The small guanosine triphosphate (GTPase) p21rac is highly expressed in human neutrophils where it is thought to play a role in cytoskeletal reorganization and superoxide production. Using the p21rac binding domain of PAK (PAK-RBD) as an activation-specific probe, we have investigated agonist-stimulated activation of p21rac. Stimulation of neutrophils with the chemoattractants fMet-Leu-Phe (fMLP) or platelet-activating factor (PAF) induced an extremely rapid and transient p21rac activation, being optimal within 5 seconds. This activation correlates with the rapid changes of intracellular free Ca2+ ([Ca2+]i) stimulated by fMLP; however, changes in [Ca2+]i were neither sufficient nor required for p21rac activation. Furthermore, fMLP-induced p21rac activation was not inhibited by broad tyrosine kinase inhibitors or specific inhibitors of ERK, p38 mitogen activated protein kinase, Src, or phosphatidylinositol 3-kinases. Surprisingly, the cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF) and tumor necrosis factor- did not cause p21rac activation or modulate fMLP-induced p21rac activation. AlF−, a potent activator of heterotrimeric G-protein -subunits, however, was found to activate p21rac. Stimulation of neutrophils with phorbol myristate acetate (PMA) strongly activated the respiratory burst, but did not induce p21rac activation, suggesting that superoxide production per se can occur independently of p21rac activation. These data suggest that in human granulocytes, G-protein coupled receptors, but not cytokine receptors, activate p21rac via a rapid, novel exchange-mechanism independently of changes in [Ca2+]i, tyrosine phosphorylation, or PI3K.
NEUTROPHILS PLAY an important role in the host defense to microbial pathogens. Stimulation of these cells induces multiple responses, including cell adhesion, migration, secretion, phagocytosis, and the generation of reactive oxygen species.1 Therefore, neutrophil function is under tight control, and deregulated activation of neutrophils is implicated in the pathogenesis of a variety of inflammatory diseases, which are characterized by tissue damage.2-5 A diverse array of receptors are expressed on the surface of neutrophils, allowing regulation by a wide range of agonists and second messengers.1 Tyrosine kinase-linked receptors such as the granulocyte-macrophage colony-stimulating factor (GM-CSF) receptor, and serpentine receptors including the N-formylmethionylleucylphenylalanine (fMLP) receptor activate both distinct and overlapping signaling pathways regulating neutrophil responses.6-14 Several small guanosine triphosphatases (GTPases) have been implicated in controlling neutrophil function. Activation of the p21ras15,16 and p21rap17 by cytokines and chemoattractants has been reported in human neutrophils, although their precise roles in neutrophil functioning are unknown.
Another small GTPase that has been implicated in neutrophil functioning is p21rac.18-20 p21rac has been shown to be present in the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex where it is thought to play a critical role in the production of oxygen radicals.18,21-26 In several cell lines, activation of p21rac has been found to be essential for superoxide production.24 27
However, measurement of the activation of p21rac in cells has been hampered by the lack of an immunoprecipitating antibody. GTP-loading of endothelial cells transfected with epitope-tagged p21rac has, however, been reported and shown to be blocked using specific inhibitors of phosphatidylinositol-3 kinase (PI3K), suggesting that p21rac is downstream of this lipid kinase.28 Like other small GTPases, p21rac activity is controlled by switching from its inactive, guanosine diphosphate (GDP) bound state to the active, GTP bound state. Guanine nucleotide exchange factors (GEFs) activate Rac by facilitating the exchange of GDP for GTP. Thus far, two GEFs for p21rac have been extensively studied: Vav and Tiam1.29,30 Vav is expressed predominantly in hematopoietic cells and its tyrosine phosphorylation is promoted by binding to PI3K-phosphorylated lipids.31 In these cells, Vav has been shown to be activated by stimuli that activate PI3K, including GM-CSF.32
While much work has evaluated the role of p21rac in cellular functioning by overexpression of dominant-negative or dominant-active mutants in cell lines, as mentioned above, it has not been possible to measure activation of p21rac by cellular stimuli. Here we use a novel assay to measure the p21rac activation.33 This assay uses the p21rac binding domain of PAK (PAKcrib), which has a high affinity for p21racGTP, but no affinity for p21racGDP, to precipitate activated p21rac from cell lysates.34-36 We show for the first time that in human neutrophils, activators of G-protein coupled receptors (GPCRs), but not cytokine receptors, activate p21rac. The activation of p21rac by fMLP is independent of the elevation of [Ca2+]i, activation of tyrosine kinases and PI3K and does not correlate with the activation of the respiratory burst. This suggests a novel pathway for activation of exchange factors regulating p21rac in these cells.
MATERIALS AND METHODS
Isolation of human neutrophils.
Blood was obtained from healthy volunteers from the Red Cross Blood Bank (Utrecht, The Netherlands). Mixed granulocytes were isolated from the buffy-coat of 500 mL 0.4% (wt/vol) trisodium citrate (pH 7.4)-treated blood as previously described.37 Mononuclear cells were removed by centrifugation over isotonic Percoll (1.078 g/mL) from Pharmacia (Uppsala, Sweden). After lysis of the erythrocytes in isotonic NaCl solution, neutrophils were washed and resuspended in incubation buffer (20 mmol/L HEPES, 132 mmol/L NaCl, 6 mmol/L KCI, 1 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 5 mmol/L glucose, 1 mmol/L CaCI2) containing 0.5% human serum albumin (HSA; Central Laboratory of The Netherlands Red Cross Blood Transfusion Service, Amsterdam). Neutrophils were incubated for 30 minutes at 37°C before stimulation. In all experiments, a concentration of 106 cells/mL was used for stimulation. Figures are representative of at least three other experiments from independent donors.
Neutrophil stimulation.
One milliliter of neutrophil suspension was stimulated with one of the following stimuli: fMLP (1 μmol/L to 10 nmol/L), platelet-activating factor (PAF) (1 μmol/L), tumor necrosis factor-α (TNF-α) (100 U/mL), phorbol myristate acetate (PMA) (100 ng/mL), thapsigargin (100 nmol/L) (all from Sigma, St Louis, MO), GM-CSF (10−10mol/L; Genzyme, Boston, MA), and ionomycin (100 nmol/L; Calbiochem, La Jolla, CA). The concentrations used are known to prime neutrophils or activate the respiratory burst.38 39 In some experiments, cells were preincubated as described in the legends of the figures with one of the following inhibitors: PP1, PD098059, and LY294002 (all from Biomol, Plymouth, PA) and SB203580 (Alexis, Laufelfingen, Switzerland). At different time points, 500 μL 3X Lysis Buffer (see below) was added, samples were vortexed and lysed on ice for 10 minutes. For the AlF4− stimulation, neutrophils were resuspended at 106 cells per 500 μL HEPES3+. Cells were stimulated by adding 500 μL aluminum fluoride (AMF) buffer (20 mmol/L HEPES, 132 mmol/L NaCl, 6 mmol/L KCl, 1 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 5 mmol/L glucose, 1 mmol/L CaCl2, 0.5% HSA, 60 mmol/L NaF, 100 μmol/L AlCl3) and incubated at 37°C. At different time points, cells were lysed by addition of 3X Lysis Buffer, vortexed, and lysed on ice for 10 minutes.
