Abstract
Several functional anomalies of B-chronic lymphocytic leukemia (B-CLL) cells may be explained by abnormalities of the B-cell receptor (BCR), a multimeric complex formed by the sIg homodimer and the noncovalently bound heterodimer Ig/Igβ (CD79a/CD79b). Because the expression of the extracellular Ig-like domain of CD79b has been reported to be absent in the cells of most CLL cases, we have investigated the molecular mechanisms that may account for this defect. Peripheral blood lymphocytes (PBL) from 50 patients and two cell lines (MEC1, MEC2) obtained from the PBL of one of them were studied. MEC1, MEC2, and 75% of CLL cases did not express detectable levels of the extracellular Ig-like domain of CD79b, which was nevertheless present in greater than 80% CD19+ cells from normal donors. In healthy subjects the expression of CD79b was equally distributed in CD5+ and CD5− B-cell subsets. Reverse transcription-polymerase chain reaction (RT-PCR) analysis of CD79b RNA from all patients and from MEC1 and MEC2 cell lines consistently yielded two fragments of different size (709 bp and 397 bp). The 709-bp band corresponds to CD79b entire transcript; the 397-bp band corresponds to an alternatively spliced form lacking exon 3 that encodes the extracellular Ig-like domain. Both fragments were also visible in normal PBL. The expression of the 397-bp fragment was increased in normal activated B cells, while no difference was seen between CD5+ and CD5− B cells. To obtain a more accurate estimate of the relative proportions of the two spliced forms, a radioactive PCR was performed in 13 normal and 22 B-CLL samples and the results analyzed using a digital imager. The mean value of the CD79b to the CD79b internally deleted ratio was 0.64 ± 0.20 SD in normal donors and 0.44 ± 0.27 SD in B-CLL (P = .01). Direct sequencing of 397-bp RT-PCR products and of genomic DNA corresponding to exon 3 from MEC1, MEC2, their parental cells, and five fresh B-CLL samples did not show any causal mutation. Single-strand conformation polymorphism analysis of exon 3 performed in 18 additional B-CLL cases showed a single abnormal shift corresponding to a TGT → TGC polymorphic change at amino acid 122. We propose a role for the alternative splicing of CD79b gene in causing the reduced expression of BCR on the surface of B-CLL cells. As normal B cells also present this variant, the mechanism of CD79b posttranscriptional regulation might reflect the activation stage of the normal B cell from which B-CLL derives.
B-CHRONIC LYMPHOCYTIC leukemia (B-CLL) is characterized by the relentless accumulation of monoclonal CD5+ B cells that express most of the membrane antigens (Ag) present on mature B cells, including activation markers, and have a number of distinctive features.1 First, they express faint to virtually undetectable amounts of surface immunoglobulins (sIg)2 often reacting to self-Ag.3 Second, they have an unusual kinetic hyporesponsiveness.4,5 The mitogenic signals that induce the proliferation of normal B cells are very weak stimuli for B-CLL cells. Third, an important mechanism of B-CLL cell accumulation is a defective apoptosis that leads to extended cell survival.3,5 Finally, B-CLL cells fail to present soluble and allo-Ag,6,7 whereas normal B cells upon activation are effective Ag-presenting cells.8
Abnormalities of the B-cell receptor (BCR) may conceivably provide a common explanation for all of these functional anomalies. The BCR of mature B cells is a multimeric complex that is formed by the sIg homodimer and the noncovalently bound heterodimer Igα/Igβ (CD79a/CD79b).9,10 Both CD79a and CD79b have an extracellular part that forms an Ig-like domain and binds to sIg.11 The heterodimer is both essential and sufficient for Ig to reach the membrane9-12 and is the signal transducing unit of the BCR.13-15 Also, CD79a and CD79b function synergistically to induce apoptosis mediated via the BCR.16Further, they influence Ag internalization and increase the efficiency of Ag presentation.17
It was first noted that B-CLL cells have defective BCR-mediated signal transduction.18 Next, it was shown that the monoclonal antibody (MoAb) SN8,19 which identifies an epitope on the extracellular domain of CD79b, fails to react with malignant B cells in more than 95% of B-CLL patients.20 The diminished display of BCR on the membrane of B-CLL cells has been recently ascribed to either reduced amounts of CD79b mRNA or the occurrence of somatic mutations predicted to affect CD79b expression.21
A CD79b truncated form (ΔCD79b) which arises by alternative splicing of the CD79b gene has been described in a variety of human B cells and B-cell lines22 23; of interest, this variant lacks exon 3 that encodes the extracellular Ig-like domain. The precise functional significance of ΔCD79b is incompletely defined and its potential significance in B-cell malignancies has not been explored. In the present work we have investigated the molecular mechanisms that may account for the absent expression of the extracellular Ig-like domain of CD79b in CLL and show that all cases are characterized by the presence of the ΔCD79b mRNA that lacks the extracellular domain. Direct sequencing of B-CLL samples did not reveal causal mutations of the CD79b gene. We propose a role for the alternative splicing of CD79b gene in causing the reduced expression of BCR on the surface of B-CLL cells.
