Abstract
Heparin-binding epidermal growth factor–like growth factor (HB-EGF) is an EGF family member expressed by numerous cell types that binds to EGF receptor 1 (HER-1) or 4 (HER-4) inducing mitogenic and/or chemotactic activities. Membrane-bound HB-EGF retains growth activity and adhesion capabilities and the unique property of being the receptor for diphtheria toxin (DT). The interest in studying HB-EGF in acute leukemia stems from these mitogenic, chemotactic, and receptor functions. We analyzed the expression of HB-EGF in L428, Raji, Jurkat, Karpas 299, L540, 2C8, HL-60, U937, THP-1, ML-3, and K562 cell lines and in primary blasts from 12 acute myeloid leukemia (AML) cases, by reverse-transcriptase polymerase chain reaction (RT-PCR) and Northern blot and by the evaluation of sensitivity to DT. The release of functional HB-EGF was assessed by evaluation of its proliferative effects on the HB-EGF–sensitive Balb/c 3T3 cell line. HB-EGF was expressed by all myeloid and T, but not B (L428, Raji), lymphoid cell lines tested, as well as by the majority (8 of 12) of ex vivo AML blasts. Cell lines (except for the K562 cell line) and AML blasts expressing HB-EGF mRNA underwent apoptotic death following exposure to DT, thus demonstrating the presence of the HB-EGF molecule on their membrane. Leukemic cells also released a fully functional HB-EGF molecule that was mitogenic for the Balb/c 3T3 cell line. Factors relevant to the biology of leukemic growth, such as tumor necrosis factor- (TNF-), 1,25-(OH)2D3, and especially all-trans retinoic acid (ATRA), upregulated HB-EGF mRNA in HL-60 or ML-3 cells. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induced HB-EGF mRNA and acquisition of sensitivity to DT in one previously HB-EGF–negative leukemia case. Moreover, the U937 and Karpas 299 cell lines expressed HER-4 mRNA. This work shows that HB-EGF is a growth factor produced by primary leukemic cells and regulated by ATRA, 1,25-(OH)2D3, and GM-CSF.
HEPARIN-BINDING epidermal growth factor–like growth factor (HB-EGF) is a heavily glycosylated EGF family member of approximately 22 kD capable of binding to heparin. It was originally identified in human monocytes and U937 monocytic cell line–conditioned medium.1-3 Subsequently, HB-EGF expression was found in a wide range of cell types, including monocytes,1 CD4+ lymphocytes,4eosinophils,5 smooth muscle cells (SMC),6 and endothelial7 and normal or neoplastic epithelial cells.1,8 HB-EGF can be released from the cell membrane through proteolytic mechanisms,9 but multiple spliced mRNAs are likely to be produced and the cDNA corresponding to a short HB-EGF form lacking intramembrane and intracytoplasmic domains has been cloned.10 HB-EGF binds to EGF receptor 1 (HER-1) and 4 (HER-4)1,2,11 eliciting different biologic responses.11 It is a potent mitogenic and a chemotactic factor for fibroblasts4 and SMC,12 mitogenic factor for keratinocytes,8 and chemotactic factor for endothelial cells13 and astrocytes.14 Moreover, HB-EGF has been shown to participate in autocrine-paracrine loops, which are active in a number of epithelial neoplasia,15 and to be involved in stromal proliferation following decidualization.16
Membrane-bound HB-EGF retains growth activity and adhesion capabilities. Macrophages infiltrating atheromatous plaques actively induce SMC hyperplasia through HB-EGF.17 Rat blastocyst implantation has been reported to be associated with HB-EGF expression and adhesion activity.18 Finally, membrane-bound HB-EGF has the unique property of acting as the receptor for the diphtheria toxin (DT),19 a protein translation inhibitor capable of triggering apoptotic death.20 CD9 coexpression enhances the mitogenic activity of membrane-bound HB-EGF,17 as well as the sensitivity to DT.21
Attention has been devoted to the role of HB-EGF in reproductive biology,16,18 wound healing,22 atheromatous phenomena,17,23 angiogenesis,13 and epithelial neoplastic proliferative events.15 The production of HB-EGF by monocytes, CD4+ lymphocytes, and eosinophils suggests that it may also be produced by other normal and neoplastic hematologic cell types. In the present study, we analyzed the expression of HB-EGF in a panel of human hematologic cell lines derived from different lineages, and in primary blast cells from patients with acute myeloid leukemia (AML). The interest in studying HB-EGF in AML stems from its mitogenic, chemotactic, and receptor functions.
