To the Editor:
A recent exchange of letters appearing in Blood1,2was of particular interest to our group. Lemons et al3 had published a report in Blood that stimulated a letter from Dr Morgenstern, to which one of the authors responded. Dr Morgenstern had no objections to the biochemical results of the article. Rather, he was concerned that the authors chose to discuss their molecular findings in the context of a morphological design with which Dr Morgenstern disagreed. In the introduction to their article the authors stated: “In one model of platelet activation, these stimuli (collagen, thrombin, and ADP) trigger morphological changes in the platelet resulting in the apparent movement of the secretory granules to the center of the cell and their subsequent fusion with the surface connected canalicular system (SCCS).”
Dr Morgenstern objected to this model. His letter stated, “The disadvantage with this model is that the fusion of membranes of secretory organelles with membranes of the SCCS (ie, open canalicular system, OCS) has never been demonstrated. In contrast, it was clearly shown in studies using rapid freezing with a time resolution in the range of milliseconds to capture fusion events, the secretory organelles (granules and dense cored bodies) fuse with the plasma membranes when the platelets were stimulated before.” He bolsters his argument with several citations of his own work4-7 and that of others,8 including one of our publications9 showing that α granules in bovine platelets that lack the OCS of human cells do fuse with the plasma membrane to secrete products to the exterior following activation in suspension.
The first paragraph of the responding letter by Whiteheart2answers the concerns of Dr Morgenstern very well. He points out: “It is well established that, subsequent to stimulation, human platelets undergo a series of morphological rearrangements that include the formation of pseudopods and centralization of secretory granules. The process culminates in the release of granule contents into the extra platelet space. . . . .”
What Whiteheart is saying, in essence, is that it doesn’t matter whether secretory organelles fuse directly with the plasma membrane or channels of the OCS because they are the same membranes. The network of surface membrane invaginations making up the OCS is unique. It is not found in any other type of blood cell. Behnke’s early studies10,11 and the work of other investigators12,13 have shown that OCS channels are not only continuous with the cell surface but are identical morphologically. That the platelet surface and linings of the OCS are identical has also been shown by ultrastructural immunocytochemistry. Monoclonal and polyclonal antibodies, together with immunogold techniques, have shown that the exposed surface and OCS channels in frozen thin sections are uniformly covered by GPIIb/IIIa14and GPIb/IX/V15 receptor complexes.
Morgenstern1 did not appear concerned about whether the SCCS and exposed surface are identical membranes. He points out: “It was concluded (ie, from his studies) that the fate of the SCCS during stimulation is to become evaginated within seconds to allow the surface enlargement necessary for formation of pseudopodia or spreading. Fully stimulated platelets do not show SCCS, but do show the membranes of degranulating organelles.” His point seems to be that OCS channels return to the exposed surface in the earliest stages of shape change after activation and are unavailable for fusion with α granules and dense bodies to facilitate secretion. These observations are incorrect.16 17
The SCCS is evaginated during platelet spreading on surfaces, but the process takes minutes, not microseconds, and elements of the SCCS remain in over 20% of fully spread platelets.17 Activation in suspension does not cause evagination of a significant number of OCS channels. Channels of the OCS do become dilated in platelets activated in suspension, and may appear to be part of the exposed surface, but are not evaginated. Had Morgenstern carefully read the articles from our laboratory cited in his letter, and others referred to in those citations, he would not have made the statements he has on this subject.
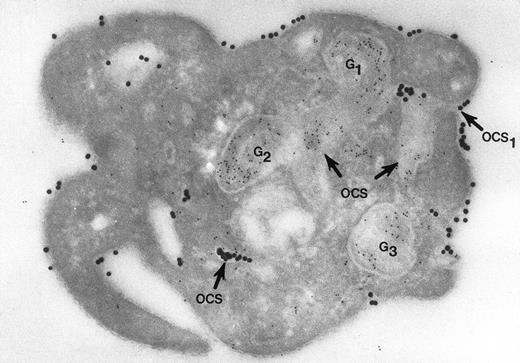
Nor would Morgenstern have made the comment that fusion of membranes of secretory organelles with membranes of the OCS has never been shown. The reports from our laboratory that he cited, and others,18 answer this concern quite well. In one of the experiments, platelets in suspension were combined with 20-nm colloidal gold particles coated with fibrinogen (Fgn/Au), then exposed to thrombin for 1, 3, and 5 minutes without stirring.19 At these intervals the platelets were fixed, frozen, and frozen thin sections prepared. The sections were stained with a polyclonal antifibrinogen antibody and then staph protein A bound to 10-nm gold particles. Thrombin stimulated uptake of 20 nm Fgn/Au gold particles into OCS channels and their transfer to α granules in the process of discharge into the OCS (Fig 1). Protein A 10-nm gold particles demonstrated fibrinogen in nonlabilized α granules and those in the process of discharge into the OCS. The work showed that the OCS was truly a final common pathway; hence, the name of the article.19