MATERIALS AND METHODS
Cells and cell lines.
Peripheral blood lymphocytes (PBL) from 50 patients with B-CLL were studied. According to the Rai staging system,24 8 patients were stage 0, 7 stage I, 13 stage II, 7 stage III, and 15 stage IV. All patients were studied either before or at least 3 months after chemotherapy.
Two B-CLL cell lines, MEC1 and MEC2, that grew spontaneously on two subsequent occasions from the PB of a patient with B-CLL in prolymphocytoid transformation (A. Stacchini, F. Caligaris-Cappio, manuscript submitted) were analyzed. The patient was Epstein-Barr virus (EBV)-seropositive, his leukemic cells were Epstein-Barr nuclear antigen (EBNA)−, but the spontaneously grown cell lines are EBNA-2+ as judged by polymerase chain reaction (PCR). In liquid culture MEC1 cells grow adherent to the container wall and as tiny clumps; MEC2 cells do not adhere and form large clumps. The doubling time of MEC1 is 40 hours and of MEC2 is 31 hours. Both cell lines express the same light (κ) and heavy chains (μ, δ) as the fresh parental B-CLL cells at the same high intensity and share the expression of mature B-cell markers (CD19, CD20, CD21, CD22). CD5 is negative on MEC1 cells (as on the vast majority of parental cells) and it has been lost by MEC2. The tumor origin of MEC1 and MEC2 has been shown by Southern Blot analysis of the IgH loci and by Ig gene DNA sequencing. The cells use the VH4 Ig family, have 94.8% homology with germ-line Ig gene 4-59, and display a complex karyotype.
The controls were: (1) Daudi, Raji, and Jurkatt cell lines; (2) four EBV-induced cell lines (lymphoblastoid cell lines [LCL]) obtained from four different normal donors; (3) fresh unstimulated PBL from 14 normal donors; (4) B lymphocytes purified from the PB of three normal donors and stimulated in vitro with murine fibroblastic L cells stably expressing both human CD40 ligand (L) and CD32/FcγRII (CD40L/CD32-L cells, kind gift of Dr J. Banchereau, Dardilly, France) (see below); and (5) CD5+ B cells purified from tonsils of three young adults undergoing tonsillectomy after antibiotic treatment.
Cell separation.
PBL were obtained by separation on Ficoll-Hypaque (FH; Pharmacia-LKB, Uppsala, Sweden) gradient and washed twice in phosphate-buffered saline (PBS).
Tonsils were teased with blunt forceps: cell suspensions were washed with RPMI 1640 medium and adhered to plastic for 1 hour. The nonadherent cells were rosetted with Sheep red blood cells (SRBC) and spun onto FH for 30 minutes. The cells at the interphase (SRBC−, adherent cell–depleted and thus B-cell–enriched lymphocytes) were further reacted with a cocktail of CD3 (Leu 4; Becton Dickinson, Mountain View, CA; cat. no. 7340), CD11b (Leu 15; Becton Dickinson; cat. no. 7550), CD14 (Leu M3; Becton Dickinson; cat. no. 7490) MoAbs. CD3+, CD11b+, and CD14+ cells were removed by goat (G)–anti-mouse (m)–IgG-coated magnetic beads. The remaining elements (>96% CD19+ B cells) were reacted with CD5 MoAb and fractionated into CD19+, CD5+, and CD19+, CD5− cells by G-anti-m–IgG-coated magnetic beads. The same technique was used to purify, within B-cell–enriched populations, CD19+, SN8+ from CD19+, SN8− cells.
Cell phenotyping.
An aliquot of cells was resuspended in PBS containing 1% bovine serum albumin (BSA; Sigma, St Louis, MO) and 0.02% sodium azide (staining medium) at the concentration of 10 × 106/mL. An appropriate amount of fluorochrome-labeled antibody (Ab), at optimal concentration, was added to 100 μL cell suspension. Negative controls were incubated with isotype irrelevant Abs. After 30 minutes of incubation at 4°C, cells were washed twice with 2 mL of staining medium and resuspended for flow cytometric analysis.
