative PCR analysis of the expression of ephrin-A4 (m) and ephrin-A4 (s) mRNA in freshly isolated and stimulated B and T lymphocytes.
(1) water control, (2) Reh pro-B cells, (3) tonsil T lymphocytes, (4) 24-hour anti-CD3– stimulated CD8+ blood T lymphocytes, (5) 24-hour anti-CD3–stimulated CD4+ blood T lymphocytes, (6) tonsil B lymphocytes, and (7) 24-hour anti-μ–stimulated blood B cells. The upper panel shows ephrin-A4 (s) specific PCR (28 cycles); the middle panel, ephrin-A4 (m) specific PCR (30 cycles); and the lower panel, β-actin specific PCR (24 cycles).
Ephrin-A4–expressing cells are detectable in situ in tonsil germinal centers and extrafollicular areas
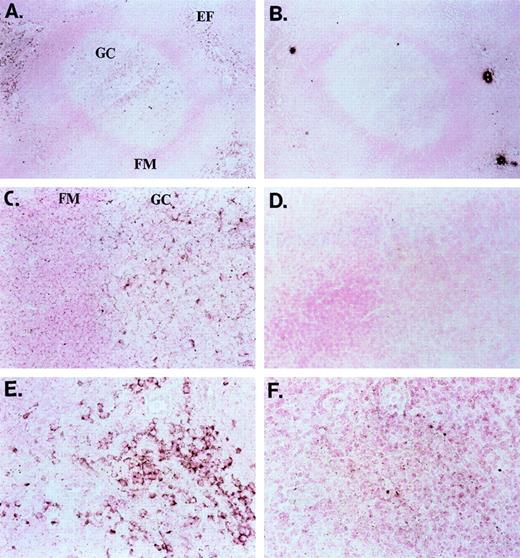
In a current model of peripheral B-cell development in secondary lymphoid organs, newly formed bone marrow–derived B cells first migrate into the extrafollicular T-cell zones. Here, the tripartite interaction between antigen-specific B and T lymphocytes and interdigitating cells leads to B-cell activation; B cells may differentiate into plasma cells or enter primary or secondary follicles to initiate or sustain a germinal center reaction.5 In-situ hybridization of sections of human tonsil with a dUTP-Dig–labeled ephrin-A4 probe unveiled strongly ephrin-A4 expressing cells in germinal centers and weakly expressing cells in the follicular mantle zone (Figures 4A and 4C). In the extrafollicular areas, scattered individual cells with a lymphoid appearance also displayed strong staining (Figures 4A and 4E). No staining was observed with a dUTP-Dig–labeled control probe (Figures4B, 4D, 4F).
In situ (mRNA) hybridization of tonsil sections with ephrin-A4 probe and control probe.
(A, B) Overview of a germinal center (GC) and extrafollicular area; objective ×25. (C, D) Close-up of a germinal center with follicular mantle (FM) zone; objective ×40. (E, F) Close-up of an extrafollicular (EF) area; objective ×40. Panels A, C, and E show the ephrin-A4 Dig-labeled probe, and panels B, D, and F, the control Dig-labeled probe. Brown staining shows ephrin-A4 mRNA hybridization in the GC and EF areas.
In situ (mRNA) hybridization of tonsil sections with ephrin-A4 probe and control probe.
(A, B) Overview of a germinal center (GC) and extrafollicular area; objective ×25. (C, D) Close-up of a germinal center with follicular mantle (FM) zone; objective ×40. (E, F) Close-up of an extrafollicular (EF) area; objective ×40. Panels A, C, and E show the ephrin-A4 Dig-labeled probe, and panels B, D, and F, the control Dig-labeled probe. Brown staining shows ephrin-A4 mRNA hybridization in the GC and EF areas.