Cryosection of blood platelet from a washed cell suspension incubated with 18- to 20-nm colloidal gold particles coated with Fgn/Au for 5 minutes, then exposed to 1 U/mL of thrombin for 60 seconds. The Fgn/Au particles bind to the cell surface and penetrate into peripheral channels of the OCS. After fixation, freezing, and cryotomy, the frozen thin section was stained with a polyclonal antifibrinogen antibody and protein A bound to 5-nm gold particles. Immunogold beads detecting endogenous fibrinogen are concentrated in intact granules (G3) and in granules (G1, G2) in the process of discharging their contents into channels of the OCS. Some of the Fgn/Au particles entering from the outside are mixed with the immunogold beads in the same OCS channels (OCS) communicating with the exterior surface. Original magnification ×60,000. (Reprinted with permission.18)
Cryosection of blood platelet from a washed cell suspension incubated with 18- to 20-nm colloidal gold particles coated with Fgn/Au for 5 minutes, then exposed to 1 U/mL of thrombin for 60 seconds. The Fgn/Au particles bind to the cell surface and penetrate into peripheral channels of the OCS. After fixation, freezing, and cryotomy, the frozen thin section was stained with a polyclonal antifibrinogen antibody and protein A bound to 5-nm gold particles. Immunogold beads detecting endogenous fibrinogen are concentrated in intact granules (G3) and in granules (G1, G2) in the process of discharging their contents into channels of the OCS. Some of the Fgn/Au particles entering from the outside are mixed with the immunogold beads in the same OCS channels (OCS) communicating with the exterior surface. Original magnification ×60,000. (Reprinted with permission.18)
The second experiment also involved human platelets.18,20Cells in suspension were combined with thrombin for 15-second to 5-minute intervals and fixed in solutions containing tannic acid. Tannic acid combines with the surface membrane glycoproteins to form a mordant dye that binds and converts osmic acid to osmium black, an electron dense stain. Tannic acid under these conditions binds only to those membranes exposed to the exterior, or membranes continuous with the outside. Thus, it stains the platelet surfaces and membranes lining channels of the OCS. It has one other desirable feature. Tannic acid also selectively stains fibrinogen and fibrin strands in the same manner as the glycocalyx.20
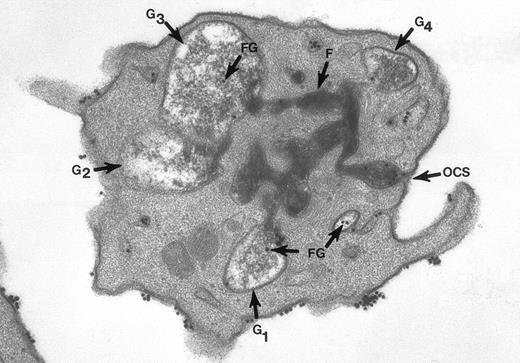
The surface and OCS membranes are the only structures stained by osmium black in resting platelets. After exposure to thrombin, the dense stain also enters α granules that have become labelized and communicate with the OCS.20 Fibrinogen and fibrin in the process of extrusion from α granules fill channels of the OCS. During this process the OCS becomes dilated, as do the labelized α granules, yielding the appearance of swollen vacuoles (Fig 2). In experiments where the platelets were combined with fibrinogen-coated gold particles before exposure to thrombin and fixation through the tannic acid staining procedure, Fgn/Au was present in OCS channels and in swollen α granules. The findings showed that the OCS is a two-way street, and α granules communicate with channels of the OCS to secrete their contents.
Thin section of a platelet from a sample of washed cells incubated with Fgn/Au particles for 15 minutes, then exposed to 5 U/mL of thrombin for 60 seconds before fixation in glutaraldehyde-tannic acid-osmium to selectively stain the platelet glycocalyx, fibrinogen, and fibrin. This example is one of several serial sections through the same platelet. It and the other serial sections show the typical features of shape change and internal transformation caused by thrombin. Fgn/Au particles are bound to the irregular surface and are in the process of entering channels of the OCS. The OCS channel indicated by an arrow (↑) is filled with fibrinogen and fibrin stained by tannic acid-osmium black. The tortuous channel is connected directly to several granules (G1, G2, G3) in various stages of labelization. Residual fibrinogen is stained by the reaction product. Fgn/Au particles carried by the OCS have entered the granules (G1, G3, G4). The direct connections between OCS channels and granules in this example and its serial sections is indisputable. Original magnification ×45,000. (Reprinted with permission.19)
Thin section of a platelet from a sample of washed cells incubated with Fgn/Au particles for 15 minutes, then exposed to 5 U/mL of thrombin for 60 seconds before fixation in glutaraldehyde-tannic acid-osmium to selectively stain the platelet glycocalyx, fibrinogen, and fibrin. This example is one of several serial sections through the same platelet. It and the other serial sections show the typical features of shape change and internal transformation caused by thrombin. Fgn/Au particles are bound to the irregular surface and are in the process of entering channels of the OCS. The OCS channel indicated by an arrow (↑) is filled with fibrinogen and fibrin stained by tannic acid-osmium black. The tortuous channel is connected directly to several granules (G1, G2, G3) in various stages of labelization. Residual fibrinogen is stained by the reaction product. Fgn/Au particles carried by the OCS have entered the granules (G1, G3, G4). The direct connections between OCS channels and granules in this example and its serial sections is indisputable. Original magnification ×45,000. (Reprinted with permission.19)
As far as Lemons et al3 are concerned, it may matter little whether α granules fuse with the exposed surface or channels of the OCS, because their membranes are identical. However, the morphological background serving as a palette for their work on the molecular machinery should be as accurate as possible. I hope this letter will help to clarify the background.