Abstract
The immunomodulatory effect of allogeneic blood transfusions (ABT) has been known for many years. However, a complete understanding of the effects of ABT on the recipient’s immune system has remained elusive. Soluble HLA class I (sHLA-I), HLA class II (sHLA-II), and Fas ligand (sFasL) molecules may play immunoregulatory roles. We determined by double-determinant immunoenzymatic assay (DDIA) sHLA-I, sHLA-II, and sFasL concentrations in different blood components. sHLA-I and sFasL levels in red blood cells (RBCs) stored for up to 30 days and in random-donor platelets are significantly (P < .001) higher than in other blood components and their amount is proportionate to the number of residual donor leukocytes and to the length of storage. Blood components with high sHLA-I and sFasL levels play immunoregulatory roles in vitro as in allogeneic mixed lymphocyte responses (MLR) and antigen-specific cytotoxic T-cell (CTL) activity, and induce apoptosis in Fas-positive cells. These data suggest that soluble molecules in blood components are functional. If these results are paralleled in vivo, they should be taken into account in transfusion practice. Blood components that can cause immunosuppression should be chosen to induce transplantation tolerance, whereas blood components that lack immunosuppressive effects should be preferred to reduce the risk of postoperative complications and cancer recurrence.
THE POSSIBILITY THAT blood transfusion (BT) might mediate donor-specific immunologic tolerance in transplantation was first suggested by Billingham et al in 1953 on the basis of some experimental evidences in the mouse system.1Thereafter, in the 1970s, several lines of evidence indicated that donor-specific transfusion (DST), as well as allogeneic blood transfusion (ABT), performed before renal allograft or during surgery improved graft survival.2 Leukocytes in the donor blood have been found to be essential for the beneficial effect.3,4 Further studies evaluated the prerequisites to obtain a tolerance-inducing effect of ABT. These studies indicate that donor and recipient must share at least one HLA-DR antigen or a haplotype and must be mismatched for the other one and that CD4+ regulatory T cells are involved in the transfusion modulatory effect.5,6 These observations have been confirmed in experiments performed in animal models providing evidence that BT may have immunomodulatory effects, like induction of transplantation tolerance, acceleration of tumor growth, and increasing susceptibility to bacterial infection.7 Whether these experimental data have any clinical counterpart is controversial.8-10 Indeed, the preliminary finding that ABT may adversely affect the clinical course of neoplastic diseases has not been confirmed by randomized studies.10-12 Moreover, although the risk of postoperative infections was increased in subjects who received a red blood cell (RBC) transfusion with respect to those who did not, no difference was observed in the prevalence of postoperative infection among subjects who received white blood cell (WBC)-reduced/buffy coat–depleted allogeneic RBCs, buffy coat–depleted allogeneic RBCs, or autologous whole blood.10-12 However, a recent study reports that transfusion of filtered leukocyte-depleted blood results in a significant reduction of postoperative bacterial infections and mortality in patients undergoing cardiac surgery.13
Nevertheless, although the fine processes by which BT modulates host-recipient interactions and induces tolerance or immune suppression are still undefined, it seems likely that such a capacity of BT is based on a combination of mechanisms. These potential mechanisms include induction of anergy, antiidiotypic antibody-mediated immunosuppression, imbalance of cytokine and/or cytokine receptor expression, T-cell clone deletion, and/or regulatory-cell activation and soluble factors.7
HLA antigens are heterodimeric and highly polymorphic glycoproteins. HLA class I antigens are expressed on the membrane of most nucleated cells,14 whereas HLA class II antigen expression is limited to the antigen-presenting cells and to activated T lymphocytes.15 The presence of soluble HLA class I and HLA class II antigens (sHLA-I and sHLA-II, respectively) in serum of healthy humans was described approximately 20 years ago.16,17 In the following years, a large number of studies have been devoted to their immunochemical and functional characterization.18-22 The serum levels of sHLA-I and sHLA-II antigens significantly increase in patients affected by infections or autoimmune diseases,23,24 as well as during acute rejection episodes following organ allograft25-30 and severe graft-versus-host disease (GVHD) following allogeneic bone marrow transplantation (BMT).31,32 Moreover, large amounts of sHLA-I and sHLA-II antigens have been detected in commercial albumin, immunoglobulin, and hemostatic preparations.33-36
In vitro studies indicate that sHLA antigens may modulate immune competent cell function in at least two ways: (1) sHLA-I and sHLA-II molecules may bind their physiologic ligands and inhibit T-cell function by receptor blockade and/or by apoptosis induction37-42; and (2) sHLA-I and sHLA-II antigens can be phagocytosed by antigen-presenting cells (APC), degraded to peptides, and presented to CD4+ T cells in the context of membrane HLA class II antigens. The latter process is known as indirect presentation43 and may lead to either immune tolerance or activation depending on the tolerogenic or stimulating capacity of the HLA-derived peptides presented by HLA class II antigens.