Rac-1 activation assay using GST-PAKcrib.
The Rac and CDC42 binding domain of PAK1b (accession no. AF 071884), crib domain (aa 56-272), was generated and used as previously described.33 GST pull-down assays were performed essentially as described for Ras and Rap.14,15 Briefly, human neutrophils (106 cells/mL) or BW5147 cell lines40 (107 cells/mL) were lysed for 10 minutes at 4°C by addition of 3X lysis buffer (1X lysis buffer: 50 mmol/L Tris pH 7.4, 10% glycerol, 200 mmol/L NaCl, 1% NP-40, 2 mmol/L MgCl2, 1 mmol/L phenylmethyl sulfonyl fluoride [PMSF], 1 mmol/L benzamidine, 10 μg/mL aprotinin, and 10 μg/mL leupeptin). Lysates were cleared by centrifugation and GST-PAKcrib protein was added for 30 minutes at 4°C and subsequently washed three times in lysis buffer. The beads were boiled in Laemmli sample buffer and protein samples were separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Immobilon-P; Millipore, Bedford, MA). The membranes were probed with anti-Rac antibodies (Transduction Laboratories, Lexington, KY) and sheep anti-mouse peroxidase-conjugated antibodies (DAKO, Glostrup, Denmark). Immune complexes were detected by enhanced chemiluminescence (Amersham, Buckinghamshire, UK). The Ras activation assay using Raf-RBD was performed as previously described.15 41 All experiments were repeated three times. Blots are representative of at least three independent experiments
For the in vitro Rac-loadings, p21rac protein was expressed and purified as previously described.42 A total of 0.2 μg of purified p21rac was added to 1 mL of lysis buffer containing either GDP or the nonhydrolyzable GTP analogue GppNHp (5′-guanosyl-[β,γ-imido]-triphosphate). GST-PAK protein was added and incubated for 30 minutes to allow binding. After washing three times, samples were analyzed by SDS-PAGE as described above.
Depletion of intracellular free Ca2+.
Intracellular free Ca2+ was depleted as previously described.43 Neutrophils were suspended in Ca2+free incubation buffer supplemented with 1 mmol/L EGTA. Indo-1/AM (Molecular Probes, Eugene, OR) was added to 1-mL aliquots of suspended cells (106 cells/mL) at a final concentration of 1.5 μmol/L. Cells were incubated for 40 minutes at 37°C. Thapsigargin (100 nmol/L) was added 10 minutes before washing to deplete internal stores. Cells were washed once with Ca2+ free incubation buffer containing EGTA. Determination of p21rac activation and Ca2+ measurements were performed in parallel with the same cells. Calcium concentration was measured by a dual excitation at a wavelength of 340 nm and detected at 390 nm using a Hitachi F4500 fluorescence spectrophotometer (Hitachi, Tokyo, Japan).43
Respiratory burst measurements.
Neutrophils were preincubated in the absence or presence of GM-CSF (10−10 mol/L for 20 minutes). fMLP (1 μmol/L), PMA (100 ng/mL), or ionomycin (100 nmol/L) was added to the cells and oxygen consumption was measured using a Clark oxygen electrode (YSI Inc, Yellow Springs, OH) as previously described.44 In addition, cells were also pretreated with or without LY294002 for 20 minutes.
RESULTS
GST-PAKcrib can be used as a specific probe for p21rac-GTP.
The responses of human phagocytes to appropriate stimuli can result in cellular migration or production of superoxide resulting in microbial killing. Both these processes have been linked to activation of the small GTPase p21rac.2,18 Previously, it has been impossible to measure the activation of this GTPase due to the inability to immunoprecipitate endogenous protein from cell lines or primary cells.28 However, it has been demonstrated that a GST-PAKcrib fusion protein specifically binds to GTP-bound, but not GDP-bound p21rac, with high affinity.33-36 Indeed, neutrophils stimulated with fMLP demonstrate activation of PAKs.45 46
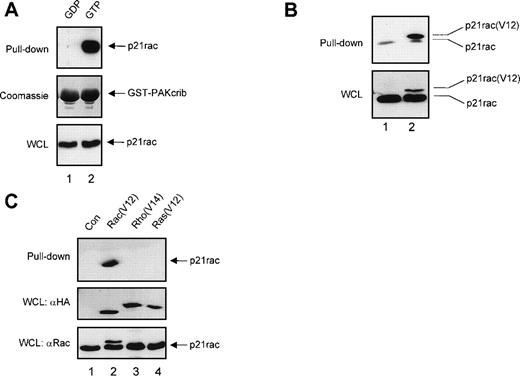
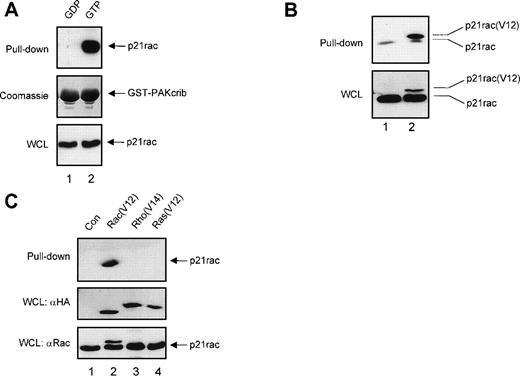
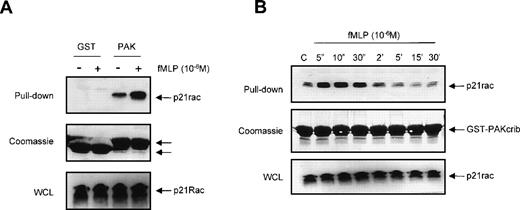
To confirm that GST-PAKcrib could be used as a specific probe to monitor the activation of p21rac, recombinant human p21rac was loaded with either GDP or the GTP analogue GppNHp. GST pull-down was performed using GST-PAKcrib, followed by anti-p21rac immunoblotting. As shown in Fig 1A, GST-PAKcrib strongly associated with the GTP-loaded p21rac, whereas no association with the GDP-loaded p21rac was observed (Fig 1A, top panel). On the same blot, Coomassie staining was performed demonstrating the use of equal amounts of GST-PAKcrib in both lanes (Fig 1A, middle panel). Before performing the GST pull-down, 1/40 of each sample was analyzed on Western blot, showing equal amounts of p21rac were present in both samples (Fig 1A, bottom panel). To determine whether this assay was also applicable for the analysis of p21rac activity in vivo, BW5147 cells stably retrovirally transduced with control vector (Fig 1B, lane 1) or with epitope tagged p21rac(V12) (lane 2) were used. GST pull-down was performed using GST-PAKcrib, followed by anti-p21rac immunoblotting. GST-PAKcrib strongly associated with p21rac(V12), despite its low expression compared with endogenous p21rac (Fig 1B, bottom panel), demonstrating that GST-PAKcrib specifically associates with activated, GTP-bound p21rac. The band running below the epitope-tagged p21rac(V12) was observed in both lanes due to the association of a small fraction of activated endogenous p21rac with GST-PAKcrib. To investigate the specificity of GST-PAKcrib toward p21rac, BW5147 cells stably retrovirally transduced with control vector (Fig 1C, lane 1), or epitope-tagged p21rac(V12) (lane 2), p21rho(V14) (lane 3), or p21ras(V12) (lane 4)40 were used. GST pull-down was performed using GST-PAKcrib followed by anti-epitope immunoblotting. Although the expression levels of p21rac(V12), p21rho(V14), and p21ras(V12) were equal (Fig 1C, middle panel), only p21rac(V12) was found to bind GST-PAKcrib. Furthermore, the antibody used for the detection of p21rac was highly specific, and did not show cross-reactivity with either p21rho or p21ras (Fig 1C, bottom panel). Because no association of GST-PAKcrib with other active small GTPases p21rho and p21ras was observed, this further shows the specificity of this assay.
