To the Editor:
A recent Rapid Communication described an adverse association between extended pretransplant interferon-α (IFN-α) therapy and outcome after unrelated donor marrow transplantation for chronic myelogenous leukemia (CML) in chronic phase.1 Although the biologic basis of the adverse effect of pretransplant IFN-α in this transplant setting could not be further elucidated, it appeared to be connected to a significantly higher risk of grades III-IV acute graft-versus-host disease (GVHD) in patients whose pretransplant IFN-α treatment duration exceeded 5 months. This communication prompted us to update and reevaluate our previously reported experience with the influence of pretransplant IFN-α treatment duration on transplant outcome in chronic phase CML patients, because we had described a strong adverse influence in patients whose pretransplant IFN-α exposure exceeded 12 months.2 To reduce inhomogeneities of the transplant procedure over time, the present analysis was restricted to 153 consecutive marrow transplant recipients who were treated with a uniform short-course methotrexate and cyclosporine acute GVHD prophylaxis as well as an identical myeloablative regimen consisting of 4 × 2.5 Gy fractionated total body irradiation and 120 mg per kilogram of recipient body weight cyclophosphamide in the time period between April 1986 and July 1996.
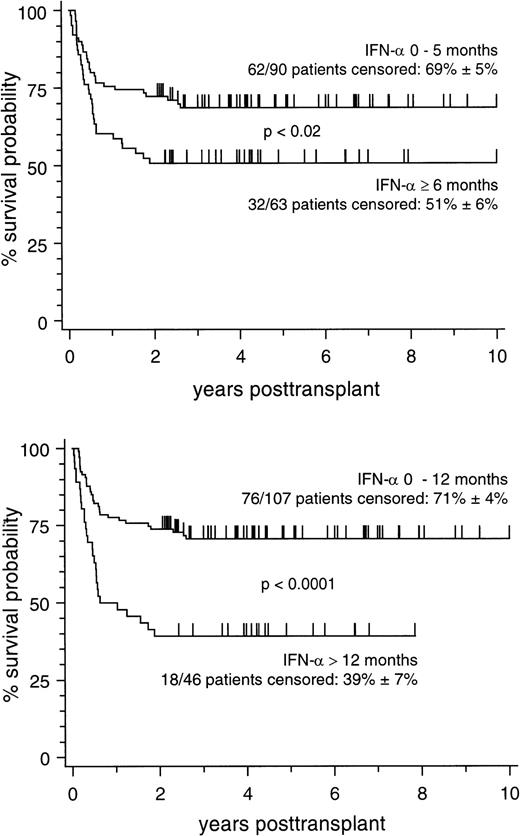
Marrow donors were genotypically HLA-identical siblings in 83 patients, partially HLA-matched extended family donors in 27 patients, and matched unrelated donors (MUD) in 43 patients, respectively. Corresponding 5-year survival estimates (±SE) of patients with different types of marrow donors were 64% ± 5%, 63% ± 9%, and 56% ± 8%, respectively (not significant), thus allowing a joint analysis on all 153 patients. Probabilities of 10-year survival according to different cut-off points of pretransplant IFN-α treatment duration are depicted in Fig 1. As illustrated by the figure, the cut-off point below 6 months of pretransplant IFN-α exposure showed a significant difference between patient subsets, but this difference was much more pronounced if the cut-off point at 12 months of pretransplant IFN-α exposure was chosen. This indicates that the adverse influence of pretransplant IFN-α treatment duration may be underestimated if patients with a treatment duration between 6 and 12 months were analyzed together with patients treated for longer time intervals.
Cumulative estimates of survival in 153 patients stratified according to the duration of pretransplant IFN- treatment. (Top) Stratification between treatment duration of 0 to 5 months and of ≥6 months. (Bottom) Stratification between treatment duration of 0 to 12 months and of >12 months.
Cumulative estimates of survival in 153 patients stratified according to the duration of pretransplant IFN- treatment. (Top) Stratification between treatment duration of 0 to 5 months and of ≥6 months. (Bottom) Stratification between treatment duration of 0 to 12 months and of >12 months.