Ephrin-A4 (s) protein is produced by freshly isolated tonsil B cells and by blood B cells after in vitro activation via the B-cell antigen receptor
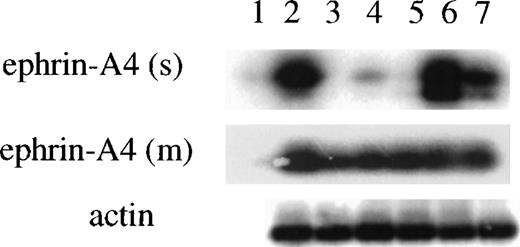
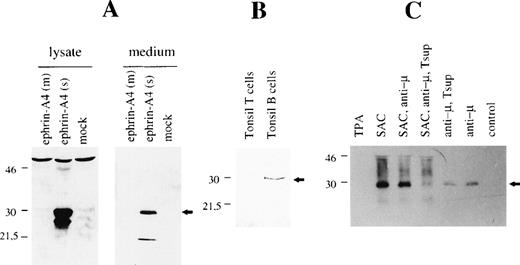
A rabbit antiserum was raised against a 15-residue peptide corresponding to a region in the carboxy terminus that is specific for the ephrin-A4 (s) protein, and it will not recognize the ephrin-A4 (m) protein. The antiserum was affinity-purified on a column with peptide-coupled sepharose beads. The specificity of the affinity-purified rabbit anti–ephrin-A4 (s) polyclonal antibody was first analyzed in Western blots of crude cell lysates and culture supernatants from COS cells transfected with ephrin-A4 (m) or ephrin-A4 (s). A band of the expected size was present in lanes containing cell lysates or culture supernatant from COS cells transfected with the ephrin-A4 (s) cDNA but not from COS cells transfected with the ephrin-A4 (m) cDNA or mock-transfected cells (Figure 5A). Note the presence of a second band in the cell lysate of ephrin-A4 transfected cells, which may represent an unprocessed product. In Western blots of lysates from purified tonsil B and T lymphocytes, the anti–ephrin-A4 (s) antiserum detected a single band of 28 kDa molecular weight in lysates from tonsil B cells but not T cells (Figure 5B). In lysates of freshly isolated blood B cells, ephrin-A4 (s) proteins were not detectable (data not shown).
Western blot analysis with ephrin-A4 (s) specific antiserum.
(A) COS cells were transfected with ephrin-A4 (m) cDNA or ephrin-A4 (s) cDNA, and cells and culture supernatant were harvested 3 days posttransfection. Cells were lysed. Either 10 μg protein was applied in each lane (left panel), or 10 μL culture supernatant was applied in each lane (right panel). (B) Tonsil T cells or tonsil B cells were isolated and lysed, and 10 μg of protein was applied in each lane. (C) 105 blood B cells were stimulated for 6 days, as indicated in the figure text. Control is no stimulation. The culture supernatant was harvested, and 10 μL was applied in each lane. All blots were stained with ephrin-A4 (s) specific polyclonal antiserum. The arrowhead denotes the ephrin-A4 (s) protein band. Molecular weight in kDa is indicated to the left of each panel. Tsup: supernatant of PHA-stimulated pooled T cells.
Western blot analysis with ephrin-A4 (s) specific antiserum.
(A) COS cells were transfected with ephrin-A4 (m) cDNA or ephrin-A4 (s) cDNA, and cells and culture supernatant were harvested 3 days posttransfection. Cells were lysed. Either 10 μg protein was applied in each lane (left panel), or 10 μL culture supernatant was applied in each lane (right panel). (B) Tonsil T cells or tonsil B cells were isolated and lysed, and 10 μg of protein was applied in each lane. (C) 105 blood B cells were stimulated for 6 days, as indicated in the figure text. Control is no stimulation. The culture supernatant was harvested, and 10 μL was applied in each lane. All blots were stained with ephrin-A4 (s) specific polyclonal antiserum. The arrowhead denotes the ephrin-A4 (s) protein band. Molecular weight in kDa is indicated to the left of each panel. Tsup: supernatant of PHA-stimulated pooled T cells.
In the supernatant of blood B cells stimulated for 6 days with anti-μ antibodies, SAC or TPA, secreted ephrin-A4 (s) protein could be detected in the culture supernatant after stimulation with anti-μ or SAC but not after stimulation with TPA (Figure 5C). Addition of supernatant from PHA-stimulated T cells (Tsup) containing cytokines that progress anti-μ stimulated B cells through the cell cycle36 had no or slightly inhibitory effect, while the combination of Tsup and SAC stimulation of B cells showed a more pronounced inhibitory effect on the expression of ephrin-A4(s) protein when compared with SAC alone or SAC and anti-μ. These protein data confirm the predicted amino acid sequence of the carboxy terminus of the ephrin-A4 (s) cDNA and show that ephrin-A4 (s) is secreted by in vitro activated B cells. Repeated attempts to utilize the anti–ephrin-A4 (s) antiserum in immunohistochemical analysis of tonsil sections or transfected COS cells were unsuccessful.