Different combinations of Abs were used in direct or indirect immunofluorescence (IF). G antisera to human (h) μ, δ, κ, and λ chains, directly conjugated with fluorescein-isothiocyanate (FITC; Tago, Burlingame, CA; cat. no. 4202, 4205, 4206, 4208). The m MoAbs used were CD5-FITC (Leu1; Becton Dickinson; cat. no. 7303), CD19-phycoerythrin (PE) (Leu12; Becton Dickinson; cat. no. 9209), CD23-PE (Leu20; Becton Dickinson, cat. no. 7797), CD3-FITC (Leu 4; Becton Dickinson; cat. no. 92-0001), HLA-DR-PE (Becton Dickinson; cat. no. 7367), CD79b-FITC (clone SN8; Dako, Glostrup, Denmark; cat. no. F7137), and CB3-125 (kind gift of M.D. Cooper, Howard Hughes Medical Institute, Birmingham, AL). Cell populations were considered CD79b+ if greater than 30% of the cells stained with the MoAb.20 The degree of intensity of sIg, SN8, and CB3-1, analyzed according to Matutes et al,26 was estimated in a histogram data display and compared to the control without Ab selecting the log scale in the fluorescence axis and geometric statistics. Accordingly, the staining intensity was defined weak (w) when the positive peak was within the first logarithmic percentile and bright (b) when the peak was within the third percentile or more.
To evaluate the possibility that the epitope detected by SN8 might be present in the cytoplasm, cells were first preincubated with 20 μL unconjugated SN8 (Dako; cat. no. 7136) to saturate CD79b molecules present on the membrane. After 30 minutes cells were washed with staining medium, fixed, and permeabilized with the Caltag Fix and Perm Cell Permeabilization kit (Caltag Laboratories, San Francisco, CA) according to the manufacturer’s instructions. Permeabilized cells were incubated with 10 μL CD79b-FITC for 30 minutes at 4°C, washed, and resuspended for flow cytometric analysis. Negative controls were performed by incubating cells with isotype irrelevant Abs: m-IgG1 (Dako; cat. no. x931) and m-IgG1-FITC (Dako; cat. no. x0927).
Flow cytometric analysis.
All samples were analyzed using a FACScan Research cytometer (Becton Dickinson) equipped with a 488 nm argon ion laser (Becton Dickinson Immunocytometry Systems [BDIS], Mountain View, CA). Data acquisition was performed using the FACScan Research Software (BDIS). Forward light scattering, orthogonal light scattering, and two fluorescence signals were determined for each cell and stored in listmode data files. Each measurement contained at least 5,000 cells. In all samples a gate was used on both light scattering parameters to obtain more events of lymphocyte populations.
Analysis of the fluorescence histograms was performed by the Kolmogorov-Smirnov test.27 The test was used for the calculation of the D value with the FACScan software, version 2.1-3/89 (Becton Dickinson). The D value corresponded to the maximum difference between the two summation curves computed from the histograms, whereas D/S(n) indicated the similarity of the two noncompared curves. The closer D/S(n) was to zero, the more alike the two curves were. The formula was: S(n) = (n1 + n2)/n1 × n2, where n1 equalled the number of events in the first graph and n2 equalled the number of events in the second graph. A D/S(n) value >10 was considered statistically significant.27
Lymphocyte stimulation.
PBL from normal donors were left for 1 hour to allow cell adherence to the plastic. The nonadherent cells were rosetted with SRBC and spun onto FH for 30 minutes. The cells at the interphase (SRBC− lymphocytes) were collected, washed twice with RPMI medium, and reacted with a cocktail of CD3, CD11b, CD14 MoAbs. CD3+, CD11b+, and CD14+ cells were removed by G-anti-m–IgG-coated magnetic beads. The remaining cells were greater than 95% CD19+ and were cultured in 96-well flat-bottom plates (Falcon, Oxnard, CA) at the concentration of 5 × 104 cells/well in the presence of 5 × 103irradiated (7,500 rad) CD40L/CD32-L cells and 100 U/mL interleukin-4 (IL-4) as described.28
RNA isolation and Northern blot analysis.
Total cellular RNA was isolated by RNAzolB procedure (Biotecx Laboratories, Friendswood, TX). Denatured total RNA samples (15 μg/well) were fractionated on a 1.5% formaldehyde containing agarose gel, transferred to a nitrocellulose filter, and hybridized with 32dCTP-labeled probe corresponding to the coding region of the CD79b gene (709-bp fragment, see Fig1) in 3× SSC (1× SSC = 0.15 mol/L NaCl, 0.015 mol/L Na citrate, pH 7.0), 0.2% sodium dodecyl sulfate (SDS), 1× Denhart’s solution, 100 μg/mL denatured salmon sperm DNA, and 50% formamide, at 42°C for 18 hours. The filters, washed twice under stringent conditions (0.1% SSC, 0.1% SDS) at 54°C, were exposed to Kodak X-OMAT films (Eastman Kodak, Rochester, NY) for 1 to 14 days at −80°C.