We found that HB-EGF was expressed by the human myeloid and T, but not B, lymphoid cell lines tested, as well as by ex vivo blast cells in a number of AML cases. Thus, HB-EGF is an additional growth factor produced by primary leukemic cells.
MATERIALS AND METHODS
Cell lines.
The following cell lines available in our laboratory were studied: B-cell lines—L428 (Hodgkin-derived)24 and Raji (non-Hodgkin’s lymphoma)24; T-cell lines—Jurkat (acute T-cell leukemia),25 Karpas 299 (anaplastic large-cell lymphoma),24 and L540 (Hodgkin-derived)24; natural killer (NK)-cell line—2C826; myeloid cell lines—HL-60 (AML)24, U937 (monocytic leukemia),24 THP-1 (acute monocytic leukemia),27 ML-3 (acute myelomonocytic leukemia),28 and K562 (blastic phase of chronic myeloid leukemia).24
Patients and isolation of leukemic cells.
The main clinical characteristics of the patients whose leukemic cells were investigated are listed in Table 1. All cases were untreated AML patients at diagnosis with a high percentage (≥90%) of blasts and minimal residual contamination by normal cells. The diagnosis of AML and its French-American-British (FAB) subtypes was based on clinical findings and on established morphologic, cytochemical, and cytofluorimetric parameters of peripheral blood and/or bone marrow cells. Viable leukemic cells from freshly heparinized peripheral blood or bone marrow were separated by centrifugation on Lymphoprep (Nycomed Pharma AS, Oslo, Norway), washed twice with phosphate-buffered saline (PBS), and either immediately used or stored frozen in liquid nitrogen. In all cases, frozen cell samples contained greater than 95% blasts.
Cell storage.
The cell lines and aliquots of ex vivo blasts were stored frozen in liquid nitrogen in 70% RPMI 1640 (Gibco-BRL Life Technologies, Paisley, UK), 20% dimethyl sulfoxide (DMSO), and 10% heat-inactivated fetal calf serum (FCS; Gibco-BRL). Frozen cells were thawed in 20% FCS/80% RPMI 1640, immediately centrifuged, and washed once with culture medium. Cell viability after thawing was always greater than 90%, as assessed by Trypan-blue staining. Freezing procedures did not modify the expression of HB-EGF.
DT sensitivity assay.
Highly purified DT at 10−11, 10−10, 10−9, and 10−8 mol/L concentration was added to 1 × 106 cells/mL (cell lines or ex vivo blasts) in a 96-well plate (Falcon, Lincoln Park, NJ) and incubated for 24 or 48 hours at 37°C in 5% CO2 in RPMI 1640 supplemented with 10% heat-inactivated FCS and penicillin (100 IU/mL)/streptomycin (100 μg/mL). The sensitivity to DT was evaluated as cytotoxic activity assessed by the modified 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Ten microliters per well of 5 mg/mL MTT solution was added and the plates were incubated at 37°C for 4 hours. After adding 100 μL/well of 0.04N HCl in isopropanol and thoroughly mixing, the plates were read (wavelengths: test, 570; reference, 630 nm) on an AutoReader III (Ortho Diagnostic Systems, Raritan, NJ).29 30 Resting cells were considered to be sensitive to DT when more than 50% of them were killed with a typical dose-response curve in the 10−11 to 10−8 mol/L DT concentration range during a 48-hour period. When the acquisition of sensitivity to DT was evaluated in stimulatory experiments on previously insensitive cells, the appearance of statistically significant cytotoxicity after stimulation was considered.
Assessment of apoptosis: DNA laddering and morphologic changes.
After 48 hours, 10−8 mol/L DT-incubated cells (1 × 106 cells/mL) and their controls were harvested and subdivided into three aliquots to evaluate apoptotic death. (a) One milliliter of cells was added with 200 μL of lysing buffer (2 mol/L NaCl, 50 mmol/L Tris/HCl, 10 mmol/L EDTA, pH 8), 50 μL of 20% sodium dodecyl sulfate (SDS), and 10 μL of proteinase K, and incubated for 45 minutes at 60°C and overnight at 37°C. Following the addition of 400 μL of 5 mol/L NaCl. the cells were centrifuged for 30 minutes at 3,500 rpm. Supernatant (SN) was transferred to a new tube, DNA precipitated for 3 hours with 5 mL ethanol 96% at −80°C, and centrifuged at 4,000 rpm for 30 minutes at 4°C. The dried pellet was resuspended in Tris-EDTA (TE)-RNAse (1 μg/μL) and incubated for 1 hour at 37°C. DNA was loaded onto 1% agarose gel and stained with ethidium bromide. (b) Cell shrinkage and acridine orange staining by phase contrast and fluorescence microscopy were evaluated. (c) Cells were analyzed with a FACScan cytometer (Becton Dickinson, San Jose, CA). A gate was depicted including the living population in the forward and side light scatter cytogram after acquiring 1 × 105 untreated cells, and the percentage of DT-treated cells falling outside the defined gate due to forward or side light scatter changes was calculated.