Fas ligand (FasL) is a type II membrane protein predominantly expressed in activated T cells44 and neutrophils,45 as well as in the stroma cells of the retina and in Sertoli cells in the testis. Fas (CD95) is a type I membrane protein expressed in various tissues.46 Binding of FasL to Fas induces apoptosis in Fas-bearing cells46 and Fas/FasL interactions are involved in the clonal deletion of T cells in the periphery and in the downregulation of cytotoxic T-lymphocyte (CTL) activity.47Recently, it has been reported that FasL is also detectable in a soluble form (sFasL) in human serum and that sFasL levels significantly increase in sera from patients with some hematologic malignancies.48
The aim of the present study was to determine the concentration sHLA-I, sHLA-II, and sFasL in different blood components and to evaluate their immunomodulatory capacity.
MATERIALS AND METHODS
Blood components.
Blood components were prepared according to the Council of Europe “Guide to the preparation, use and quality assurance of blood components” (Strasbourg, France, June 8, 1994). The following blood components were analyzed: (1) washed RBCs (W-RBC, no. 11); (2) RBCs stored for 5 days (RBC-5, no. 18); (3) RBCs stored for 30 days (RBC-30, no. 19); (4) prestorage leukodepleted RBCs stored for 30 days (LD-RBC, no. 5); (5) random-donor platelets (PLT, no. 21); and (6) fresh-frozen plasma (FFP, no. 24). Controls were blood donors’ sera (no. 16). The concentrations of sHLA-I, sHLA-II, and sFasL were determined in samples of blood components’ supernatant after centrifugation at 12,000g for 2 minutes. The supernatants of blood components to be used in functional assays were extensively dialyzed to remove the additive solutions and, in some experiments, were immunodepleted with anti-HLA class I monoclonal antibody (MoAb) W6/32 and/or with anti-FasL MoAb NOK-1 coupled to cyanogen bromide–activated Sepharose 4B (Pharmacia, Uppsala, Sweden).
Antibodies.
MoAb W6/3249 to a monomorphic determinant of HLA class I heavy chains was purchased from Serotec (Oxford, UK). MoAb TP25.9950 to a nonpolymorphic determinant expressed on HLA class I heavy chains, MoAb NAMB-1 to β2-microglobulin,51 MoAb Q5/13 to HLA class II α chain,52 and MoAb LGII-612.14 to HLA class II β chain53 were developed and characterized as described and were a kind gift of Dr S. Ferrone (New York Medical College, Valhalla, NY). MoAb NOK-1 and MoAb NOK-2 to different epitopes of sFasL molecule were purchased from PharMingen (San Diego, CA). MoAb CH11 and ZB4 to human Fas were purchased from Kamiya (Thousand Oaks, CA). MoAb NAMB-1, MoAb Q5/13, and MoAb NOK-1 were conjugated to biotin,54 and MoAb TP25.99 was labeled with 125I using the chloramine T method55 at the specific activity of 5 μCi/mg. Fluorescein isothiocyanate–conjugated goat antimouse immunoglobulin antibodies (GAM-FITC) were purchased from Coulter (Hialeah, FL). Annexin-V-biotin and streptavidin-R-phycoerythrin were purchased from Boehringer Mannheim (Monza, Italy).
Cells.
Human peripheral blood mononuclear cells (PBMCs) were obtained by centrifugation on a Ficoll-Hypaque gradient as described.56Human T-lymphoid Jurkat cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (GIBCO-BRL, Gaithersburg, MD) at 37°C in a 5% CO2 atmosphere.
Flow cytometric assays.
Indirect immunofluorescence was performed by incubating 5 × 105 cells sequentially with MoAb and with GAM-FITC. Each incubation was for 30 minutes at 4°C. Following three washings, cells were analyzed on an Epics Elite flow cytometer (Coulter).
Determination of sHLA-I, sHLA-II, and sFasL concentrations.