The high expression of ephrin-A4 mRNA in anti-CD3 stimulated T cells led us to perform the same experiment with these cells. T cells (both CD4+ and CD8+) were stimulated for 6 days with anti-CD3–coated beads (Dynabeads) or TPA. Ephrin-A4 (s) protein could not be detected in the supernatants or cell lysates of these cells (data not shown). These observations correspond to the semiquantitative PCR data showing no detection of ephrin-A4 (s) message in anti-CD3–stimulated T cells.
Staining of cells with an ephrin-A4-Fc chimeric protein
To detect cells capable of binding ephrin-A4, a fusion protein, which contained the shared sequence between the membrane and soluble forms of the molecule and the constant region of mouse IgG2b, was generated (ephrin-A4-Fc). As a negative control, we generated a fusion protein comprising the amino terminal 30 residues of human CD19 and the murine IgG2b constant region (CD19short-Fc). The fusion proteins were first analyzed for reactivity with different cell lines of the B-cell, T-cell, and myeloid lineage. The B lymphoma cell line Bjab specifically bound the ephrin-A4-Fc fusion protein, while the T-cell line Jurkat did not. The negative control protein, CD19short-Fc, did not bind to either of the cell lines (Figure 6).
Ephrin-A4-Fc binding to cell lines.
The Jurkat and the Bjab cell line were stained with either the control CD19short-Fc (dotted line) or the ephrin-A4-Fc (bold line) fusion protein.
Ephrin-A4-Fc binding to cell lines.
The Jurkat and the Bjab cell line were stained with either the control CD19short-Fc (dotted line) or the ephrin-A4-Fc (bold line) fusion protein.
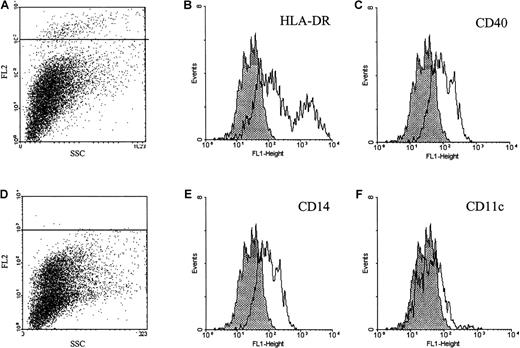
In tonsil, purified B and T lymphocytes did not bind the ephrin-A4-Fc fusion protein (results not shown). Within the population of tonsil cells that adhered to collagen-coated tissue flasks, approximately 5% of cells stained with the ephrin-A4-Fc protein. Double immunofluorescent staining with different markers for adherent cell populations in the tonsil showed that the ephrin-A4+ cells expressed low to intermediate levels of CD40, high or intermediate levels of HLA-DR, and barely detectable levels of CD14. Staining was not observed with anti-CD11c, anti-CD19, anti-CD4, anti-CD2, anti-CD13, anti-CD31, anti-CD34, anti-CD86, anti-CD21, anti-CD45, anti-CD38, anti-CD71, and anti-CD123 antibodies (Figure 7). Immunohistochemical staining of frozen tonsil sections with ephrin-A4 fusion protein was not successful. Ephrin-A4-Fc did not stain cytospin preparations of the Bjab cell line using different fixation protocols, indicating that the receptor-ligand interaction is abrogated under these conditions.
Immunofluorescent analysis of ephrin-A4 binding tonsil adherent cells.
Tonsil adherent cells were stained with ephrin-A4-Fc (A) or ephrin-CD19short-Fc (D) chimeric proteins in combination with a panel of fluorochrome-labeled monoclonal antibodies. (B, C, E, F) Histograms represent the staining patterns of ephrin-A4 binding cells gated in the upper left panel (A): shaded histograms represent ephrin-A4 binding cells costained with relevant FITC control, and open histograms represent costaining of ephrin-A4 binding cells with FITC-labeled antibodies directed to indicated surface markers.