Transwell cultures and HB-EGF activity detection.
Transwell cultures of the Balb/c 3T3 cell line, proliferation of which is induced by HB-EGF,2 and of leukemic cells were performed to assess whether HB-EGF released by the ML-3 cell line and ex vivo AML blasts retained its mitogenic capability on Balb/c 3T3 cells. Confluent Balb/c 3T3 cells were trypsinized and resuspended at 5 × 104 cells/mL. Aliquots of 200 μL were plated in Dulbecco’s modified Eagle’s medium (DMEM)/10% FCS in 24-well plates (Falcon). The cells were incubated for 5 days after reaching confluence to deplete the media of growth factors. Transwells (12-mm diameter, 0.4-μm pore size; Costar, Cambridge, MA) were introduced into the wells of the 24-well plate containing Balb/c 3T3 cells and ML-3 or myeloid blast cells (5 × 105 cells/mL) were cocultured with or without 40 ng/mL phorbol myristate acetate (PMA) in order to favor HB-EGF release from the cell membrane.31,32A 100-μg/mL quantity of goat antihuman HB-EGF neutralizing antibody (R&D Systems, Minneapolis, MN) was used to block HB-EGF activity. Such activity was evaluated after 72 hours by measuring the number of Balb/c 3T3 cells using the modified MTT assay,29 30 after constructing a standard curve based on the absorbance/cell number ratio. The results were expressed as percentage increase in cell number as opposed to control.
Stimulation of cells in culture.
The cell lines and ex vivo blasts were cultured in RPMI medium supplemented with 10% FCS at a concentration of 1 × 106 cells/mL. When indicated, cells were treated with PMA (40 ng/mL), all-trans retinoic acid (ATRA, 10−5 mol/L), tumor necrosis factor-α (TNF-α, 100 U/mL), interferon-gamma (IFN-γ, 1,000 U/mL), granulocyte-macrophage colony-stimulating factor (GM-CSF, 100 ng/mL), 1α,25-(OH)2D3 (vitamin D3, 10−1 mol/L), DMSO (1.6%), or a combination of two of these factors. After culture for the indicated times, the cells were harvested and RNA was extracted, as previously reported,33 and analyzed for HB-EGF gene expression by reverse-transcriptase polymerase chain reaction (RT-PCR) or Northern blot.
RT-PCR analysis of HB-EGF and HER-4; plasmid insertion of HB-EGF cDNA.
Total cellular RNAs were isolated and 4 μg of RNA was reverse-transcribed (universal primers, 1.25 U of AMV RT [Gibco-BRL Life Technologies]) as previously described.33,34 cDNA was PCR-amplified using the following primers (Genenco, m-medical, Florence, Italy). (1) HB-EGF sense 5′-TGGTGCTGAAGCTCTTTCTGG-3′ and antisense 5′-GTGGGAA TTAGTCATGCCCAA-3′; these primers were designed to span exons 1 to 5 of the gene giving a fragment of 605 bp (complete form of HB-EGF cDNA)35 or a fragment of 605 + 94 bp (short form of HB-EGF cDNA).10 (2) HB-EGF antisense 5′-TCAAGTAACATCTTTCTGCCCAGC-′3 specific for a sequence on the 94-bp insert present in the short HB-EGF10 expected to give a 407-bp fragment when associated with the above-specified sense primer. (3) HER-4 sense 5′-AGATGGAGGTTTTGCTGCTGAA CA-3′ and antisense 5′-TTACACCACAGTATTCCGGTGTCT-3′ (726-bp fragment)36; (4) vimentin sense 5′-GCTCAGATTCAGGAACAGCAT-3′, and antisense 5′-TAAGGGCATCCACTTCACAGG-3′ (266-bp fragment). The cDNA was denatured for 5 minutes at 94°C before 35 runs in a thermal cycler (GeneAmp PCR System 2400; Perkin Elmer, Norwalk, CT) using 1.25 U of Taq polymerase (Perkin Elmer, Branchburg, NJ) in 50 μL (94°C 40 seconds, 57°C 40 seconds, 72°C 50 seconds) followed by 5 minutes at 72°C. PCR products were separated by electrophoresis on 1.5% agarose gel. The HB-EGF RT-PCR product was analyzed for theSmaI (Gibco-BRL) restriction site (which gave the expected HB-EGF fragments of 388 and 217 bp), and was sequenced (Sequenase 2.0 sequencing kit; USB, Cleveland, OH) as a plasmid insert (TA cloning kit; Invitrogen, San Diego, CA) from which the HB-EGF probe was generated for Northern blot analysis.