GST-PAKcrib is a p21rac activation-specific probe. (A) Recombinant p21rac (0.2 μg) was generated and loaded in vitro with either GDP or the nonhydrolyzable GTP analogue, GppNHp, as described in Materials and Methods. p21rac was isolated using GST-PAKcrib and subsequently detected by Western blot analysis. (Top) GST pull-down of GDP-p21rac (lane 1) or GTP-p21rac (lane 2). (Middle) Coomassie staining of the same blot showing equal GSP-PAKcrib protein in both lanes. (Bottom) A fraction (1/40) of the sample used for the assay was probed with anti-rac antibody to show equal amounts of p21rac were present in both lanes. (B) BW5147 cells (107) stably retrovirally transduced with either control virus or epitope-tagged p21rac(V12)40 were lysed and active p21rac was isolated using GST-PAKcrib as described in Materials and Methods. p21rac was detected by Western blot analysis. (Top) GST pull-down of p21rac from control (lane 1) or p21rac(V12) transfected cells (lane 2). p21rac was detected using anti-rac antibody. (Bottom) A fraction of the lysate used for the pull-down assay was probed with anti-p21rac. (C) (Top) GST pull-down of p21rac from BW5147 cells stably retrovirally transduced with control virus (lane 1), p21rac(V12) (lane 2), p21rho(V14) (lane 3), or p21ras(V12) (lane 4).40 p21rac was detected using an antibody directed against the epitope-tag. (Middle) A fraction of the lysate used for the pull-down was probed with antibody directed against the epitope-tag. (Bottom) The same blot was subsequently probed with anti-rac to demonstrate the specificity of the anti-rac antibody.
GST-PAKcrib is a p21rac activation-specific probe. (A) Recombinant p21rac (0.2 μg) was generated and loaded in vitro with either GDP or the nonhydrolyzable GTP analogue, GppNHp, as described in Materials and Methods. p21rac was isolated using GST-PAKcrib and subsequently detected by Western blot analysis. (Top) GST pull-down of GDP-p21rac (lane 1) or GTP-p21rac (lane 2). (Middle) Coomassie staining of the same blot showing equal GSP-PAKcrib protein in both lanes. (Bottom) A fraction (1/40) of the sample used for the assay was probed with anti-rac antibody to show equal amounts of p21rac were present in both lanes. (B) BW5147 cells (107) stably retrovirally transduced with either control virus or epitope-tagged p21rac(V12)40 were lysed and active p21rac was isolated using GST-PAKcrib as described in Materials and Methods. p21rac was detected by Western blot analysis. (Top) GST pull-down of p21rac from control (lane 1) or p21rac(V12) transfected cells (lane 2). p21rac was detected using anti-rac antibody. (Bottom) A fraction of the lysate used for the pull-down assay was probed with anti-p21rac. (C) (Top) GST pull-down of p21rac from BW5147 cells stably retrovirally transduced with control virus (lane 1), p21rac(V12) (lane 2), p21rho(V14) (lane 3), or p21ras(V12) (lane 4).40 p21rac was detected using an antibody directed against the epitope-tag. (Middle) A fraction of the lysate used for the pull-down was probed with antibody directed against the epitope-tag. (Bottom) The same blot was subsequently probed with anti-rac to demonstrate the specificity of the anti-rac antibody.
p21rac is activated by serpentine, but not cytokine receptors in human neutrophils.
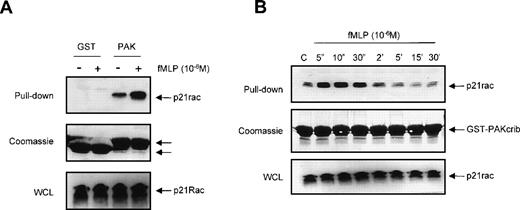
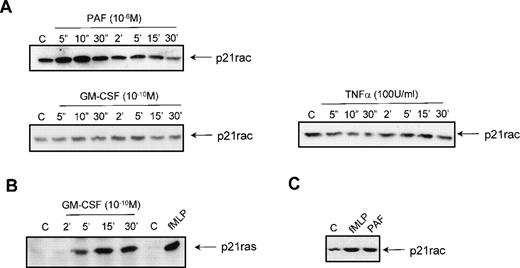
We investigated whether GST-PAKcrib could be used to monitor p21rac-GTP levels in human neutrophils in vivo after stimulation by the chemotaxin fMLP. Resting neutrophils isolated from human peripheral blood were left untreated or stimulated with fMLP for 10 seconds as indicated (Fig 2A, top panel). p21rac was isolated using either GST or GST-PAKcrib, followed by anti-Rac immunoblotting. The rac-antibody used reacts with both rac1 and rac2, thus allowing the simultaneous measurement of both isoforms. p21rac did not associate with the GST alone, further demonstrating the specificity of this assay. However, p21rac was pulled down when GST-PAKcrib was used. Although a basal level of p21rac-GTP was observed in unstimulated neutrophils, the amount of p21rac associating with GST-PAKcrib was greatly enhanced when cells were stimulated with 1 μmol/L fMLP. On the same blot, Coomassie staining was performed to show the use of equal amounts of GST or GST-PAKcrib in all samples (Fig 2A, middle panel). All samples contained equal amounts of p21rac (Fig 2A, bottom panel). To investigate the kinetics of fMLP-induced p21rac activation in human neutrophils, resting neutrophils isolated from human peripheral blood were stimulated with fMLP for various periods of time. p21rac was isolated with GST-PAKcrib, followed by anti-Rac immunoblotting. Stimulation with 1 μmol/L fMLP induced an extremely rapid and transient activation of p21rac (Fig 2B, top panel), declining after 10 seconds. All samples contained equal amounts of p21rac (Fig2B, middle panel) and equal amounts of GST-PAKcrib were used to precipitate p21rac (Fig 2B, bottom panel).