Immunofluorescent analysis of ephrin-A4 binding tonsil adherent cells.
Tonsil adherent cells were stained with ephrin-A4-Fc (A) or ephrin-CD19short-Fc (D) chimeric proteins in combination with a panel of fluorochrome-labeled monoclonal antibodies. (B, C, E, F) Histograms represent the staining patterns of ephrin-A4 binding cells gated in the upper left panel (A): shaded histograms represent ephrin-A4 binding cells costained with relevant FITC control, and open histograms represent costaining of ephrin-A4 binding cells with FITC-labeled antibodies directed to indicated surface markers.
EphA2 is a candidate ephrin-A4 receptor in human tonsils
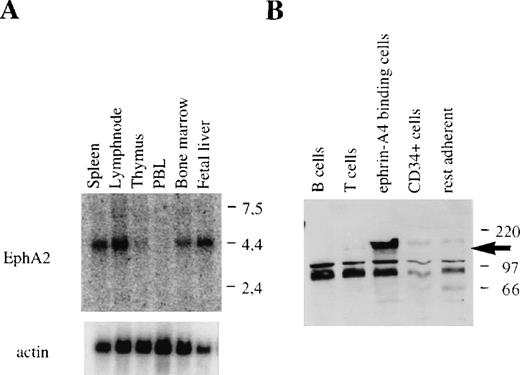
Ephrin-A4 has been shown to bind to 6 different members of the family of Eph receptor tyrosine kinases. Binding of ephrin-A4 to 3 of these, EphA2, EphA5, and EphA6, reportedly results in autophosphorylation of the receptor and has been interpreted to reflect high-affinity, functional receptor-ligand interaction.18 In humans, expression of EphA5 has been detected in the brain and placenta.9 While expression data have been reported for the EphA6 gene in humans, high levels of expression in rats and mice are noted in the brain.37,38 EphA2 mRNA has been found in different rat tissues, including the spleen; in human cell lines of epithelial origin; and in human umbilical vein endothelial cells.22 39 In Northern blots, we detected high levels of EphA2 mRNA in adult bone marrow, spleen, and lymph node, and in fetal liver, whereas no message was found in adult thymus and peripheral blood leukocytes (Figure 8A). The expression pattern of EphA2 in lymphoid tissues was similar to the expression pattern observed for ephrin-A4.
EphA2 mRNA expression in hematopoietic tissues and EphA2 protein production by purified populations of cells.
(A) EphA2 mRNA expression in different hematopoietic tissues. The upper panel shows EphA2 hybridization, and the lower panel, β-actin hybridization to the same blot. Molecular weight in kb is indicated to the right. (B) Western blot analysis of lysates of purified tonsil cell subpopulations using the EphA2-specific anti-serum. No specific staining was observed with lysates from purified T or B lymphocytes, CD34+ (endothelial) cell, and collagen-adherent cells depleted of CD34+ cells and ephrin-A4 binding cells (“rest adherent'). The arrow indicates the EphA2 protein specifically detected in the fraction of ephrin-A4 binding adherent cells. Molecular weight in kDa is indicated to the right.
EphA2 mRNA expression in hematopoietic tissues and EphA2 protein production by purified populations of cells.
(A) EphA2 mRNA expression in different hematopoietic tissues. The upper panel shows EphA2 hybridization, and the lower panel, β-actin hybridization to the same blot. Molecular weight in kb is indicated to the right. (B) Western blot analysis of lysates of purified tonsil cell subpopulations using the EphA2-specific anti-serum. No specific staining was observed with lysates from purified T or B lymphocytes, CD34+ (endothelial) cell, and collagen-adherent cells depleted of CD34+ cells and ephrin-A4 binding cells (“rest adherent'). The arrow indicates the EphA2 protein specifically detected in the fraction of ephrin-A4 binding adherent cells. Molecular weight in kDa is indicated to the right.