An analysis on the estimates (±SE) of transplant-related mortality (TRM) revealed an increase from 25% ± 7% (9 of 38 deceased patients) after pretransplant IFN-α exposure of up to 12 months to 62% ± 7% (28 of 46 deceased patients), if IFN-α treatment duration exceeded 12 months (P < .0009). In contrast, for patients previously not exposed to IFN-α, the categorized duration of pretransplant treatment using hydoxyurea and/or busulfan had no adverse effect on this endpoint. In this patient subset, the estimates of TRM were 29% ± 9% (7 of 24 deceased patients) after a pretransplant treatment duration of up to 12 months and 26% ± 7% (11 of 45 deceased patients) if pretransplant hydroxyurea and/or busulfan exposure exceeded 12 months. These figures clearly support that pretransplant IFN-α treatment duration and not pretransplant treatment duration per se adversely affected TRM in our patient population.
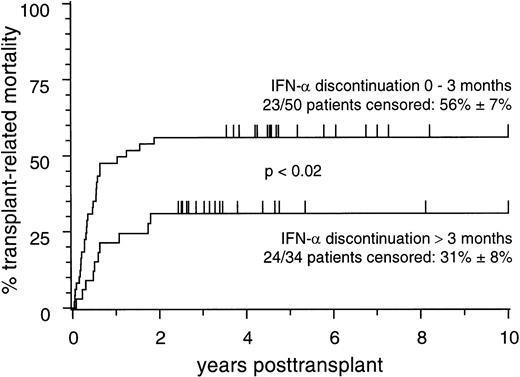
Notably, and in contrast to the results of Morton et al, the adverse influence of pretransplant IFN-α treatment on TRM was less pronounced in patients in whom IFN-α treatment had been discontinued more than 3 months before transplant compared with those in whom the time interval between IFN-α discontinuation and transplant was shorter (Fig2).
Cumulative estimates of transplant-related mortality in 84 patients who received pretransplant IFN- treatment and who were stratified according to discontinuation of IFN- treatment within 0 to 3 months or >3 months before transplant.
Cumulative estimates of transplant-related mortality in 84 patients who received pretransplant IFN- treatment and who were stratified according to discontinuation of IFN- treatment within 0 to 3 months or >3 months before transplant.
To account for potential interactions between pretransplant disease characteristics (disease duration, Sokal’s score at diagnosis, spleen size, and degree of marrow fibrosis at transplant), the type of marrow donors, the individual duration of exposure to IFN-α, hydroxyurea, or busulfan pretransplant, as well as the time intervals between discontinuation of therapy and transplant, we further applied proportional hazard general linear model (PHGLM) analysis on TRM as the analytical endpoint. Acute (categorized as grades 0-II v grades III-IV) and chronic GVHD as major posttransplant predictors of TRM were analyzed as time-dependent covariates in this analysis, which corroborated only an independent adverse influence of more than 12 months pretransplant IFN-α exposure (relative risk, 3.0 [95% confidence limits, 1.8 to 5.1], P < .0001) and of grades III-IV acute GVHD (relative risk, 4.7 [95% confidence limits, 2.5 to 8.6], P < .0001) on TRM. Multivariate analysis on grades III-IV as an endpoint showed no influence of the duration of pretransplant IFN-α exposure on the development of grades III-IV acute GVHD.
Despite these differences between both analyses, which may be partially explained by the restriction to patients with matched unrelated donors in the analysis of Morton et al, it is of note that in both patient populations, extended pretransplant IFN-α treatment significantly increased TRM, and this occurred preferably during the first posttransplant year. In considering the pleiotropic biologic effects of IFN-α, several mechanisms may be responsible for the observed adverse influence of pretransplant IFN-α on transplant outcome. The present analysis suggests that these effects become more evident with increasing IFN-α treatment duration, and this may be best explained by chronic cumulative IFN-α toxicity, which manifests especially in marrow transplant patients.