The concentrations of sHLA-I, sHLA-II and sFasL molecules were determined by double-determinant immunoassay (DDIA). DDIA to measure sHLA-I and sHLA-II molecules was performed as described57 58 with minor modifications. MoAb W6/32 and MoAb LGII-612.14 and biotinylated MoAb NAMB-1 and MoAb Q5/13 were used to capture and to detect sHLA-I and sHLA-II molecules, respectively. Results were expressed as micrograms per milliliter. To measure the concentration of sFasL, a DDIA was developed. Briefly, 96-well polyvinylchloride microtiter plates (Becton Dickinson, Oxnard, CA) were coated overnight at 4°C with a solution of MoAb NOK-2 (10 μg/mL) in NaHCO3 buffer. After three washings with 0.05% Tween 20/phosphate-buffered saline (PBS), the wells were blocked by 10% AB serum for 1 hour at 37°C, then 100 μL of blood components’ supernatant were added in duplicate to wells and incubated for 1 hour at 37°C. Following three washings, biotinylated MoAb NOK-1 (5 μg/mL) was added and incubated for 1 hour at 37°C. After additional washings, peroxidase-conjugated streptavidin (Pierce, Rockford, IL) was added at a concentration of 1 μg/mL for 1 hour at 37°C. Plates were developed with 100 μL of 1 mg/mL orthophenylenediamine in 50 mmol/L citrate-phosphate buffer (pH 5.0) containing 0.03% H2O2 and stopped with 100 μL of 2N H2SO4. Optical density was measured at 490 nm on an automated enzyme-linked immunosorbent assay (ELISA) reader. Serial dilutions (0.1 to 100 ng/mL) of human recombinant sFasL (Alexis Co, Läufelfingen, Switzerland) were used to construct the standard curve and results were expressed as nanograms per milliliter.
Immunochemical assays.
Immunoprecipitation of HLA class I molecules with MoAb W6/32 coupled to cyanogen bromide–activated Sepharose 4B followed by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described.59 Immunoblotting was performed according to Towbin et al60 using a 0.45-μm nitrocellulose membrane (Transblot Transfer Medium; Biorad, Milan, Italy). Following protein blotting, membranes were incubated with 125I-labeled MoAb TP25.99 (2 × 106 cpm/mL). Membranes were then autoradiographed using X-omat AR x-ray film (Eastman Kodak, Rochester, NY).
Mixed lymphocyte reactions.
Mixed lymphocyte reactions (MLR) were performed by incubating in 96-well U-bottom plates (Becton Dickinson, Oxnard, CA) irradiated stimulator PBMC (5,000 rad, 1 × 105 cells) with allogeneic responder PBMC (1 × 105 cells) in 100 μL of RPMI 1640 culture medium supplemented with 10% fetal bovine serum, 2% glutamine, and 1% penicillin-streptomycin for up to 10 days at 37°C in a 5% CO2 atmosphere. Samples (100 μL) of blood components’ supernatant and samples of the same supernatants immunodepleted of sHLA-I and/or sFasL molecules were added at the beginning of the culture period. All of the experiments were performed in triplicate and MLR response was evaluated every 24 hours. [3H]thymidine (0.5 μCi) was added to each well for the last 10 hours of incubation, cells were harvested with an automated cell harvester (Titertek; Flow Lab, Milan, Italy), and incorporated radioactivity was measured by a β-counter.
Cytotoxic assays.
CTL activity was evaluated by a standard 4-hour 51Cr release assay. Effector cells were Epstein-Barr virus (EBV)-specific CD8+ T lymphocytes, and target cells were autologous EBV-transformed B cells (gift of Dr F. Manca, Department of Immunology, University of Genoa, Italy). CD8+ effector T lymphocytes expressed Fas (CD95) antigen as determined by flow cytometry after anti-Fas MoAb ZB4 staining. Target cells were labeled with 100 μCi Na2CrO4 (ICN, Biomedicals Inc, Costa Mesa, CA) for 1 hour at 37°C, washed, resuspended in RPMI 1640 medium (5 × 104 cells/mL), and plated in 96-well U-bottom plates at 30:1, 10:1, and 3:1 effector:target cell ratios for 4 hours at 37°C. Samples (100 μL) of blood components’ supernatant and samples of the same supernatants immunodepleted of sHLA-I and/or sFasL molecules were added at the beginning of the assay. All assays were performed in triplicate. Plates were then centrifuged for 5 minutes at 1,800 rpm and 51Cr release was evaluated by counting 100 μL of supernatant in a γ-counter. Spontaneous and maximum 51Cr release were measured by incubating target cells in RPMI 1640 medium or in 0.1% Triton X-100 (Sigma Chemical Co, St Louis, MO). The percentage of lysis was calculated by the following formula: Lysis (%) = (sample51Cr release − minimum 51Cr release)/(maximum 51Cr release − minimum51Cr release).
Detection of apoptosis and cell viability.