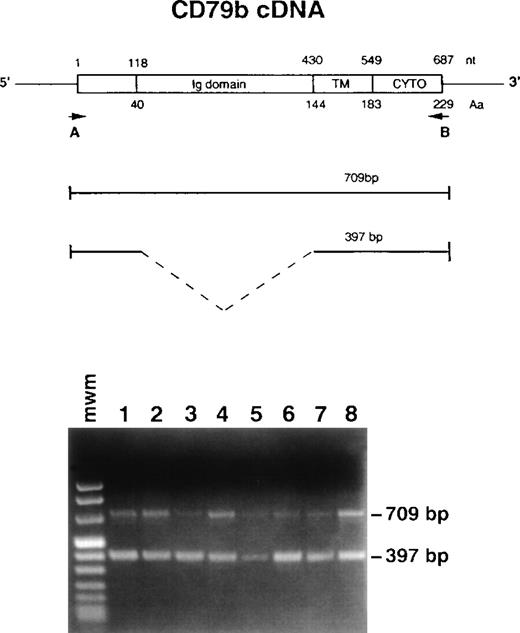
Strategy to amplify CD79b cDNA by RT-PCR and results. (Top) Numbers above the cDNA scheme represent nucleotide (nt) position (with 1 corresponding to the initiation ATG codon). Numbers below the scheme indicate amino acid (Aa) position. Ig domain indicates the extracellular domain, TM the transmembrane, and CYTO the cytoplasmic segment. Positions of the primers are shown by the arrows. (Bottom) RT-PCR analysis showing the two detected fragments: 709 bp and 397 bp. Lanes 1 through 3 and 5 through 8, different CLL cases; lane 4, normal PBL.
Strategy to amplify CD79b cDNA by RT-PCR and results. (Top) Numbers above the cDNA scheme represent nucleotide (nt) position (with 1 corresponding to the initiation ATG codon). Numbers below the scheme indicate amino acid (Aa) position. Ig domain indicates the extracellular domain, TM the transmembrane, and CYTO the cytoplasmic segment. Positions of the primers are shown by the arrows. (Bottom) RT-PCR analysis showing the two detected fragments: 709 bp and 397 bp. Lanes 1 through 3 and 5 through 8, different CLL cases; lane 4, normal PBL.
Reverse transcription-(RT-PCR) and cDNA analysis.
Two micrograms of total cellular RNA was reverse-transcribed using 12.5 U of AMV-RT (Promega, Madison, WI) and 0.5 μg of (oligo)dT as primer for 45 minutes at 42°C.
PCR was performed in a 50-μL reaction mixture containing 5 μL of the obtained cDNA fragments, 25 pmol of primers, 1 U of Thermus Aquaticus (Taq) polymerase (AmpliTaq; Perkin-Elmer, Branchburg, NJ), 200 mmol/L of dNTPs (Pharmacia Biotech, Uppsala, Sweden), and GENE Amp 10× PCR Buffer II (Perkin-Elmer). The conditions of PCR were: 1 cycle at 95°C for 3 minutes, followed by 25 cycles at 94°C for 1 minute-65°C for 30 seconds-72°C for 1 minute and a final extension cycle at 72°C for 7 minutes. Amplified products were analyzed by 1.5% agarose electrophoresis gel and visualized under UV rays after ethidium bromide staining.
Primers used to amplify CD79b cDNA were designed to encompass all the coding region as shown in Fig 1. Their sequence was: A = 5′ GTGACCATGGCCAGGCTGGCGTTGT 3′ and B = 5′ GGCGACCTGGCTCTCACTCCTGGC 3′ corresponding, respectively, to nucleotides 11-35 and nucleotides 696-719 in the cDNA sequence29 (accession no. M80461). To amplify the 3′ UTR region the primers used were: C = 5′ GGAAGAGTCCCAGAACGAAT 3′ at nucleotide position 304-323 and D = 5′ GCCTCCCTGGGGGTGGGAGTGGTT 3′ corresponding to nucleotides 1117-1140.
In a set of experiments primer A was end-labeled with γ33PATP (Amersham, Little Chalfont, UK) for 1 hour at 37°C using T4 polynucleotide kinase (USB, Cleveland, OH). Reaction conditions for radioactive PCR with primers A/B were identical to those described above. PCR products were electrophoresed on 5% polyacrylamide gels. After electrophoresis, gels were dried at 80°C, and analyzed by Instant Imager (Camberra-Packard, Grove Hills, IL) to quantify the CD79b/CD79bΔ ratio.
Analysis of CD79b genomic DNA.
The genomic sequences corresponding to CD79b exon 3 were analyzed by single-strand conformation polymorphism (SSCP) and direct sequencing. The primers used were designed on genomic sequences.30 Exon 3 was amplified into two overlapping fragments using the following primers: Ex3I F: 5′ GAATGCTGAGCCTGACCTTG 3′ (position 2176-2195); ex3I R: 5′ TTGTCCTCAAACCGGATGCC 3′ (2418-2437) (ref 30); Ex3II F: 5′ GGAAGAGTCCCAGAACGAAT 3′ (2375-2394); ex3II R: 5′ GCTCTCCTGAGTGCTCTAG 3′ (2601-2619) (ref 30).
SSCP of exon 3 was performed using one fifth of the PCR product adding 1 μL of 35S-dATP (Amersham) and 0.2 U of Taq polimerase for 10 more cycles.31 After denaturation at 95°C for 5 minutes, 1 μL of the mixture was loaded on a 6% polyacrylamide gel in the absence of glycerol. Gels were dried and exposed for 3 days at −80°C.