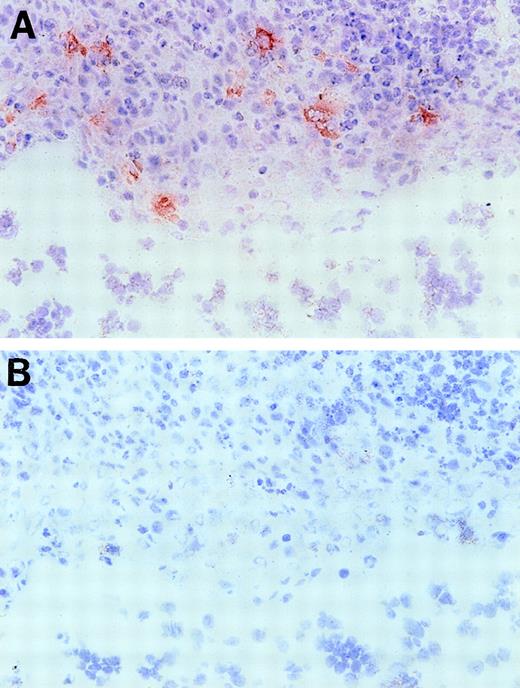
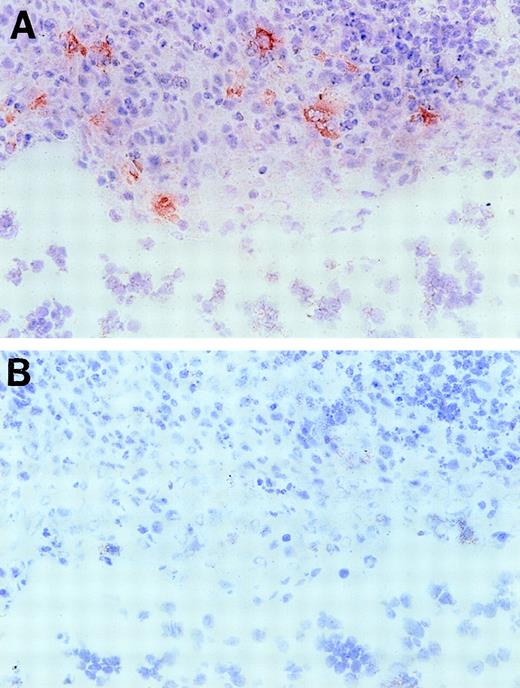
Based on the presence of EphA2 message in these lymphoid organs, we further investigated EphA2 as a putative candidate receptor for the ephrin-A4 ligand in human tonsil. First, we confirmed that COS cells transiently transfected with the ephrin-A4 (m) cDNA are capable of binding an EphA2-Fc fusion protein, confirming results previously published by Gale et al18 (results not shown). In immunohistochemical staining of frozen tonsil sections, an anti-EphA2 antiserum showed reactivity with cells in the crypts of the tonsils (Figure 9), whereas, tonsil B and T lymphocytes completely lacked reactivity with this antibody.
Immunohistochemical staining of a tonsil section with anti-EphA2 antibodies.
Frozen tonsil sections were stained with EphA2 specific antiserum (A) or normal rabbit serum Ig (B) (objective ×40. Red EphA2+ cells are present in the tonsil crypt area only.
Immunohistochemical staining of a tonsil section with anti-EphA2 antibodies.
Frozen tonsil sections were stained with EphA2 specific antiserum (A) or normal rabbit serum Ig (B) (objective ×40. Red EphA2+ cells are present in the tonsil crypt area only.
The immunohistochemical data were further confirmed by isolating ephrin-A4 binding cells from the fraction of adherent tonsil cells by immunomagnetic separation using beads (Dynal) coupled to the ephrin-A4-Fc chimeric protein. Lysates of ephrin-A4-Fc binding cells strongly reacted with the anti–EphA-2 antibody in Western blot analysis, whereas isolated CD34+ endothelial cells, or the tonsil adherent cell fraction depleted of both ephrin-A4-Fc binding cells and CD34+ cells, did not react or only weakly reacted with this antibody (Figure 8B).