The GST-PAKcrib assay can be used to measure p21rac activation in human neutrophils. (A) Human neutrophils were isolated as described and stimulated with fMLP (1 μmol/L) for 10 seconds as indicated. Cells were lysed and GST pull-down was performed using either GST or GST-PAKcrib as described in Materials and Methods. (Top) The isolated p21rac was detected by Western blot analysis using anti-rac antibody. (Middle) A Coomassie staining of the blot. The arrowheads indicate the GST and GST-PAKcrib proteins. (Bottom) A fraction of the lysate used for the pull-down was probed with anti-Rac. (B) Neutrophils were isolated as described and stimulated with fMLP (1 μmol/L) for the indicated time. Cells were lysed and active p21rac was isolated using GST-PAKcrib as described in Materials and Methods. (Top) The GST-PAKcrib–associated p21rac was detected by Western blot analysis using anti-Rac antibody. (Middle) A fraction of the lysate used for the pull-down was probed with anti-Rac. (Bottom) Coomassie staining of the blot, the arrowhead indicates the GST-PAKcrib protein.
The GST-PAKcrib assay can be used to measure p21rac activation in human neutrophils. (A) Human neutrophils were isolated as described and stimulated with fMLP (1 μmol/L) for 10 seconds as indicated. Cells were lysed and GST pull-down was performed using either GST or GST-PAKcrib as described in Materials and Methods. (Top) The isolated p21rac was detected by Western blot analysis using anti-rac antibody. (Middle) A Coomassie staining of the blot. The arrowheads indicate the GST and GST-PAKcrib proteins. (Bottom) A fraction of the lysate used for the pull-down was probed with anti-Rac. (B) Neutrophils were isolated as described and stimulated with fMLP (1 μmol/L) for the indicated time. Cells were lysed and active p21rac was isolated using GST-PAKcrib as described in Materials and Methods. (Top) The GST-PAKcrib–associated p21rac was detected by Western blot analysis using anti-Rac antibody. (Middle) A fraction of the lysate used for the pull-down was probed with anti-Rac. (Bottom) Coomassie staining of the blot, the arrowhead indicates the GST-PAKcrib protein.
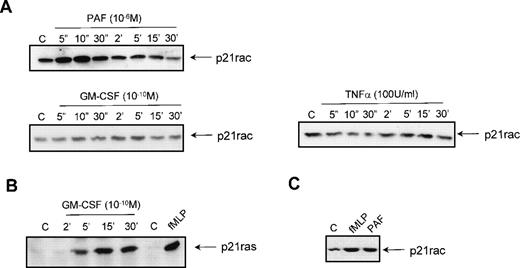
To investigate the effect of other stimuli on the activation of p21rac, resting neutrophils were isolated from human peripheral blood and stimulated with different ligands for various periods of time. Stimulation with 1 μmol/L PAF also resulted in a rapid activation of p21rac similar to the activation by 1 μmol/L fMLP (Fig 3A). Unexpectedly, stimulation of the cells with GM-CSF (10−10 mol/L) or TNF-α (100 U/mL) did not result in p21rac activation (Fig 3A), although these cytokines have been shown to be potent activators of PI3K at these concentrations in human neutrophils.6,15 To show that GM-CSF could activate other small GTPases in these cells, we performed a p21ras-activity assay using GST-Raf(RBD) as an activation-specific probe.15,41 Stimulation of neutrophils with 10−10 mol/L GM-CSF resulted in a transient activation of p21ras (Fig 3B). TNF-α was not able to activate p21ras (data not shown). To determine if other human phagocytes also demonstrate chemoattractant-mediated p21rac activation, human eosinophils were isolated from peripheral blood as described previously.43Stimulation of these cells for 10 seconds with fMLP or PAF also resulted in a potent activation of p21rac (Fig 3C). Thus, it appears that while both cytokine and chemokine receptors can activate overlapping signaling pathways that include activation of tyrosine and lipid kinases, only GPCRs are capable of potently activating p21rac at physiologically relevant ligand concentrations.
Activation of p21rac by diverse stimuli in human neutrophils. (A) Neutrophils were isolated as described and stimulated with either PAF (1 μmol/L), GM-CSF (10−10 mol/L), or TNF- (100 U/mL) for the indicated time. Cells were lysed and active p21rac was isolated using GST-PAKcrib as described in Materials and Methods. p21rac was detected by Western blot analysis. (B) Neutrophils were stimulated with either GM-CSF (10−10 mol/L) for the indicated time or with fMLP (1 μmol/L) for 10 seconds. Cells were lysed and active p21ras was isolated using GST-Raf1-RBD. p21ras was detected by Western blot analysis. (C) Eosinophils were stimulated with either fMLP (1 μmol/L) or PAF (1 μmol/L) for 10 seconds. Cells were lysed and active p21rac was detected as above.
Activation of p21rac by diverse stimuli in human neutrophils. (A) Neutrophils were isolated as described and stimulated with either PAF (1 μmol/L), GM-CSF (10−10 mol/L), or TNF- (100 U/mL) for the indicated time. Cells were lysed and active p21rac was isolated using GST-PAKcrib as described in Materials and Methods. p21rac was detected by Western blot analysis. (B) Neutrophils were stimulated with either GM-CSF (10−10 mol/L) for the indicated time or with fMLP (1 μmol/L) for 10 seconds. Cells were lysed and active p21ras was isolated using GST-Raf1-RBD. p21ras was detected by Western blot analysis. (C) Eosinophils were stimulated with either fMLP (1 μmol/L) or PAF (1 μmol/L) for 10 seconds. Cells were lysed and active p21rac was detected as above.
fMLP activates p21rac in a dose-dependent manner independent of GM-CSF priming.