Direct sequencing.
Five microliters of PCR fragments was incubated with 10 U of exonuclease I and 2 U of shrimp alkaline phosphatase (SAP) for 15 minutes at 37°C, followed by heating at 80°C for 15 minutes to inactivate the enzymes. One microliter of the mixture was directly sequenced using a ThermoSequenase sequencing kit (Amersham Life Science, Cleveland, OH).
Restriction enzyme digestion.
PCR products were digested with the restriction enzyme CAC8 I (New England Biolabs, Beverly, MA), according to the manufacturer’s recommendations.
Statistical analysis.
The CD79b/ΔCD79b ratio in CLL patients versus healthy donors was compared by both the Mann-Whitney-Wilcoxon and the Kruskal-Wallis statistical tests.
RESULTS
CD79b protein expression.
We used SN8 MoAb in double staining with CD19 to evaluate the expression of the extracellular domain of CD79b on the cell surface of MEC1 and MEC2 cell lines, and 40 fresh CLL samples (Table1). All cases coexpressed CD5, CD19, and CD23 on the surface of most cells. Thirty-one samples had faint sIgM; 12 of them also expressed very low levels of sIgD. In 4 cases the sIg amount was too low to be discernible. The remaining 5 cases expressed μ (3 cases) or μ,δ (2 cases) heavy chains with strong intensity. The two cell lines MEC1 and MEC2, obtained from the PBL of one of these patients (patient 34, Table 1), retained the same intensity of sIg expression as fresh PBL.
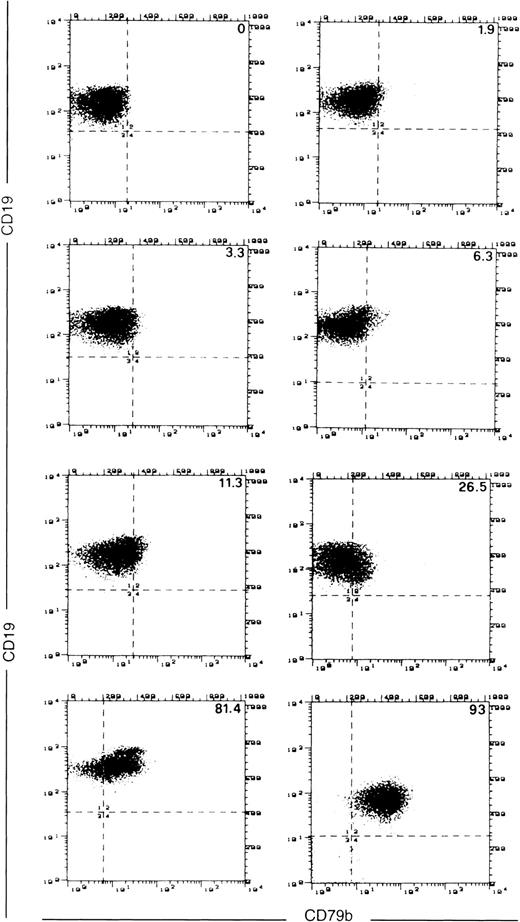
MEC1 and MEC2 were SN8− (<1% positive cells); the proportion of SN8+ elements among parental cells was 3.6%. Consistent with the observation of a larger study,20 30 of 40 CLL cases (75%) were SN8− (Table 1; Fig2). However, in a number of cases (10 of 40: 25%) more than 30% of the cells stained (albeit weakly) with SN8, thereby indicating a variability more pronounced than expected (Table1). Such a variability may merely reflect the smaller number of cases analyzed compared with literature data.20 SN8 positivity was unrelated to disease stage as well as to sIg isotype and intensity. In 10 cases CD19+ malignant cells were tested in double staining with both SN8 and CB3.1 MoAbs. Only minor differences in the percentage of positive cells were detected. In 12 SN8−cases, the cells were also permeabilized and stained to explore the possibility that the SN8-reactive epitope might be located in the cytoplasm. No cytoplasmic reactivity was detected (Fig3).
Cytofluorimetric analysis of CD79b expression by SN8 MoAb in a number of different representative CLL cases. The numbers in the upper right corner of each panel are the percentage of CD19+, SN8+ cells.
Cytofluorimetric analysis of CD79b expression by SN8 MoAb in a number of different representative CLL cases. The numbers in the upper right corner of each panel are the percentage of CD19+, SN8+ cells.