Discussion
We have identified a mRNA splice variant of the human ephrin-A4 gene, denoted ephrin-A4 (s), a member of a family consisting of at least 8 predominantly membrane-bound ligands for Eph receptor tyrosine kinases. Characterization of the genomic structure of the ephrin-A4 gene unveiled that this splice variant lacks 146 nucleotides at the 3′ end of the open reading frame in the first part of exon IV when compared to the published ephrin-A4 sequence, here denoted ephrinA-4 (m).32 This results in an altered carboxy terminus of 37 amino acids compared to the ephrin-A4 (m) protein and the absence of the GPI-signal sequence present in ephrin-A4 (m). The prediction that this mRNA encodes a soluble molecule was substantiated by the finding that COS cells transfected with ephrin-A4(s) cDNA produced soluble ephrin-A4 protein, and more importantly, in vitro activated human blood B-lineage cells were found to secrete the ephrin-A4 (s) protein into the culture supernatant. Moreover, cell lysates of freshly isolated human tonsil B lymphocytes containing a large fraction of in vivo activated B cells were shown by Western blotting to contain the ephrin-A4 (s) protein.
The significance of these findings is two-fold. First, this is only the second member of the ephrin gene family that can reportedly exist in a membrane-bound and soluble form, and it is the first soluble ephrin ligand that originates from an alternatively spliced mRNA. Ephrin-A1, a cytokine-inducible endothelial cell product, appears to be shed from the cell membrane.22 Second, in the peripheral B-cell compartment, ephrin A4 (s) expression is associated with B-cell activation. So far, ephrins and their ligands have almost exclusively been studied in the developing embryo, where they act as instructive molecules that guide the topographic movement of cells and growth cones and play a role in defining compartments. The current data add to the more recent notion that Eph receptors and ephrins play a role outside the developing nervous system, ie, in hematopoiesis and angiogenesis.22-24
The analysis of ephrin-A4 (m) and ephrin-A4 (s) expression at the mRNA and protein level in peripheral B-cell populations yielded important clues as to the possible functional role of these molecules. Very weak ephrin-A4 mRNA expression was observed in blood B cells, and ephrin-A4 (s) protein could not be detected in lysates of freshly isolated blood B cells. In contrast, tonsil B cells appeared to express mRNA for soluble ephrin-A4, and ephrin-A4 (s) protein could be readily detected in lysates of freshly purified tonsil B cells. Tonsil and blood T cells expressed barely detectable levels of ephrin-A4 mRNA in Northern blot analysis and did not produce detectable levels of ephrin-A4 (s) protein. Anti-CD3 stimulated T cells expressed high levels of ephrin-A4 mRNA, but neither ephrin-A4 (s) mRNA nor protein could be detected from these cells. Because of the lack of ephrin-A4 (m) specific antibodies, we were not able to assess whether activated T cells produce membrane-bound ephrin-A4 protein.
To further elucidate the nature of the ephrin-A4 producing cells, we examined B-cell populations in human tonsils, a lymphoid organ that represents a working model for in vivo peripheral B-cell activation and differentiation. With a probe detecting both ephrin-A4 mRNA species, strongly ephrin-A4+ cells, with a round, lymphoid appearance, were found in germinal centers and in extrafollicular areas in tonsil by in situ hybridization. Weakly ephrin-A4 mRNA expressing cells were observed in the follicular mantle zone. Of note, the extrafollicular ephrin-A4+ cells may represent naive B-cell blasts that have been activated by T cells and interdigitating cells.5
The suggestion that ephrin-A4 (s) secretion is associated with B lymphocytes in an activated state was further supported by the observation that resting blood B cells could be induced to secrete the soluble ligand through agents that cross-link the B-cell antigen receptor and induce B-cell activation. In that respect it is noteworthy that the phorbol ester TPA, a potent B-cell antigen receptor-independent stimulator of B-cell proliferation, did not induce secretion of ephrin-A4 (s) protein. Thus, B-cell antigen receptor cross-linking appears to be a prerequisite for the induction of ephrin-A4 secretion, presumably mediated by B-cell receptor-associated Src-family kinases such as Lyn, Fyn, and Blk.
Eph receptors have been shown to bind multiple ligands, and ephrin ligands are capable of binding multiple Eph receptors. Binding of ephrin-A4 to EphA2, EphA5, and EphA6 has been shown to result in autophosphorylation of the receptor and has been interpreted to reflect high-affinity, physiologically relevant ligand-receptor interaction. The overlap in expression pattern of EphA2 (lymphoid organs but not in thymus or blood) and ephrin-A4 prompted us to investigate whether EphA2 could be a candidate receptor for ephrin-A4 in human tonsils. In immunohistochemical analysis, EphA2+ cells were detectable in the crypts of tonsils with a polyclonal antibody specific for the intracellular portion of the EphA2 receptor. An ephrin-A4 IgG fusion protein did not perform in immunohistochemistry, but ephrin-A4 binding cells could be found by immunofluorescence analysis in the adherent fraction of isolated tonsil MNC. In lysates of purified ephrin-A4 binding cells, EphA2 protein was detectable in Western blotting, whereas in lysates of the remaining purified CD34+ tonsillar endothelial cells, EphA2 receptor protein was not detectable.