Human T-lymphoid cells Jurkat that are susceptible to FasL-mediated apoptosis were suspended in RPMI 1640 medium (2 × 106cells/mL). Aliquots of Jurkat cells (50 μL) were incubated for 24 hours at 37°C with (1) blood components’ supernatant (50 μL); (2) anti-Fas apoptosis inducing MoAb CH11 (50 ng/mL); and (3) RPMI medium (50 μL). Apoptosis was detected by flow cytometry after permeabilization and propidium iodide (PI) (Sigma Chemical Co) staining, as described.61 Early apoptotic events were evaluated by annexin-V labeling method according to the manufacturer’s protocol, and viable apoptotic cells were differentiated from necrotic cells by flow cytometry after PI staining of nonpermeabilized cells.
Statistical analysis.
Results are reported as the mean ± SD. Statistical analysis was performed by one-way analysis of variance (ANOVA) and comparisons among groups were performed by Bonferroni’s multiple-comparisont-test. A two-tailed P value less than .05 was considered significant.
RESULTS
Determination of sHLA-I, sHLA-II, and sFasL molecules in blood components.
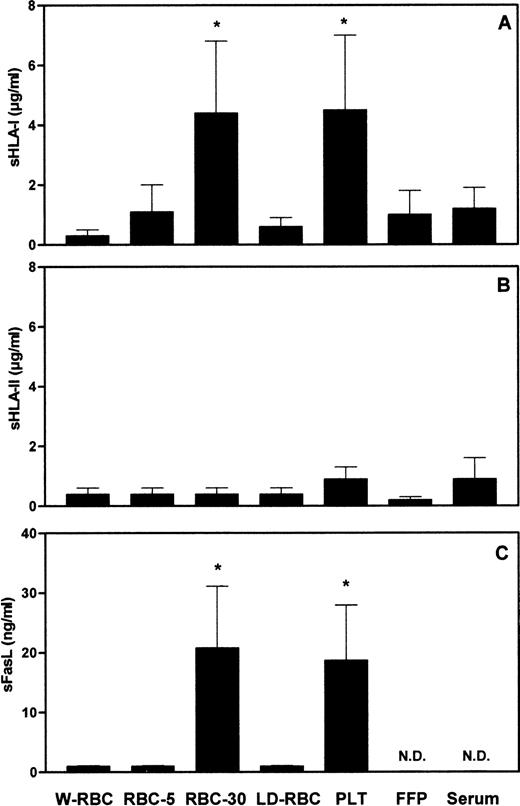
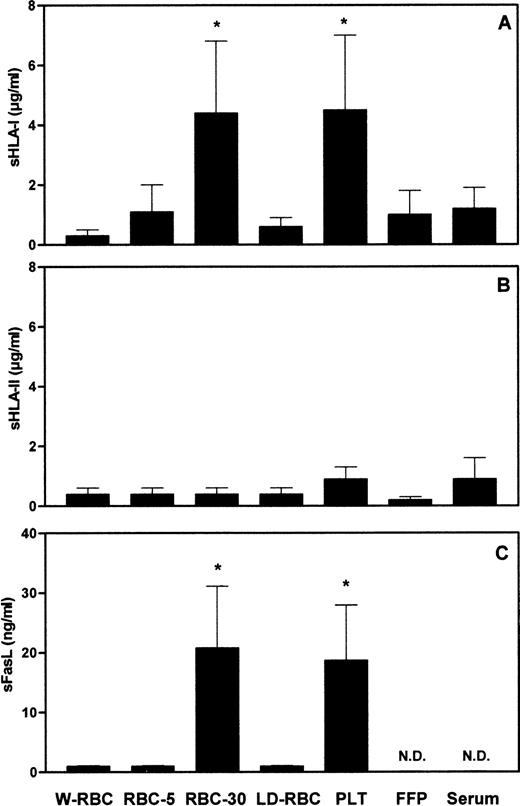
To assess whether the length of storage and the residual leukocyte number might affect the amount of soluble molecules in blood components, we measured the concentration of sHLA-I, sHLA-II, and sFasL molecules in different blood components. The concentration of sHLA-I molecules in RBC-30 and in PLT was significantly (P < .001) higher than that in W-RBC, RBC-5, LD-RBC, FFP, and control sera (Fig1A). The concentrations of sHLA-II molecules were comparable among the blood components tested and control sera (Fig 1B). The concentration of sFasL in RBC-30 and in PLT was significantly (P < .001) higher than that in W-RBC, RBC-5, and LD-RBC. sFasL was undetectable in FFP and in control sera (Fig 1C). The highest concentrations of sHLA-I and sFasL molecules were found in blood components that contain elevated amounts of leukocytes (1 to 3 × 109 cells/U), like RBC and PLT, and that have been stored for a long period of time (up to 30 days). Therefore, it can be hypothesized these molecules might derive from residual leukocytes that undergo membrane changes during storage. The release of sHLA-I antigens adsorbed on PLT and RBC membrane62-68 might also contribute to their detection in blood components. The low concentration of sHLA-II molecules in blood components might be attributable to their defective expression on polymorphonuclear leukocytes and on resting T lymphocytes that represent the major components of residual leukocytes.