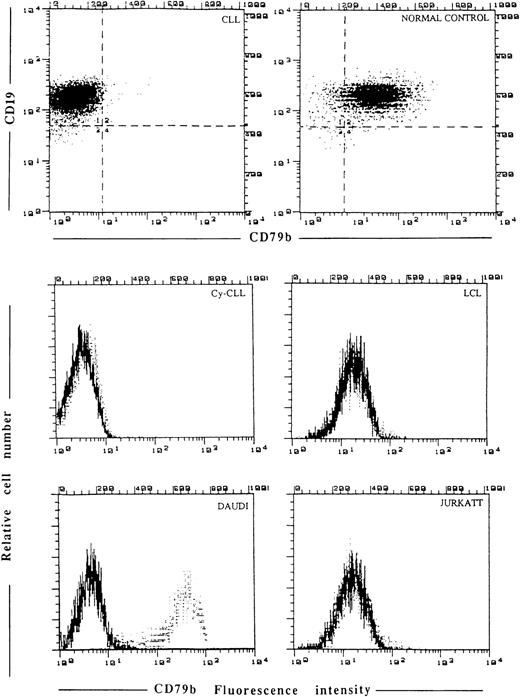
Cytofluorimetric analysis of CD79b expression by SN8 MoAb. Membrane staining in CLL fresh cells, normal control, LCL, Daudi, and Jurkatt cell lines. In the same CLL cells the possibility that the epitope detected by SN8 might be present in the cytoplasm (Cy-CLL) was ruled out.
Cytofluorimetric analysis of CD79b expression by SN8 MoAb. Membrane staining in CLL fresh cells, normal control, LCL, Daudi, and Jurkatt cell lines. In the same CLL cells the possibility that the epitope detected by SN8 might be present in the cytoplasm (Cy-CLL) was ruled out.
Unstimulated CD19+ cells from 20 normal donors were greater than 80% SN8+ (and CB3.1, data not shown) (Table 1 and Fig3); the expression of SN8 was equally distributed in CD5+and CD5− tonsil B cells (data not shown). In three normal CD19+ B-cell samples stimulated by the CD40L/CD32-L cell system the percentage of CD19+, SN8+ cells was as low as 19%, 21%, and 18%, respectively. The proportion of SN8+ cells of the four LCL cell lines from normal individuals was less than 5% (Fig 3). Daudi (Fig 3) and Raji cells reacted very strongly with SN8, while Jurkatt cells were completely negative (Fig 3).
CD79b transcript analysis.
The absence or reduced expression of CD79b membrane protein led us to examine the CD79b transcript. In agreement with literature data,32 Northern blot analysis of MEC1 and MEC2 cell lines and five fresh B-CLL samples showed significant amounts of CD79b mRNA (data not shown).
To investigate the type of CD79b mRNA present in B-CLL, total RNA from 50 fresh samples (including the 40 cases analyzed by flow cytometry) was retrotranscribed to cDNA and amplified by PCR using primers able to detect the two alternatively spliced transcripts (Fig 1). Two fragments of different size (709 bp and 397 bp) were found (Fig 1). The 709-bp band corresponds to the expected CD79b entire transcript and the 397-bp band corresponds to the alternatively spliced form lacking exon 3, which codes for amino acids (aa) 40-143 of the extracellular domain (Fig 1).23 In all 50 samples examined, as well as in MEC1 and MEC2 cell lines, the 397-bp band was always detectable. The 397-bp fragment was virtually undetectable in the Raji cell line that shows very high levels of SN8 reactivity. As for normal PBL, both fragments were visible. Although PCR-based techniques are not quantitative, a coamplification of two fragments using the same primers offers a rough estimate of the amount of the corresponding template. A comparison of normal and B-CLL samples show that the 397-bp fragment was generally more evident in B-CLL. In normal B cells stimulated in vitro by the CD40L/CD32-L cell system as well as in the four LCL examined, the expression of the 397-bp fragment was increased (not shown).
Estimation of the CD79b/ΔCD79b mRNA ratio.
To obtain a more accurate estimate of the relative proportions of the two spliced forms, a radioactive PCR was performed and the results were analyzed using a digital imaging system in 13 normal and 22 B-CLL samples. As shown in Table 2, the mean value of the CD79b/ΔCD79b ratio was 0.64 ± 0.20 SD in normal donors and 0.44 ± 0.27 SD in B-CLL. The difference was statistically significant (P = .01). However, no statistically significant correlation was observed between SN8 expression and CD79b/ΔCD79b ratio.
CD79b and ΔCD79b mRNA in normal CD5+B cells.
Considering that most CLL are believed to originate from the CD5+ B-cell subset,3 we investigated whether ΔCD79b RNA expression could be a feature of this population. To this end, we analyzed the transcript expression on tonsil CD5+and CD5− purified B-cell populations from three different healthy subjects. As shown in Table 3, the results of radioactive PCR analyzed by a digital system revealed that both fragments were present in both B-cell subsets. Thus, the presence on the membrane of CD5 does not discriminate B cells prevalently expressing one of the two fragments. The relative prevalence of one of the two forms is variable on an individual basis.