Northern blot analysis.
Total RNA preparation and Northern blot analysis (10 μg of RNA per lane) were performed as previously described.33 The RNA blots were hybridized with the 32P-labeled cDNA probe to HB-EGF obtained as specified above and with a 32P-labeled plasmid containing a cDNA probe to G6PDH or beta-actin.
Immunostaining.
Surface expression of HER-1 and CD9 was assessed by incubation of 1 × 106 cells with 10 μL fluorescein isothiocyanate (FITC)-conjugated anti–HER-1 monoclonal antibody (mAb) (Medac, Hamburg, Germany) and 10 μL phycoerythrin (PE)-conjugated anti-CD9 mAb (SBA, Birmingham, AL) for 30 minutes at 4°C. Cells were washed twice in PBS. Irrelevant FITC- or PE-conjugated IgG2b mAbs (Immunotech, Westbrook, MA) were used as a control. The analysis was performed with a FACScan cytometer (Becton Dickinson).
Statistics.
Student’s t-test, the Mann-Whitney U test, and Kruskall-Wallis analysis of variance (ANOVA) by ranks were used. When needed, a logarithmic transformation was performed. Differences were considered statistically significant when theP value was less than .05.
RESULTS
HB-EGF mRNA in cell lines and blasts.
We examined the presence of mRNA for HB-EGF in a panel of cell lines and in ex vivo AML blasts. The main findings are listed in Tables 1 and2 for patients and cell lines, respectively. The results of mRNA analyses in AML blasts and cell lines are detailed in Figs 1 and2. HB-EGF mRNA expression by cell lines was studied using RT-PCR. As shown in Fig 1, B-derived cell lines (L428, Raji) were negative, whereas the remaining cell lines (Jurkat, Karpas 299, L540, 2C8, HL-60, U937, THP-1, ML-3, and K562) shared a band of 605 bp corresponding to cDNA encoding the complete form of HB-EGF. At the resolution level adopted, only one clear-cut 605-bp band for HB-EGF was amplified, corresponding to the complete HB-EGF molecule. In no cell lines were we able to reamplify an HB-EGF cDNA corresponding to the short form of the molecule.10 Restriction and base sequence analysis of the PCR product confirmed that it was amplified from HB-EGF mRNA. The 605-bp cDNA obtained from PCR was used as a probe for HB-EGF in the Northern blot analysis of the leukemic cells from patients. HB-EGF transcript, evaluated by Northern blot, was present in 8 of 12 cases with a distribution apparently independent of FAB subtype (Table 1 and Fig 2).
(A) RT-PCR analysis of HB-EGF mRNA expression in a number of leukemic, Hodgkin-, and NK-derived cell lines. HB-EGF: 605 bp; vimentin: 266 bp; marker: 100-bp DNA ladder. (B) 48-hour DT dose-sensitivity curve as evaluated by the MTT method. Only HB-EGF mRNA-positive cell lines were sensitive to DT (ie, >50% death in the range of tested concentrations), indicating membrane expression of the HB-EGF molecule.
(A) RT-PCR analysis of HB-EGF mRNA expression in a number of leukemic, Hodgkin-, and NK-derived cell lines. HB-EGF: 605 bp; vimentin: 266 bp; marker: 100-bp DNA ladder. (B) 48-hour DT dose-sensitivity curve as evaluated by the MTT method. Only HB-EGF mRNA-positive cell lines were sensitive to DT (ie, >50% death in the range of tested concentrations), indicating membrane expression of the HB-EGF molecule.