Concentrations of sHLA-I (A), sHLA-II (B), and sFasL (C) in blood components. *Statistical significance of one-way ANOVA (P < .001). N.D., not detectable.
Concentrations of sHLA-I (A), sHLA-II (B), and sFasL (C) in blood components. *Statistical significance of one-way ANOVA (P < .001). N.D., not detectable.
Immunochemical characterization of sHLA-I molecules in blood components.
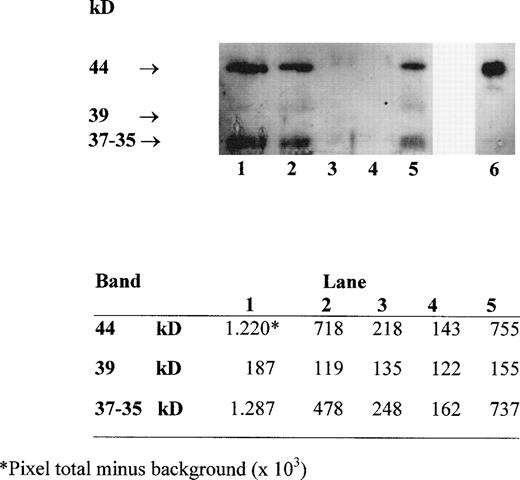
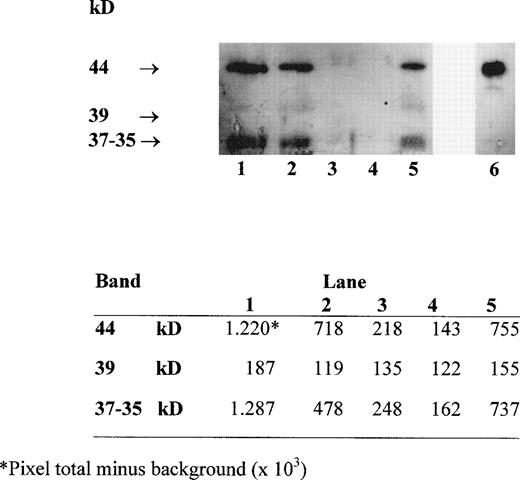
Several forms of sHLA-I heavy chains circulate in serum. These include intact chains of 44 kD, which are shed from the cell membrane, chains of 39 kD, which lack their transmembrane portion and are generated by an alternative RNA splicing pathway, and chains of 37 to 35 kD, which represent proteolytic breakdown products of cellular and/or serum 44-kD class I molecules.19 Western blot analysis with MoAb TP25.99 of HLA class I heavy chains immunoprecipitated from the supernatant of blood components with MoAb W6/32 identified the three forms of sHLA-I heavy chains (Fig 2). The densitometric analysis of the bands, performed using the UN-SCAN-IT software (Silk Sci. Corp, Orem, UT), indicated that their intensity was higher in RBC-30, RBC-5 and PLT, which contain a high number of residual leukocytes (1 to 3 × 109 cells/U) (Fig 2, lanes 1, 2, and 5), than in W-RBC and in LD-RBC, which contain less residual leukocytes (<1 × 105 cells/unit) (Fig2, lanes 3 and 4). Moreover, the intensity of the 44-kD and 37- to 35-kD bands was higher in RBC-30 than in RBC-5 (Fig 2, lanes 1 and 2). These results confirm that the major source of sHLA-I molecules might be the residual leukocytes that undergo membrane damage during storage.
Western blot analysis with MoAb TP25.99 of HLA class I heavy chains immunoprecipitated with MoAb W6/32 from RBC-30, RBC-5, W-RBC, LD-RBC, and PLT (lanes 1, 2, 3, 4, 5, respectively) and from PBMC lysate as control (lane 6). Densitometric analysis of the bands is shown in the lower panel.
Western blot analysis with MoAb TP25.99 of HLA class I heavy chains immunoprecipitated with MoAb W6/32 from RBC-30, RBC-5, W-RBC, LD-RBC, and PLT (lanes 1, 2, 3, 4, 5, respectively) and from PBMC lysate as control (lane 6). Densitometric analysis of the bands is shown in the lower panel.
Apoptosis-inducing capacity of blood components.