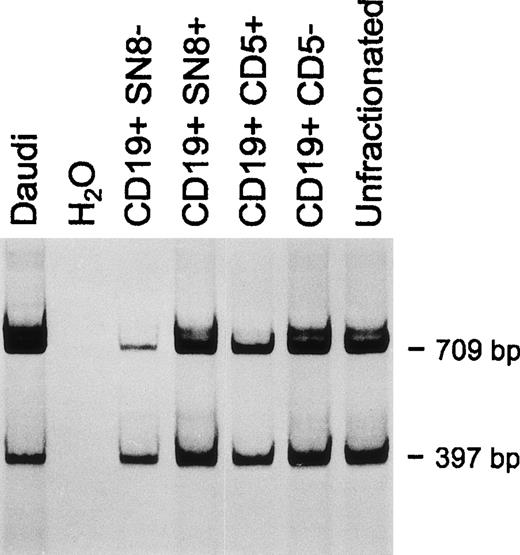
In one sample, the relative proportions of the two fragments were also estimated on purified CD19+, SN8+ and CD19+, SN8− cells. As shown in Fig4, CD19+, SN8+cells had an increased prevalence of the 709-bp band (ratio 1.51), while CD19+, SN8− cells had an increased prevalence of the 397-bp fragment (ratio 0.52).
CD79b and ▵CD79b transcript expression in normal CD5+ compared with CD5− B cells and CD19+, SN8+ compared with CD19+, SN8− normal B cells. One representative experiment of three consecutive samples is shown.
CD79b and ▵CD79b transcript expression in normal CD5+ compared with CD5− B cells and CD19+, SN8+ compared with CD19+, SN8− normal B cells. One representative experiment of three consecutive samples is shown.
CD79b cDNA and DNA sequencing.
mRNA from MEC1 and MEC2, their parental cells (Table 1: patient 34), five additional fresh B-CLL samples (Table 1: patients 1, 17, 18, 30, 33), and normal donor PBL amplified in RT-PCR reactions were directly sequenced. Because of technical difficulties in obtaining material from the 709-bp fragment sufficient for direct sequencing, the strategy devised was to sequence the 397-bp band followed by sequencing of genomic DNA corresponding to exon 3 to cover the coding region not represented in the 397-bp fragment. In cell lines the 3′ UTR region was also investigated up to position 1110.
Sequence analysis of the 379-bp fragment exactly matched the reported CD79b sequence29 and, as expected, lacked nucleotides 118-429 corresponding to exon 3. As for the genomic exon 3 analysis, at variance with the reported sequence (30), a TGT → TGC change was observed in the third exon at position corresponding to aa 122 in all but one of the samples examined. This silent substitution does not change the codified cysteine and was already reported as a normal sequence.21 29 The 3′ UTR region investigated by sequencing the two B-CLL cell lines was normal.
To confirm the absence of mutations, SSCP analysis of exon 3 was performed in 18 further B-CLL cases. An abnormal shift was commonly observed and shown to correspond to the TGT → TGC change at aa 122. Restriction enzyme analysis with CAC8 II endonuclease, whose cleavage site is affected by the nucleotide change, was then performed in 18 B-CLL and 6 normal samples. This analysis confirmed that that the T → C substitution is a common polymorphism.
DISCUSSION
CD79b is a crucial component of the BCR.9-17 The largest series of B-CLL so far studied provides evidence that the membrane expression of CD79b is greatly diminished or even absent in most typical B-CLL.20 Although a certain degree of variability exists among different series21,33 (and Table 1), conceivably because of different MoAbs, number of patients, and laboratory techniques, a general consensus exists on the fact that the majority of CLL cells express low to undetectable amounts of the extracellular epitope of CD79b. Because CD79b is essential for the transport of Ig to the membrane,9-11 signal transduction,13-15 and the process of apoptosis,16 it is reasonable to predict that its absence has a central role in the pathophysiology of the disease. In this work we have investigated the mechanisms that may cause the lack of CD79b expression and show that CLL cells consistently have two mRNA forms: one that corresponds to the full coding region and a form that lacks the extracellular Ig-like domain (ΔCD79b). The ΔCD79b RNA, which is the product of alternative splicing, has been shown in a variety of human B cells and B-cell lines using an RNAse protection assay.22,23 Experiments of BCR reconstitution have shown that murine fibroblast L cells transfected with ΔCD79b cDNA fail to express CD79b on the membrane, whereas L cells transfected with the entire CD79b cDNA do express membrane CD79b.23 These findings establish an unequivocal relationship between the presence of internally deleted mRNA and the absence of detectable CD79b on the membrane. On this basis it is tempting to speculate that the formation of CD79b mRNA lacking the extracellular domain plays a role in the BCR phenotypic abnormalities in B-CLL.