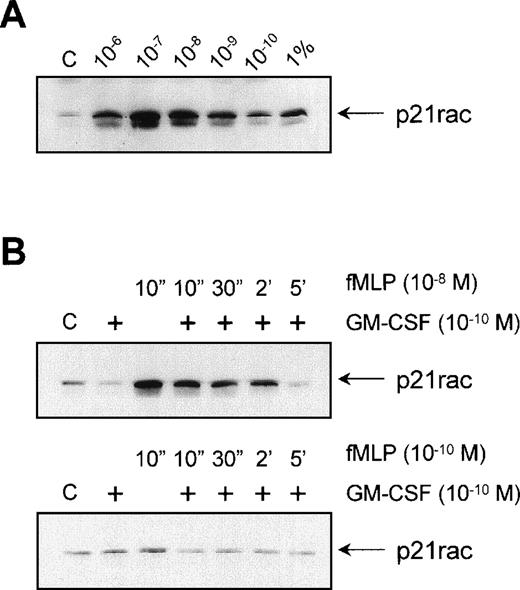
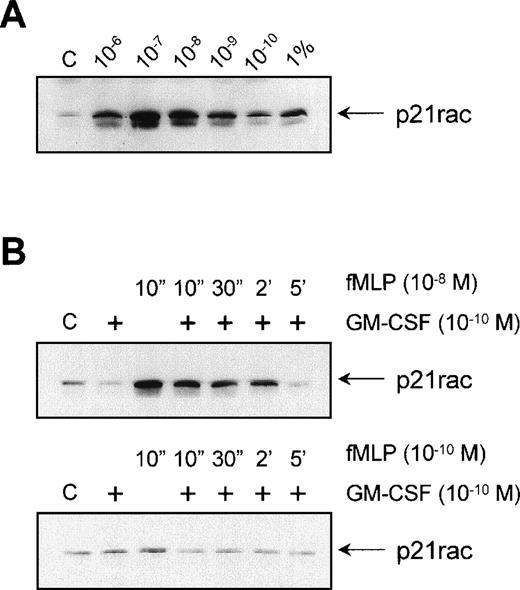
To determine the optimal concentration at which fMLP activates p21rac, we stimulated neutrophils with different concentrations of fMLP. Interestingly, p21rac activation showed a bell-shaped fMLP-concentration profile, typical for serpentine receptor-induced responses such as chemotaxis, with an optimal activation between 10−7 and 10−8 mol/L fMLP. Approximately 3% to 5% of p21rac present in whole cell lysates was found to bind GST-PAK after 10−7 mol/L fMLP stimulation of neutrophils (Fig 4A, compare last lane with third lane). GM-CSF is known to have a strong priming effect on both the fMLP-induced respiratory burst in human neutrophils and chemotaxis47 (and references therein). The mechanism of this priming is largely unknown. As p21rac is thought to play a role both in the respiratory burst and cytoskeletal reorganization associated with migration, we looked at whether GM-CSF had a priming effect on the fMLP-induced activation of p21rac. Neutrophils were primed with 10−10 mol/L GM-CSF before stimulation with either 10−8 mol/L fMLP or a suboptimal dose of 10−10 mol/L fMLP. We found no enhanced or extended activation when cells were stimulated with 10−8 mol/L fMLP (Fig 4B, top panel). Similarly, GM-CSF did not enhance the sensitivity of the cells toward a suboptimal dose of 10−10 mol/L fMLP (Fig 4B, bottom panel). Thus, the mechanism of GM-CSF–mediated priming of the respiratory burst does not appear to involve activation of p21rac.
Effect of GM-CSF on fMLP-induced p21rac activation. (A) Neutrophils were isolated as described and stimulated with the indicated final concentrations of fMLP for 10 seconds. p21rac activation was determined as described in the legend to Fig 1. One percent of a total cell lysate was used for comparison with the amount of activated p21rac. (B) Neutrophils were pretreated for 20 minutes with 10−10 mol/L GM-CSF after stimulation with either 10−8 mol/L fMLP (top) or 10−10 mol/L fMLP (bottom) for the time indicated.
Effect of GM-CSF on fMLP-induced p21rac activation. (A) Neutrophils were isolated as described and stimulated with the indicated final concentrations of fMLP for 10 seconds. p21rac activation was determined as described in the legend to Fig 1. One percent of a total cell lysate was used for comparison with the amount of activated p21rac. (B) Neutrophils were pretreated for 20 minutes with 10−10 mol/L GM-CSF after stimulation with either 10−8 mol/L fMLP (top) or 10−10 mol/L fMLP (bottom) for the time indicated.
fMLP-induced p21rac activation is not inhibited by kinase inhibitors.
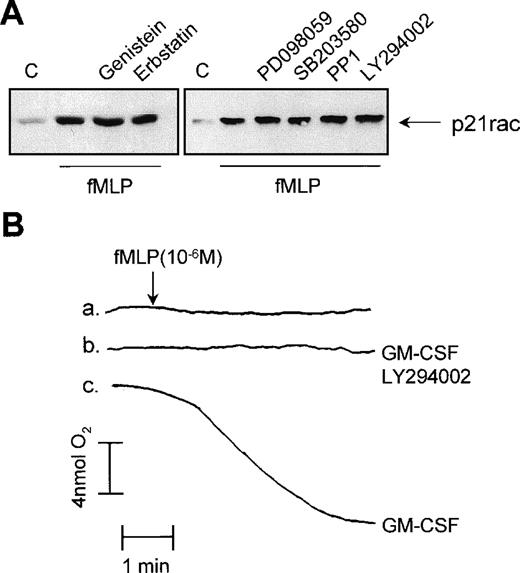
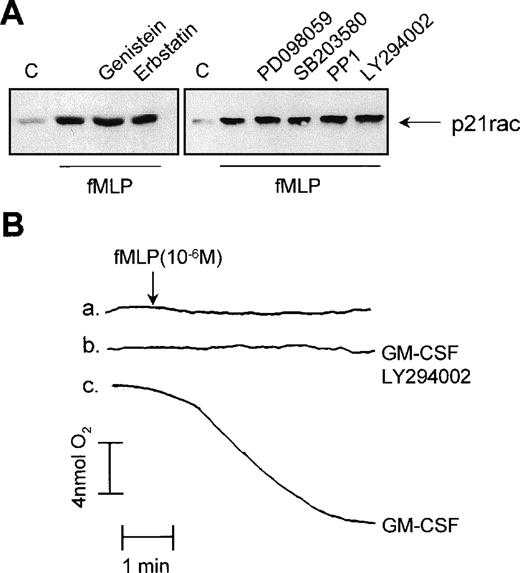
We used several protein and lipid kinase inhibitors to determine the mechanism by which p21rac is activated by fMLP in human neutrophils. First, resting human neutrophils were treated with the broad specificity tyrosine kinase inhibitors, genistein and erbstatin. Engagement of fMLP receptors has been shown to induce tyrosine phosphorylation-mediated signaling events in neutrophils.48These inhibitors effectively block or greatly reduce tyrosine phosphorylation in human neutrophils in response to diverse stimuli.16 Pretreatment of the cells with 100 μmol/L genistein or erbstatin had no effect on fMLP-induced p21rac activation (Fig 5A, left panel). Studies in various cell lines have suggested that p21rac activation may lie downstream of the lipid kinase PI3K.28,49,50 Furthermore, inhibition of PI3K with the specific inhibitor, LY294002, has been shown to block the fMLP-induced respiratory burst in neutrophils completely51(see also Fig 5B). As fMLP induces PI3K activation in human neutrophils,52 we investigated whether this kinase may play a role in the activation of p21rac. Addition of LY294002 to human neutrophils at concentrations that inhibit PI3K53 had no effect on p21rac activation (Fig 5A, right panel). The same was observed for wortmannin, another potent PI3K inhibitor (data not shown). However, as expected, LY294002 completely blocked the fMLP-induced respiratory burst (Fig 5B).