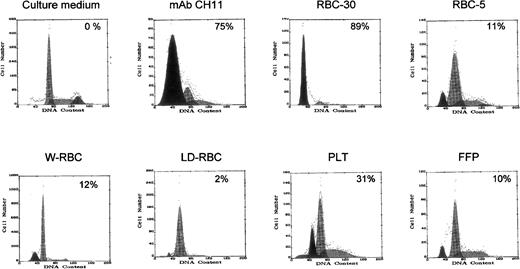
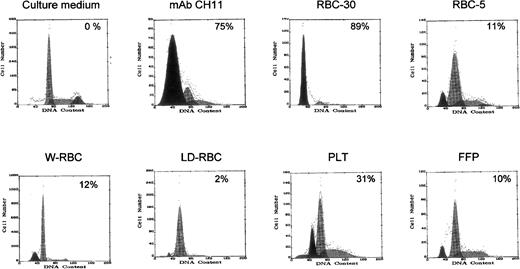
The functional capacity of sFasL molecules detected in blood components was then assessed. To this end, Jurkat cells, which are susceptible to FasL-induced apoptosis, were cultured in the presence of blood components’ supernatant, and apoptotic cells were detected by flow cytometry after permeabilization and PI staining. The supernatants of RBC-30 and PLT, which contain high amounts of sFasL molecules, induced apoptosis in 89% and 30% Jurkat cells, respectively, whereas the supernatants of the other blood components induced apoptosis in 2% to 12% of target cells (Fig 3). These results suggest that the apoptotic-inducing activity of blood components is proportionate to their content in sFasL and that sFasL molecules are functionally active.
Apoptosis-inducing capacity of blood components on Jurkat cells. Apoptotic cells with hypodiploid DNA are shown as a black peak and their percentage is indicated. Apoptosis induced by MoAb CH11 is shown as positive control.
Apoptosis-inducing capacity of blood components on Jurkat cells. Apoptotic cells with hypodiploid DNA are shown as a black peak and their percentage is indicated. Apoptosis induced by MoAb CH11 is shown as positive control.
Immunomodulatory activity of blood components.
The potential immunomodulatory activity of blood components was assessed by analyzing their effects on the lymphocyte response in MLR and on the cytotoxic activity of EBV-specific CTL. MLR can be considered as an in vitro model of allogeneic cell recognition in which CD8+ and CD4+ T lymphocytes proliferate in response to endogenous peptides presented in the context of allogeneic HLA class I molecules.69 CTL assay evaluates the lysis of target cells by CD8+ T lymphocytes, which recognize antigenic peptides presented in the context of autologous HLA class I molecules.70
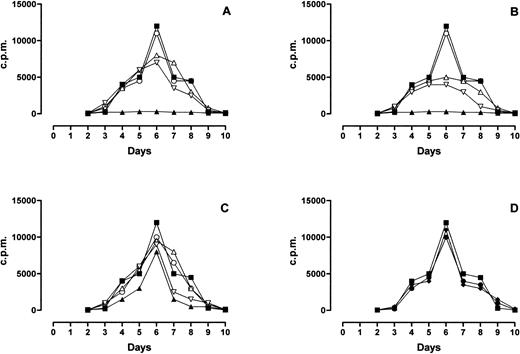
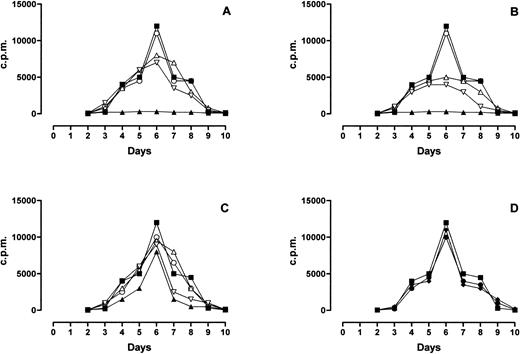
MLR response was completely inhibited by PLT and RBC-30 (Fig 4A and B, respectively) whereas it was partially inhibited by RBC-5 (Fig 4C) and unaffected by LD-RBC and FFP (Fig 4D). Of interest, the inhibitory effect of PLT, RBC-30, and RBC-5 on MLR response was reduced by the immunodepletion of either sHLA-I or sFasL and abolished by the depletion of both molecules (Fig 4A, B, and C). These findings indicate that sHLA-I and sFasL in blood components may negatively interfere with alloreactive T-cell responses.
Effect of blood components on MLR response. The behavior of MLR performed in absence (▪) or presence of PLT (▴, A), RBC-30 (▴, B), RBC-5 (▴, C), LD-RBC (•, D), and FFP (⧫, D) is shown. The behavior of MLR performed in presence of PLT, RBC-30, and RBC-5 immunodepleted of sHLA-I (▿), sFasL (▵), or sHLA-I and Fas-L (○) is also shown (A, B, C, respectively).