A large array of mutations preventing the formation of functional CD79b in mRNA clones or undetectable CD79b mRNA levels has been recently proposed as an explanation of the reduced or absent expression of CD79b in B-CLL.21 However, in the RT-PCR systems used,21 the internally deleted variant escaped detection as forward primers partially encompassed the deleted Ig-like domain. It is also intriguing that no attempt was made in this study to confirm the mutations detected on mRNA clones in the patient’s genomic DNA.21 In our experiments, direct sequencing of CD79b coding region in six B-CLL and two B-CLL cell lines did not show causal mutations. Direct sequencing of PCR products prevents the possibility of identifying artefactual nucleotide changes that may occur when sequencing clones of PCR products. In addition, mutation screening by SSCP, focused on the region encompassing exon 3, showed in our series a single abnormal shift corresponding to a polymorphic change. Finally, in agreement with previous findings using Northern blot,32we have observed normal levels of CD79b mRNA in both B-CLL cell lines and fresh samples. Taken together, our findings exclude, at least in our ethnic group, the presence of causal mutations.
The possibility that alternative splicing may have a role in the mechanisms that lead to the lack of CD79b expression in B-CLL is supported by the observations on normal B lymphocytes. Resting normal B cells, which express the CD79b extracellular epitope in more than 80% of the elements, have been shown to have low amounts of ΔCD79b.23 Also, both SN8 and ΔCD79b (Table 3) are equally distributed in normal CD5+ and CD5− B cells, indicating that their expression is not restricted to a phenotypically distinct B-cell subset. SN8+ normal B cells have a prevalence of the mRNA that corresponds to the full coding region, whereas ΔCD79b prevails in SN8− normal B cells (Fig 4). Our semiquantitative experiments (Table 2) document that lower amounts of ΔCD79b are expressed by normal unactivated B cells compared with B-CLL cells. Activated B cells have higher levels of internally deleted mRNA23: in these cells we have observed that the proportion of elements that fail to express the extracellular epitope of CD79b is also increased. High levels of ΔCD79b are observed in activated B cells irrespective of the activation stimuli delivered, as they are found with the CD40L/CD32-L cell stimulation system, with EBV and with lipopolysaccharide (LPS),23suggesting that the process of B-cell activation per se favors the posttranscriptional negative regulation of CD79b. In keeping with this possibility, LCL obtained from patients with acquired immunodeficiency syndrome (AIDS) have a decreased expression of BCR.34,35 Even more important, lymphoid tissue sections stained with CD79b MoAb reveal a strong staining of mantle zone cells and a barely detectable staining of germinal centers (GC),36 indicating that in vivo–activated GC cells are essentially CD79b−. All of these observations concur to suggest that the mechanism of alternative splicing which produces a CD79b form lacking the Ig-like extracellular domain is a physiological mechanism during B-cell activation. Posttranscriptional regulation through alternative splicing is a general mechanism that operates in several gene systems, including the T-cell receptor37 and several cytokine receptors,38-40 to modulate gene expression according to the cell requirement.41 Thus, it is conceivable that B cells physiologically use alternative splicing to downregulate BCR expression upon activation. This would allow proliferation and differentiation to proceed undisturbed after the encounter with an individual triggering Ag. On these bases, it becomes consequent to conclude that the lack of CD79b in B-CLL is not causal to the disease, but likely reflects the state of activation of the B cell where the malignant transformation has occurred.
The final point that needs to be discussed is the abnormality of BCR formation that appears to characterize CLL cells. It remains to be explained why CD79b extracellular epitope is not expressed (or expressed at very low levels) despite the presence of some amounts of the normal mRNA species and, conversely, why in some cases the cells express normal levels of membrane CD79b despite the presence of the short-sized mRNA fragment. In terms of rank distribution, the correlation between SN8 positivity and the CD79b/ΔCD79b ratio is not as strict as one would expect. However, the biochemical properties of the protein encoded by the ΔCD79b transcript are unknown and the half life of both CD79b protein and mRNA are undefined. Likewise, the dynamics of the assembly of the BCR components has been explored in detail in Ramos cell line,34 where it has been shown that the association of Ig heavy and light chains together with CD79a and CD79b is required and sufficient to permit the exit of BCR complex out of the endoplasmic reticulum.34 The dynamics and features of BCR assembly in human fresh normal and malignant B cells are unknown. It is possible that a threshold level of ΔCD79b has to be reached to disturb the proper assembly of the BCR components, a process that appears to be profoundly disturbed in CLL cells. Irrespective of the mechanisms operating, the emerging abnormalities of BCR organization appear to be central to the pathophysiology of B-CLL cells by influencing the behavior of the malignant cell population.
ACKNOWLEDGMENT
The secretarial assistance of G. Tessa, Fondazione R. Favretto, is gratefully acknowledged.
The first two authors contributed equally to this work.
Supported by A.I.R.C., Milano; PF Biotechnologie, CNR; MURST 40%; MURST 60%; Grant EC-Biotec BI04CT950100.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to A. Amadori, MD, Dipartimento di Scienze Oncologiche e Chirurgiche, Sezione di Oncologia, Via Gattamelata 64, 35128 Padova, Italy; e-mail: albido@uxl.unipd.it.