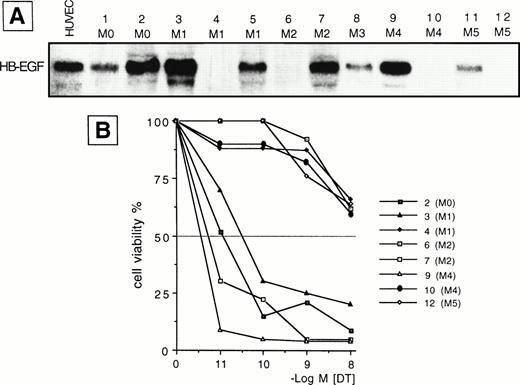
(A) Northern blot analysis of HB-EGF mRNA expression in different subtypes of ex vivo AML blasts. Human umbilical vein endothelial cells (HUVEC) were used as positive control. (B) 48-hour DT dose-sensitivity curve for 8 representative cases (2, 3, 4, 6, 7, 9, 10, 12 in A), as evaluated by the MTT method. Only HB-EGF mRNA-positive leukemic cells (cases 2, 3, 7, 9) were sensitive to DT, indicating membrane expression of the HB-EGF molecule.
(A) Northern blot analysis of HB-EGF mRNA expression in different subtypes of ex vivo AML blasts. Human umbilical vein endothelial cells (HUVEC) were used as positive control. (B) 48-hour DT dose-sensitivity curve for 8 representative cases (2, 3, 4, 6, 7, 9, 10, 12 in A), as evaluated by the MTT method. Only HB-EGF mRNA-positive leukemic cells (cases 2, 3, 7, 9) were sensitive to DT, indicating membrane expression of the HB-EGF molecule.
Membrane-bound HB-EGF assessed by sensitivity to DT.
To evaluate whether the HB-EGF molecule was expressed on cells positive for HB-EGF mRNA, we tested their sensitivity to DT. We found that all T-cell lines and patient blasts positive for HB-EGF mRNA were sensitive to DT-induced cytolysis, as evaluated at 24 and 48 hours by a dose-response curve comprising 10−11 to 10−8 mol/L concentrations of DT. By contrast, B-cell lines and four patients negative for HB-EGF mRNA expression were insensitive to DT (Tables 1 and 2, Figs 1 and 2). The sole exception was the myeloid cell line K562, which, though positive for HB-EGF mRNA, was fairly resistant to DT (Fig 1). DT-related apoptosis was evaluated by different techniques. Microscopy and flow cytometry analysis showed morphologic changes usually associated with apoptotic death in the majority of DT-sensitive cases. In the same cases, DNA laddering was documented. No evidence of apoptosis in DT-insensitive cases was observed.
Expression of HER-1, HER-4, and CD9.
Since HB-EGF binds to HER-1 or HER-4, we evaluated whether these receptors were expressed by cell lines and ex vivo AML cells. We failed to detect HER-1 expression by such cells (Tables 1 and 2). HER-4 mRNA was detectable in U937 and Karpas 299 cell lines in basal conditions, whereas a very low expression was found in ML-3 and HL-60 (Table 2). CD9, a coreceptor of membrane-bound HB-EGF,3,17 21 was present on a minority of cell lines (Table 2) and ex vivo AML cells (Table 1).
Regulation of HB-EGF expression in leukemic cells.
We analyzed whether the spontaneous expression of HB-EGF mRNA in HL-60 and ML-3 cell lines could be modified by exogenous agents. Cell lines were stimulated with a panel of molecules, including DMSO, PMA, ATRA, IFN-γ, 1α,25-(OH)2D3, and TNF-α, known to induce biologic effects on HL-60 or ML-3 cells, such as proliferation or differentiation. PMA, DMSO, and, more interestingly, TNF-α, 1α,25-(OH)2D3, and especially ATRA and costimulation with TNF-α and ATRA induced an increase in transcripts for HB-EGF (Figs 3 and4). However, TNF-α antagonized the effects of ATRA in ML-3 cells (Fig 4). When we used GM-CSF to stimulate AML blasts from a patient shown to be negative for HB-EGF mRNA and insensitive to DT (patient no. 6 in Table 1), we induced both expression of HB-EGF mRNA and sensitivity to DT (GM-CSF–treatedv untreated blasts, P = .002) (Fig5).