In double-immunofluorescent staining analysis, ephrin-A4 binding cells expressed intermediate to high levels of HLA-DR, intermediate to low levels of CD40, and barely detectable CD14, but lacked other markers for myeloid or endothelial cells or markers specific for B- and T-lineage cells. The localization of the EphA2 receptor to cells in the crypts and the HLA-DR++ or HLA-DR+/CD40+/CD14low/lineage phenotype suggests that these cells may be dendritic cells. The phenotype of the EphA2+ adherent cells partially overlaps with the phenotype of interdigitating dendritic cells, including the characteristic presence of HLA-DR++ and HLA-DR+ cells, but lacks other characteristics of interdigitating dendritic cells such as expression of low levels of CD4 and CD86.40 Thus, within the tonsil micro environment, EphA2+ cells with a dendritic cell phenotype may communicate with activated B cells through the soluble ephrin-A4 protein.
It is noteworthy that B-ephrins, anchored to the cell membrane via a transmembrane region, can transduce signals initiated by cell–cell contact.20,41 Although not proven, it has been suggested that A-type ephrins, such as ephrin-A4, may exert a similar activity through association with transmembrane proteins.42 In B and T cells from blood and tonsils, the membrane-bound form of ephrin-A4 was detectable, indicating that putative signaling events may be mediated via this ligand.
The functional consequence of the interaction between soluble ephrin-A4 and the Eph2+ cells in tonsil with a dendritic cell phenotype remains to be elucidated. The ephrin-A1 soluble ligand, the only other reported naturally occurring soluble ephrin, has been shown to act as an angiogenic factor in vivo,22 as a growth factor for melanoma cell lines,43 and as a neurotrophic factor in cultures of rat spinal cord neurons.44 Along the same line, it may be envisaged that in tonsil, ephrin-A4 (s) acts as a stimulator or chemoattractant of dendritic cells. Indeed, dendritic cells in tonsil have recently been shown to be capable of directly interacting with B cells and inducing immunoglobulin production.45 Alternatively, ephrin-A4 (s) may act as an antagonist of the EphA2 receptor, similar to what has been proposed for (nonnatural) recombinant soluble ephrin ligands, such as ephrin-B1 and ephrin-A3,19 46 produced in vitro. The observation that EphA2-Fc fusion protein does not appear to bind to any cell population in tonsil lends support to a model of interaction between soluble ephrin-A4 and EphA2+ cells, without cell–cell interactions involving EphA2+ and ephrin-A4 (m)+ cells. The notion that the only reported naturally occurring soluble ephrins, ephrin-A1 and ephrin-A4, both appear to act through the EphA2 receptor expressed on different cell types supports the view that the spatially and temporarily regulated expression of Eph receptors and their ligands is a key determinant in the specificity of their interactions. Whatever the outcome of this interaction, the data reported here add to the growing notion that Eph receptors and their ligands may play an important role outside the developing nervous system, and they appear to have a role in the hematopoietic system.
Acknowledgments
We thank Dick van Wichen for expert help with in situ hybridizations; Kari Hildrum for help with producing the ephrin-A4 (s) antiserum; and Goril Olsen, Toril Holien, and Ruth Solien for expert technical assistance. We also thank Espen Bekkevold at LIIPAT, The Norwegian National Hospital, for invaluable help with the isolation of adherent cells from tonsils.
H. C. A. is a post-doctoral fellow at the Norwegian Cancer Society, and E. M. is a doctoral student supported by the Norwegian Counsel of Research.
Reprints:Hans-Christian Aasheim, PhD, Department of Immunology, Institute for Cancer Research, The Norwegian Radium Hospital, 0310 Oslo, Norway; e-mail: h.c.asheim@labmed.uio.no.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.