Effect of tyrosine kinase inhibitors on p21rac activation. (A) Neutrophils were pretreated for 20 minutes with 100 μmol/L genistein, 100 μmol/L erbstatin, 50 μmol/L PD098059, 10 μmol/L SB203580, 50 μmol/L PP1, or 10 μmol/L LY294002 after stimulation with fMLP (1 μmol/L) for 10 seconds. (B) Neutrophils were either untreated (a), pretreated with 10 μmol/L LY294002 for 20 minutes and then GM-CSF (10−10 mol/L) for 20 minutes (b), or with GM-CSF (10−10 mol/L) for 20 minutes (c). At the time indicated by the arrowhead, samples were stimulated with fMLP (1 μmol/L). Oxygen consumption was measured using a Clark oxygen electrode as previously described.
Effect of tyrosine kinase inhibitors on p21rac activation. (A) Neutrophils were pretreated for 20 minutes with 100 μmol/L genistein, 100 μmol/L erbstatin, 50 μmol/L PD098059, 10 μmol/L SB203580, 50 μmol/L PP1, or 10 μmol/L LY294002 after stimulation with fMLP (1 μmol/L) for 10 seconds. (B) Neutrophils were either untreated (a), pretreated with 10 μmol/L LY294002 for 20 minutes and then GM-CSF (10−10 mol/L) for 20 minutes (b), or with GM-CSF (10−10 mol/L) for 20 minutes (c). At the time indicated by the arrowhead, samples were stimulated with fMLP (1 μmol/L). Oxygen consumption was measured using a Clark oxygen electrode as previously described.
Using a variety of specific pharmacologic inhibitors including the ERK inhibitor, PD098059, the p38 mitogen activated protein kinase (MAPK) inhibitor, SB203580, and the Src inhibitor, PP1, we investigated the role of these kinases in mediating p21rac activation. We and others have previously shown these inhibitors to be potent and specific in human neutrophils at these concentrations.15 54 None of these compounds had an inhibitory effect on the fMLP-induced p21rac activation (Fig 5A, right panel). These data suggest a novel mechanism for p21rac activation independent of tyrosine phosphorylation or PI3K. Furthermore, it indicates that the mechanism by which inhibition of PI3K results in abrogation of superoxide production does not involve p21rac, but apparently another component of the NADPH oxidase system.
p21rac activation is independent of changes in intracellular Ca2+.
Although the fMLP receptor has been shown to activate a variety of tyrosine-phosphorylation–mediated signaling events, activation of protein kinase C (PKC) and an increase in intracellular free Ca2+ ([Ca2+]i) have also been described.55 Stimulation of neutrophils with fMLP results in a very rapid and transient increase in intracellular free [Ca2+]i that correlates with the activation of p21rac (Fig 6A). To investigate a possible role for Ca2+ and/or PKC in fMLP-induced p21rac activation, we depleted neutrophils of Ca2+ by pretreatment with 1 mmol/L EGTA, 1.5 μmol/L indo-1/AM, and 100 nmol/L thapsigargin. This causes a decrease of [Ca2+]i to less than 10 nmol/L, and fMLP stimulation no longer induces an increase in [Ca2+]i (Fig 6B).
Effect of Ca2+ depletion on p21rac activation. (A) Neutrophils were stimulated with fMLP (10−8 mol/L) for the time indicated. p21rac activation was determined as previously described. In cells from the same donor change in [Ca2+]i was measured after stimulation. The arrowhead indicates addition of fMLP (10−8 mol/L). (B) Neutrophils were Ca2+-depleted before stimulation with fMLP (10−8 mol/L). p21rac activation and [Ca2+]i were measured as described above.
Effect of Ca2+ depletion on p21rac activation. (A) Neutrophils were stimulated with fMLP (10−8 mol/L) for the time indicated. p21rac activation was determined as previously described. In cells from the same donor change in [Ca2+]i was measured after stimulation. The arrowhead indicates addition of fMLP (10−8 mol/L). (B) Neutrophils were Ca2+-depleted before stimulation with fMLP (10−8 mol/L). p21rac activation and [Ca2+]i were measured as described above.
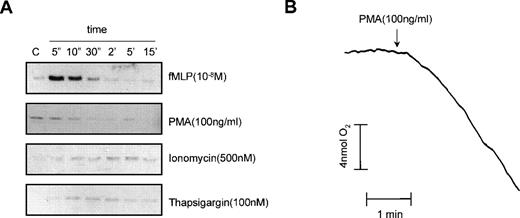
As shown in Fig 6B, Ca2+-depletion did not affect the fMLP-induced p21rac activation. It has been shown for the activation of the Rap1 small GTPase in neutrophils that although Ca2+ is not essential for the activation of Rap1 by fMLP, Ca2+influx induced with ionomycin or thapsigargin resulted in Rap1 activation.17 Therefore, we investigated whether ionomycin or thapsigargin could activate p21rac. As shown in Fig 7A, neither ionomycin nor thapsigargin induced p21rac activation compared with an fMLP (10−8mol/L) control. Surprisingly, PMA, an activator of PKC that strongly induces the respiratory burst in human neutrophils, did not induce p21rac activation. As a control, we measured the respiratory burst induced by PMA in cells from the same donor. As shown in Fig 7B, PMA strongly activated the respiratory burst under conditions that did not activate p21rac. This demonstrates that superoxide production per se in human neutrophils can occur independently of p21rac activation.