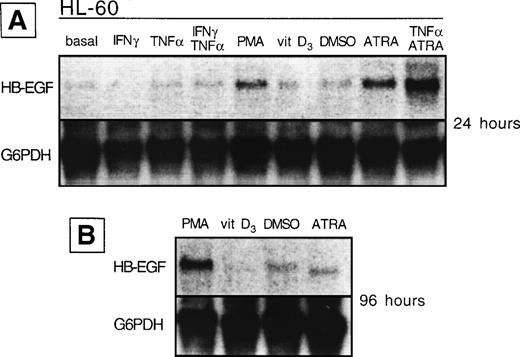
HB-EGF mRNA expression by HL-60 cell line in basal conditions and after different stimuli. (A) After 24 hours, PMA, ATRA, and ATRA + TNF- were the strongest inducers of HB-EGF mRNA. (B) After 96 hours, PMA-induced band was more evident than at 24 hours.
HB-EGF mRNA expression by HL-60 cell line in basal conditions and after different stimuli. (A) After 24 hours, PMA, ATRA, and ATRA + TNF- were the strongest inducers of HB-EGF mRNA. (B) After 96 hours, PMA-induced band was more evident than at 24 hours.
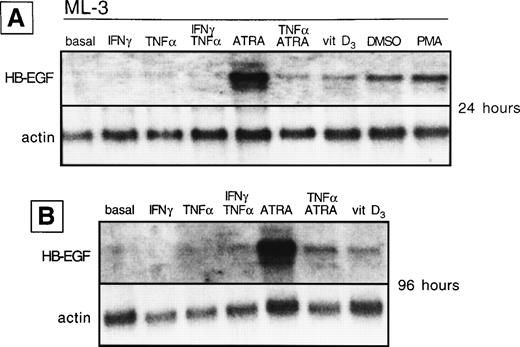
HB-EGF mRNA expression by ML-3 cell line in basal conditions and after different stimuli. (A) After 24 hours, HB-EGF mRNA was strongly induced by ATRA, 1,25-(OH)2D3(vitamin D3), DMSO, and PMA. (B) After 96 hours, ATRA- and 1,25-(OH)2D3-induced bands were still present.
HB-EGF mRNA expression by ML-3 cell line in basal conditions and after different stimuli. (A) After 24 hours, HB-EGF mRNA was strongly induced by ATRA, 1,25-(OH)2D3(vitamin D3), DMSO, and PMA. (B) After 96 hours, ATRA- and 1,25-(OH)2D3-induced bands were still present.
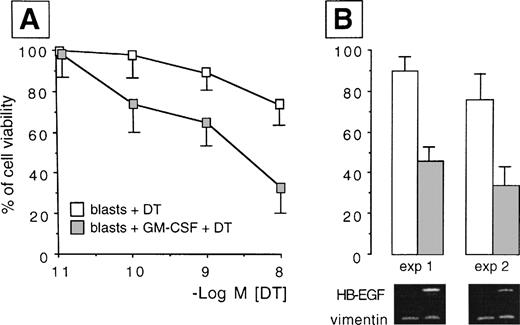
HB-EGF induction by GM-CSF treatment in AML blasts. Resting blasts were negative for HB-EGF mRNA and insensitive to DT, but after GM-CSF they acquired HB-EGF transcripts and sensitivity to DT-induced cytolysis (P = .002). (A) Dose-response curves testing the different sensitivity to DT in ex vivo myeloid blasts from case 6 in Table 1 before and after treatment with GM-CSF. Results were expressed as percentage of controls and represented the mean ± SD of 5 experiments. (B) The percentage of cell viability at the 10−8 mol/L DT concentration in two representative experiments is associated with the corresponding pattern of RT-PCR analysis, showing the induction of the transcripts for HB-EGF after exposure to GM-CSF (HB-EGF: 605 bp; vimentin: 266 bp).
HB-EGF induction by GM-CSF treatment in AML blasts. Resting blasts were negative for HB-EGF mRNA and insensitive to DT, but after GM-CSF they acquired HB-EGF transcripts and sensitivity to DT-induced cytolysis (P = .002). (A) Dose-response curves testing the different sensitivity to DT in ex vivo myeloid blasts from case 6 in Table 1 before and after treatment with GM-CSF. Results were expressed as percentage of controls and represented the mean ± SD of 5 experiments. (B) The percentage of cell viability at the 10−8 mol/L DT concentration in two representative experiments is associated with the corresponding pattern of RT-PCR analysis, showing the induction of the transcripts for HB-EGF after exposure to GM-CSF (HB-EGF: 605 bp; vimentin: 266 bp).