Effect of blood components on MLR response. The behavior of MLR performed in absence (▪) or presence of PLT (▴, A), RBC-30 (▴, B), RBC-5 (▴, C), LD-RBC (•, D), and FFP (⧫, D) is shown. The behavior of MLR performed in presence of PLT, RBC-30, and RBC-5 immunodepleted of sHLA-I (▿), sFasL (▵), or sHLA-I and Fas-L (○) is also shown (A, B, C, respectively).
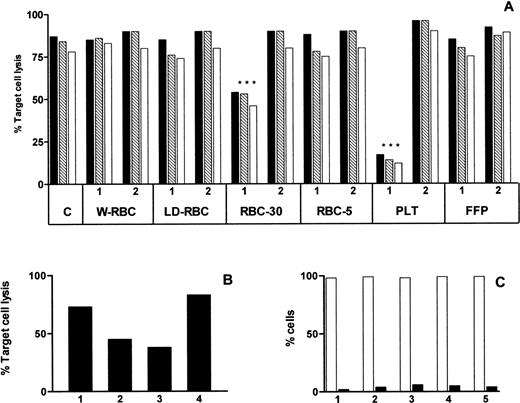
The cytotoxicity assay was inhibited by PLT and RBC-30 (P < .001) and the inhibitory effect was abolished by the depletion of sHLA-I molecules (Fig 5A). To better define the mechanism underlying the inhibition of cytotoxicity, effector or target cells were preincubated with RBC-30 supernatant for 1 hour at 37°C, washed, and used in the cytotoxic assay. The preincubation of effector cells, but not of responder cells, inhibited the cytotoxic assay suggesting that the activity of EBV-specific CTL was specifically affected by RBC-30 supernatant (Fig 5B). Moreover, to assess whether the reduction of CTL activity was attributable to their death and/or apoptosis, EBV-specific CD8+ Fas (CD95)+ T lymphocytes were incubated for 4 hours at 37°C with RBC-30 supernatant before and after immunodepletion of sHLA-I and/or sFasL molecules. Cell viability, evaluated after PI staining of nonpermeabilized cells, was greater than 95% and apoptosis, evaluated by annexin-V labeling, was less than 5% in all experimental conditions (Fig 5C). These findings strongly suggest that sHLA-I molecules in blood components may be responsible for the inhibition of EBV-specific CTL activity, whereas sFasL molecules do not seem to play a major role in this phenomenon.
Effect of blood components on CTL activity. Cytotoxic activity of EBV specific CTL evaluated by a 4-hour 51Cr release assay performed at 30:1 (▪), 10:1 (▧), and 3:1 (□) effector:target cell ratio in absence (C) and in presence of different blood components before (1) and after (2) immunodepletion of sHLA-I molecules (A). Cytotoxic activity of EBV specific CTL evaluated in absence (lane 1) and in presence (lane 2) of RBC-30 and after preincubation of either effector (lane 3) or target (lane 4) cells with RBC-30 (B). Percentage of viable (□) and apoptotic (▪) EBV-specific CTL after incubation for 1 hour with RPMI medium (1), RBC-30 (2), and RBC-30 immunodepleted of sHLA-I (3), sFasL (4), or sHLA-I and sFasL (5) (C).
Effect of blood components on CTL activity. Cytotoxic activity of EBV specific CTL evaluated by a 4-hour 51Cr release assay performed at 30:1 (▪), 10:1 (▧), and 3:1 (□) effector:target cell ratio in absence (C) and in presence of different blood components before (1) and after (2) immunodepletion of sHLA-I molecules (A). Cytotoxic activity of EBV specific CTL evaluated in absence (lane 1) and in presence (lane 2) of RBC-30 and after preincubation of either effector (lane 3) or target (lane 4) cells with RBC-30 (B). Percentage of viable (□) and apoptotic (▪) EBV-specific CTL after incubation for 1 hour with RPMI medium (1), RBC-30 (2), and RBC-30 immunodepleted of sHLA-I (3), sFasL (4), or sHLA-I and sFasL (5) (C).
DISCUSSION
The aim of the present study was to analyze the potential mechanisms underlying the immunomodulatory effects of ABT. The main findings can be summarized as follows: (1) elevated concentrations of sHLA-I and sFasL molecules are found in some blood components; (2) the level of sHLA-I and sFasL molecules is proportionate to the amount of leukocytes in each blood component and to the length of storage; and (3) sHLA-I and sFasL molecules detected in blood components are functional and play immunomodulatory effects in vitro.