PMA activates the respiratory burst independently of p21rac. (A) Neutrophils were stimulated with fMLP (10−8mol/L), PMA (100 ng/mL), ionomycin (500 nmol/L), or thapsigargin (100 nmol/L) for the time indicated. p21rac activation was determined as described. (B) In cells from the same donor, oxygen consumption was measured after stimulation with PMA (100 ng/mL).
PMA activates the respiratory burst independently of p21rac. (A) Neutrophils were stimulated with fMLP (10−8mol/L), PMA (100 ng/mL), ionomycin (500 nmol/L), or thapsigargin (100 nmol/L) for the time indicated. p21rac activation was determined as described. (B) In cells from the same donor, oxygen consumption was measured after stimulation with PMA (100 ng/mL).
Role of heterotrimeric G-protein α-subunits in fMLP-mediated activation of p21rac.
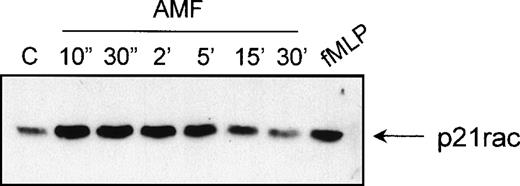
fMLP and PAF both act via serpentine receptors coupled to heterotrimeric G-protein subunits. Recently, a conserved motif was found between a GEF for Rho GTPases (p115RhoGEF) and members of the Regulators of G protein Signaling (RGS) family.56,57 It is suggested that p115RhoGEF links the inactivation of Gα12and Gα13 proteins to the activation of Rho through the direct association with the activated Gα13 subunits. To determine whether direct activation of heterotrimeric G protein α-subunits can in itself play a role in the activation of p21rac, we stimulated cells with AlF4−, which causes specific activation of the α-subunits of heterotrimeric G-proteins.58 Indeed, AlF4−has previously been demonstrated to induce actin-polymerization via G-proteins in human neutrophils.59 We found that AlF4− activates p21rac with similar kinetics, as does fMLP (Fig 8). This suggests, although indirectly, that there is a potential direct role for Gα-subunits in the activation of p21rac by serpentine receptors.
p21rac can be activated AlF4−. Neutrophils were stimulated with AlF4− as described in Materials and Methods for the indicated times. As a control, neutrophils were stimulated with fMLP (10−8mol/L) for 10 seconds. p21rac activation was determined as previously described.
p21rac can be activated AlF4−. Neutrophils were stimulated with AlF4− as described in Materials and Methods for the indicated times. As a control, neutrophils were stimulated with fMLP (10−8mol/L) for 10 seconds. p21rac activation was determined as previously described.
DISCUSSION
This is the first report to describe the activation of p21rac by chemoattractants in vivo. Activation of p21rac results in the exchange of bound GDP for GTP. Conventionally, activation of small G-proteins is measured by the immunoprecipitation of the protein and measurement of GTP loading. However, as endogenous p21rac cannot be immunoprecipitated from cell lines or primary cells,28 direct measurement of the GTP loading of p21rac has not been possible. We therefore used a novel assay to measure p21rac activation in vivo. This assay is based on the finding that GTP-p21rac specifically associates with the Cdc42/p21rac interactive binding (CRIB) motif of PAK, whereas GDP-p21rac does not (Fig 1).34 35 In stably retrovirally transduced BW5137 cells, p21rac(V12), but not p21ras(V12) or p21rho(V14), associated with GST-PAKcrib, demonstrating the specificity of this interaction (Fig 1). Although the expression level of p21rac(V12) was extremely low as compared with the endogenous p21rac, p21rac(V12) was shown to potently bind GST-PAKcrib. We believe this is not a consequence of aspecific binding of GDP-p21rac to the GST-PAKcrib, as we observed no interaction between the recombinant GDP-p21rac and GST-PAKcrib, nor did a GST control pull-down any p21rac from a neutrophil lysate (see Figs 1A and 2A). The low background binding of endogenous p21rac that was nevertheless observed is probably due to serum activation of the endogenous p21rac.
An extremely rapid activation of p21rac was observed on stimulation of human neutrophils with the chemoattractants fMLP or PAF, with an optimal activation within 5 to 10 seconds. Previous studies in cell lines have suggested that activation of p21rac lies downstream of PI3K.28,49,50 Unexpectedly, stimulation of neutrophils with TNF-α or GM-CSF had no effect on p21rac activity, although we and others have previously reported that both of these cytokines are potent activators of the lipid kinase PI3K in neutrophils.6,15This suggests that activation of PI3K per se is not sufficient to induce p21rac activation in neutrophils. This is supported by a recent report, showing that the dissociation of gelsolin from actin filaments, which initiates actin polymerization, is regulated by p21rac independently of PI3K.60 Recent reports have also indicated that PI3K can indeed act downstream of p21rac. Inhibition of PI3K in cells expressing dominant active p21rac blocked the rac-induced spreading and the formation of actin structures,61 and bombesin-stimulated membrane ruffling, which requires p21rac, is not blocked by wortmannin.62 Furthermore, a direct association between GTP-p21rac and PI3K has been reported in neutrophils, which caused an increase in PI3K activity.63 In addition, tyrosine kinase inhibitors, genistein and erbstatin, and specific inhibitors of MEK, p38 MAPK, Src, and PI3K had no effect on the fMLP-induced p21rac activation, suggesting that in human neutrophils fMLP-induced p21rac activation is via an alternative pathway.