HB-EGF proliferation assay.
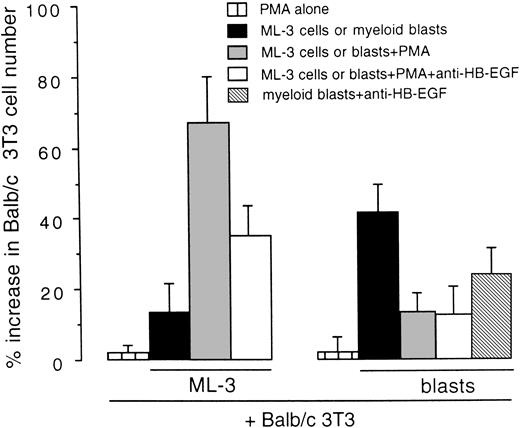
We examined whether HB-EGF expressed by cell lines and leukemic cells was released as a functional molecule mitogenic for Balb/c 3T3 cells in coculture tests. As shown in Fig 6, the stimulation of HB-EGF–positive ML-3 cells with PMA, which increases HB-EGF expression and release from the cell membrane,31,32induced a 1.67-fold increase in Balb/c 3T3 cell number as compared with controls (P < .01). In addition, antihuman HB-EGF antibody inhibited this proliferative effect, as expected.8 37 Ex vivo AML blasts presented a different pattern, characterized by a higher HB-EGF proliferative effect on Balb/c 3T3 in basal conditions than ML-3 cells, whereas PMA had no effect on HB-EGF activity (Fig 6).
Mitogenic effect on Balb/c 3T3 cells of the HB-EGF molecule released by the ML-3 cell line and by ex vivo myeloid blasts. ML-3 and AML blasts were stimulated with PMA to induce both new transcripts and release of HB-EGF (see text). Results were expressed as percentage increase in Balb/c 3T3 cell number versus untreated controls ± SD (P < .01). Each test was replicated five times.
Mitogenic effect on Balb/c 3T3 cells of the HB-EGF molecule released by the ML-3 cell line and by ex vivo myeloid blasts. ML-3 and AML blasts were stimulated with PMA to induce both new transcripts and release of HB-EGF (see text). Results were expressed as percentage increase in Balb/c 3T3 cell number versus untreated controls ± SD (P < .01). Each test was replicated five times.
DISCUSSION
In this study, we found that all human hematologic cell lines investigated, except for the B-derived ones, and blasts from a substantial proportion of AML cases produce, bear on their membrane, and release a fully functional HB-EGF molecule. This evidence is based on the following : (1) the demonstration of HB-EGF mRNA expression, (2) the cytolytic effect of DT exposure exerted solely on HB-EGF mRNA-expressing cells, and (3) the proliferative effect of HB-EGF released by ML-3 cells or by AML blasts on the Balb/c 3T3 cell line. We also found that factors relevant to the biology of leukemic growth modified the expression of HB-EGF mRNA. TNF-α, 1α,25-(OH)2D3, and especially ATRA increased the expression of HB-EGF mRNA in HL-60 and ML-3 cells. GM-CSF induced HB-EGF mRNA and acquisition of sensitivity to DT in one previously HB-EGF–negative AML case. At variance with another report,10 we failed to demonstrate the expression of the spliced short HB-EGF mRNA in the Raji and Daudi (the latter was studied only for this purpose) cell lines, at least at the agarose gel electrophoresis level. Finally, the U937 and Karpas 299 cell lines expressed HER-4 mRNA.
HB-EGF has been shown to play a role in the context of proliferative and chemotactic phenomena related to the inflammatory response.3 More recently, a number of reports have demonstrated that HB-EGF is involved in epithelial neoplastic proliferation15 and may stimulate angiogenesis through the induction of vascular-endothelial growth factor (VEGF) in vascular SMC.13 In such contexts, HB-EGF plays a relevant part as a cytokine whose activity is mediated through paracrine, cell-to-cell, or autocrine interactions.