Our findings are in agreement with most published literature data reporting that functional soluble molecules are detectable in blood components33-36,71-73 and that the immunomodulatory effect of ABT is linked to the presence of donor leukocytes.3,4,13,72,73 However, they are in disagreement with Dzik et al,74 who reported that HLA antigens in blood components are not shed from leukocytes during storage. Different experimental protocols and reagents used to measure sHLA-I antigens might explain this discrepancy. We propose that HLA class I and FasL antigens are released from residual donor blood leukocytes membrane during storage. This hypothesis is supported by several findings as follows. First, the amount of sHLA-I and sFasL molecules is proportionate to the number of donor leukocytes in blood components; second, it is related to the duration of refrigerated storage, which leads to the damage of leukocyte membrane and to cell death73; third, the immunochemical profile of HLA class I heavy chains in blood components suggests that they are mainly generated by shedding or proteolysis from membrane HLA class I antigens19; and last, the concentrations of sHLA-I and sFasL molecules are low in FFP, in W-RBC, and in prestorage leukodepleted RBC after a 30-day storage. The release of HLA class I antigens adhered on residual donor platelets membrane and RBC might also contribute to elevate the concentration of sHLA-I molecules in some blood components.62-68
Of interest, sHLA-I and sFasL in blood components are functional and may exert immunoregulatory effects in vitro as shown by the inhibition of MLR response and CTL activity and by the induction of apoptosis in Fas-expressing cells. MLR evaluates the proliferation of responder CD8+ and CD4+ T lymphocytes, which recognize endogenous peptides presented in the context of allogeneic HLA class I molecules,69 whereas CTL assay evaluates the lysis of target cells by antigen-specific CD8+ T lymphocytes, which recognize antigenic peptides presented in the context of autologous HLA class I molecules.70 Different mechanisms may underlie the inhibitory effects of sHLA-I and sFasL on MLR response and CTL activity. sHLA-I molecules may bind to CD8 molecules on alloreactive and antigen-specific CD8+ T lymphocytes,37-39,75 interfering with the recognition of and the response to peptides presented in the context of allogeneic and autologous HLA molecules. Moreover, according to previously published results, sHLA-I molecules may induce apoptosis in cytotoxic T lymphocytes42 and in activated autologous and allogeneic CD8+ T cells.76 Finally, functional sFasL molecules may trigger apoptosis in activated Fas-positive T lymphocytes, which are generated during the alloresponse.47 77
These findings strongly suggest that sHLA-I and sFasL molecules in blood components might contribute to some of the immunomodulatory effects of ABT in vivo. It has to be emphasized that the potential immunomodulatory effects induced by ABT in vivo are related not only with the kind, but also with the amount of blood components infused. This statement is consistent with the estimated average increase of 2.5% in postoperative infections per unit transfused7 and with the results of a randomized clinical trial reporting a significant decreased mortality following cardiac surgery in patients receiving less than 3 U of blood.13 In this regard, FFP, which contains a low level of soluble molecules, might also play an immunomodulatory role when infused in high amounts as required in particular clinical conditions. The immunomodulatory effects of ABT are also to be considered as a public health issue, as they are associated with increased costs owing to a major rate in cancer recurrence and postoperative infection.7 On the other hand, tolerance induced by pretransplant ABT is expected to reduce health cost due to a reduced rate of graft rejection and failure.
In conclusion, according to our data, the following potential immunomodulatory effects of ABT should be taken into account in clinical practice: (1) transfusion of fresh RBC containing viable donor leukocytes and a low amount of soluble molecules can cause alloimmunization but may also induce anergy or tolerance; (2) transfusion of nonleukodepleted or poststorage leukodepleted RBC containing dead donor leukocytes and a high amount of functional soluble molecules is more likely to induce strong immunosuppression; (3) transfusion of prestorage leukodepleted or W-RBC should prevent the immunosuppressive effect; and (4) transfusion of random-donor platelets, which contain viable donor leukocytes and elevated concentrations of soluble molecules, can induce alloimunization and immunosuppression as well. Therefore, blood components that can cause immunosuppression, anergy, or tolerance should be chosen in candidates for allografts to induce transplantation tolerance, whereas blood components lacking immunosuppressive effects should be preferred in patients undergoing surgery to reduce the risk of postoperative complications and in neoplastic patients to reduce the risk of recurrence.
Supported by grants from the Ministero della Sanità-Istituto Superiore di Sanità, IX Progetto di ricerche sull’AIDS (no. 9403-58), and from the MURST National Program “Meccanismi umorali e cellulari di modulazione dell’immunoflogosi” (no. 9706117821-001).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to F. Puppo, MD, Di.M.I.-Viale Benedetto XV, n.6, 16132 Genova, Italy; e-mail: metclin@unige.it.