During the preparation of this report, an article reported that in transfected COS-7 cells expressing the fMLP receptor, fMLP-induced cortical actin reorganization was blocked by dominant-negative mutants of PI3Kγ, Vav, and p21rac.50 These data suggests that fMLP activates p21rac via PI3Kγ-mediated activation of Vav. This is in contrast to the data reported herein concerning the mechanisms of fMLP-mediated p21rac activation in neutrophils. One explanation could be that expression of the fMLP receptor in COS cells, a cell that does not normally respond to fMLP, results in the utilization of signaling pathways not normally used for p21rac activation. Indeed, activation of fMLP receptors overexpressed in RBL cells has been shown to cause an abnormally sustained activation profile relative to cells endogenously expressing the receptor.64
p21rac is known to regulate actin assembly in a variety of cells, resulting in cell spreading and the formation of lamellipodia that may play a role in cell migration (see Hall65). It is interesting to note that, while GM-CSF and TNF-α promote chemokinesis (random cell migration) and do not activate p21rac, PAF and fMLP are strong chemoattractants (directed movement of cells) and do activate p21rac15 (Figs 2 and 3). Possibly the activation of p21rac plays an important role in the regulation of chemotaxis rather than random migration, suggesting interesting mechanistic variations between these two methods of cell movement. Indeed, it has been demonstrated that migration of fibroblasts towards platelet-derived growth factor (PDGF) is inhibited by expression of dominant-negative N17rac1.66 Furthermore, in macrophage cell lines, microinjection of dominant-negative N17rac1 inhibited CSF-1–induced polarization and migration.67
Another cellular response that has been linked to the activation of p21rac is the production of superoxide.21,22 Studies in cell-free extracts have suggested that p21rac is necessary for the assembly of the NADPH oxidase complex.23-26 The involvement of p21rac in the oxidase is also supported by the parallel reduction of p21rac expression and NADPH oxidase activation in Epstein-Barr virus (EBV)-transformed B cells cultured in the presence of antisense p21rac oligonucleotides.27 Indeed, interaction of p21rac with the p67phox component of the NADPH oxidase system has also been shown.68,69 This association is then thought to target the p67phox and associated p47phox proteins to the membrane where another component of the oxidase system, flavocytochrome b558, is present. In a patient suffering from chronic granulomatous disease, a single amino acid deletion was found in p67phox, which disrupts the interaction with p21rac.70 Neutrophils isolated from this patient failed to produce superoxide. Furthermore, overexpression of the dominant negative form of p21rac in HL60 cells inhibited the production of superoxide by these cells.24 We show that although inhibition of PI3K does not inhibit p21rac, it completely blocks the respiratory burst in neutrophils. This again suggests that p21rac is not downstream of PI3K in fMLP-stimulated neutrophils, but may act in parallel with this kinase to activate production of superoxide. fMLP stimulation of neutrophils results in changes in intracellular-free [Ca2+] levels,55 which are necessary for the respiratory burst to occur.71 Although the time frame of the fMLP-induced p21rac activation and the increase in intracellular Ca2+ are similar, Ca2+depletion did not affect the fMLP-induced p21rac activation, showing that p21rac can be activated independently of changes in [Ca2+]i. Similarly, Ca2+-influx induced by ionomycin or calcium release from intracellular stores induced by thapsigargin did not induce p21rac activation. Furthermore, PMA failed to activate p21rac, although in the same cells, PMA leads to a strong activation of the respiratory burst. This demonstrates that it is possible to activate superoxide production independently of p21rac using certain stimuli. In support of this finding, Philips et al72 showed that although fMLP stimulation of human neutrophils induced a transient increase in membrane-bound p21rac, stimulation with PMA did not cause p21rac translocation. Furthermore, in a cell-free assay, bacterially expressed unprenylated p21rac, which is not membrane-associated, can effectively activate the oxidase system25 calling into question both the necessity for p21rac to be translocated to the membrane fraction and the need for GTPase function. Thus, the precise mechanism of action of p21rac in the activation of the respiratory burst still needs to be determined. It has been reported that GTP loading of p21rac is necessary for the interaction between p21rac and p67phox.68 On the other hand, Bromberg et al23 have shown that GDP-p21rac complexed to the GDP dissociation inhibitor for p21Rho (Rho GDI) can activate the NADPH oxidase system in vitro. It is tempting to speculate that in neutrophils, p21rac does not need to be activated (GTP-bound) for the assembly and activation of the NADPH oxidase complex, but rather in a GDP-bound state complexed to Rho GDI.
Because fMLP-induced p21rac activation is not mediated by tyrosine kinases or induced by changes in [Ca2+]i, the mechanism of its activation remains elusive. Recently, a novel mechanism was reported for the activation of small GTPases by serpentine receptors. A conserved motif found between a GEF for Rho GTPases (p115RhoGEF) and members of the RGS family suggested that p115RhoGEF links the inactivation of Gα12 and Gα13 proteins to the activation of Rho through the direct association with the activated Gα-subunits.56,57Furthermore, a direct association was shown between Gα13and p115RhoGEF. Indeed, we found a similar homology between the exchange factor for p21rac, Vav, and several members of the RGS family (not shown). To explore the possibility that Gα-subunits may directly activate p21rac, human neutrophils were treated with AlF4−. It is known that AlF4− causes specific activation of the α subunits of heterotrimeric G-proteins by binding to Gα-GDP resulting in a GTP-like transition state.58Previously, it has been shown that AlF4−treatment of human neutrophils results in a pronounced increase in F-actin content.59 Interestingly, AlF4− treatment also resulted in the rapid and transient activation of p21rac with a time course that closely resembles that observed for fMLP and PAF (Fig 8). Our data suggest that this may be due to G-protein activation of p21rac. Although indirect, these data add weight to the idea that that activation of Vav by serpentine receptors may occur via an alternative direct mechanism requiring neither tyrosine phosphorylation nor 3-phosphorylated lipids, but rather occurring via a direct Gα-mediated mechanism.
ACKNOWLEDGMENT
We thank Tim Reid for the generation of GST-PAKcrib and Reza Ahmadian for the generation of recombinant p21rac and technical assistance with the GDP/GTP loading. Finally, we thank Laura M’Rabet for helpful discussions and Leo Houben for technical assistance.
Supported by GlaxoWellcome b.v., The Netherlands.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Paul J. Coffer, PhD, Department of Pulmonary Diseases, University Hospital Utrecht, G03.550, Heidelberglaan 100, 3584 CX Utrecht, The Netherlands; e-mail:P.Coffer@hli.azu.nl.

![Fig. 6. Effect of Ca2+ depletion on p21rac activation. (A) Neutrophils were stimulated with fMLP (10−8 mol/L) for the time indicated. p21rac activation was determined as previously described. In cells from the same donor change in [Ca2+]i was measured after stimulation. The arrowhead indicates addition of fMLP (10−8 mol/L). (B) Neutrophils were Ca2+-depleted before stimulation with fMLP (10−8 mol/L). p21rac activation and [Ca2+]i were measured as described above.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/94/3/10.1182_blood.v94.3.1121.415k04_1121_1130/5/m_blod41504006w.jpeg?Expires=1769449720&Signature=0cUE8izhrIzxib77Lde50lA~Ly78jkm8uvqDTKgwyMD9XVOCw~3i6ssE~tB7f5gB2g-kfE4x~ktPxnMJbyTIsKcyl2qcAgSPx2Wo~jYU2R40VcaVpK~PzTAMan1W4JvFDdTw8e0Nmu-rS9C0lac0QddBMBZ3v8666khC9nYlT7O2egxb3S~E~W1MFIn7TAZWyUkIFJs8YbIAZnyET3xRTUDZrmdV5ImqcK6JTrTQZ5nieRFRgmYQfpZnCiAXa9lzzzJDVXSDupr0gJaYOVgTVUyBMw3efD1-qQrkn-x8t1sw9oLK39C4a8bg9tblOQBI~Gq2jgZ0~ez7HX3qUqaNxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)