The role, if any, played by HB-EGF in leukemic expansions is less intuitive. The lack of HER-1 on AML cells suggests that this cytokine is not directly involved in leukemic proliferation. However, it has been shown that THP-1 cell line can be induced to express HER-4 upon adequate stimulation36 and we found HER-4 mRNA at least in resting U937 and Karpas 299 cell lines. Leukemia-derived HB-EGF may be active on bystander cells via paracrine or juxtacrine mechanisms either directly or through induction of secondary factors, including VEGF, which is expressed by AML cells38 and, though in a different context, has been shown to be induced by HB-EGF.13 Transwell costimulatory experiments demonstrated that HB-EGF was released by both the ML-3 cell line and ex vivo AML blasts inducing the Balb/c 3T3 cell line to proliferate. Ex vivo blasts released more HB-EGF as compared with ML-3 cells in basal conditions, but PMA was less effective on AML blasts, possibly due to a direct cytotoxic effect (Fig 6). Interestingly, factors involved in differentiation or proliferation of acute leukemia, such as ATRA, 1α,25-(OH)2D3, TNF-α, and GM-CSF, could modify the expression of HB-EGF mRNA in leukemic cell lines. In HB-EGF promoter, putative binding sites for NFkB and AP1 sites have been identified.3,35 In addition, it has been shown that HB-EGF could be induced through Ras pathway activation.3,39,40Actually, TNF-α has been reported to mobilize NFkB41; the receptors for vitamins A and D (including 1α,25-(OH)2D3) recognize common response elements containing the AP1 site42; by binding to the beta subunit of its receptor, GM-CSF activates Ras and Raf-1 and the MAP kinase pathway.43 Thus, it is likely that TNF-α, ATRA, 1α,25-(OH)2D3, and GM-CSF had the HB-EGF gene as a downstream target. Whereas HB-EGF induction by TNF-α has been reported by others,7 35 to the best of our knowledge the role of ATRA, 1α,25-(OH)2D3, and GM-CSF has yet to be characterized.
These findings may lead to a better understanding of a number of biologic aspects of leukemic blasts, including their outgrowth capability in the context of skin or mucosal tissues or the induction of fibrosis, which characterize some FAB subtypes33 34 and are believed to play a part in limiting the effectiveness of the therapy. However, it is not clear how and to what extent AML cells may be influenced by HB-EGF or by factors similar to, or induced by, HB-EGF.
The presence of HB-EGF protein on the membrane of leukemic cells was also assessed by evaluation of the degree of cytolysis induced by DT.20 In general, DT cytolysis was associated with DNA laddering. We found a clear-cut correlation between the expression of HB-EGF mRNA and the susceptibility to death from DT in both cell lines and ex vivo AML cells. Worthy of note is the fact that the K562 cell line was the only case in which HB-EGF mRNA encoding for the complete molecule was expressed, but DT did not induce cytotoxicity. Chang et al have reported that actually DT is internalized by K562 cells in which DT severely reduces protein synthesis.20 The lack of a cytolytic effect in this cell line might be related to the expression of antiapoptotic factors, such as the chimeric protein BCR/ABL being translated as a consequence of t(9;22), which is known to confer resistance to a variety of apoptotic signals.44,45 Hence, our data suggest, albeit indirectly, that DT internalization after binding to HB-EGF and inhibition of protein translation do not imply straightforward apoptotic death in leukemic cell lines.20 46 DT receptor density, coreceptors, efficiency of internalization, and, in general, the status of pathways allowing transduction of apoptotic signals (ie, overexpression of antiapoptotic factors) should be considered in a given cell type. Yet, on the whole, apoptotic mechanisms activated in response to DT were extremely efficient in the HB-EGF–positive leukemic cells tested.
In summary, HB-EGF was expressed in the majority of myeloid blasts. It was inducible in HB-EGF–negative leukemic cells, at least by TNF-α, GM-CSF, ATRA, and 1α,25-(OH)2D3. It was released by leukemic blasts in a fully functional form mitogenic for the Balb/c 3T3 cell line. The expression of at least HER-4 by a number of leukemic cell lines adds significance to these findings. Finally, membrane-bound HB-EGF was found to be a functional DT receptor efficiently mediating DT-induced cytotoxicity in ex vivo AML blasts. Since DT per se is not practical for therapeutic purposes, due to the wide range of cell types expressing HB-EGF, DT fusion proteins binding to GM-CSF and interleukin-3 (IL-3) receptors have been generated and reported to be effective and more selective in targeting leukemic cells.46-50
Supported by grants from Associazione Italiana per la Ricerca sul Cancro (AIRC, Milano), Progetto Sanità 96/97, Fondazione Cariverona (Verona), and Italy-USA Program on “Therapy of Tumors” (’96-’98).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Fabrizio Vinante, MD, Cattedra di Ematologia, Ospedale Policlinico, 37134 Verona, Italy.