To the Editor:
Ganju et al1 reported in BLOOD that the ectoenzyme CD10 (neutral endopeptidase 24.11, CALLA) expressed on the surface of lymphoid progenitors, mature granulocytes, and several nonhematopoietic cell types is associated with the protein tyrosine kinase (PTK) Lyn and with at least two other unidentified 40-kD and 75/80-kD phosphoproteins. These CD10-associated proteins (Lyn, p40, and p75/80) become tyrosine phosphorylated under the conditions of in vitro kinase assay on CD10 immunoprecipitates obtained from detergent lysates of the pre-B–cell line Nalm-6. Ganju et al1suggest that coassociation between CD10 and a tyrosine phosphoprotein complex may link CD10 with so far poorly defined peptide-mediated signal transduction pathways and note that the mechanism of CD10 association with the Lyn-containing phosphoprotein complex is unclear because the CD10 cytoplasmic domain lacks characteristic structural motifs (SH2 and SH3 domains or their ligands) that could be responsible for such association. We offer here an explanation for these associations.
We observed very similar patterns of tyrosine phosphorylated protein zones (major 80 kD [so far unidentified], 55 kD [corresponding to autophosphorylated Lyn], and weak unidentified 40 kD) in vitro-kinase reactions of CD10 immunoprecipitates obtained from detergent lysates of pre-B–cell (Nalm-6) or B-cell (Ramos, Raji) lines. This pattern was identical to that obtained after in vitro kinase reactions of materials immunoprecipitated by monoclonal antibodies to some glycosphingolipids (eg, CDw75) and glycosylphosphatidylinositol (GPI)-anchored proteins (CD48, CD55; Fig 1A), indicating that all these molecules may be components of a common large complex. Indeed, preclearing of the Raji cell lysate by an anti-CD10 immunosorbent led to a substantial decrease of the kinase activity associated with CD48, CD55, and CDw75 immunoprecipitates (not shown). Moreover, a GPI-anchored protein, CD48, and the PTK Lyn could be readily demonstrated in the anti-CD10 immunoprecipitates (Fig2). These multicomponent complexes were very large, as judged by gel chromatography on Sepharose 4B (Fig 1B), and buoyant, as judged by sucrose gradient ultracentrifugation (Fig1C). The presence of a fraction of CD10 in these large complexes (together with Lyn and GPI-anchored molecules) could be demonstrated by Western blotting (Fig 3). Therefore, we conclude that a fraction of CD10 is a component of large, detergent-resistant “GPI-microdomains”2 (also called glycolipid rafts,3 glycolipid-enriched membranes [GEM],4 or detergent-insoluble glycosphingolipid complexes [DIG]3), membrane specializations rich in cholesterol, glycosphingolipids, GPI-anchored proteins, PTKs of the Src family, and some other signaling molecules but devoid of most transmembrane proteins.2-4 These membrane microdomains appear to be involved in signaling via GPI-anchored proteins5 and glycolipids,6 but may play an essential role also in signaling via Fc-receptors7 and TCR.8 CD10 appears to be one of very few transmembrane proteins present in these microdomains. It is not clear what determines whether a transmembrane protein can be a component of the GPI-microdomains, but posttranslational modification by palmitylation is a likely possibility; actually, a slight difference in electrophoretic mobility of CD10 present in the buoyant microdomains versus that excluded from them (Fig 3) is compatible with such a modification.
(A) The results of in vitro kinase assays on the indicated immunoprecipitates obtained from NP40-solubilized Raji cells (analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE] and autoradiography). Note major in vitro phosphorylated zones of 75-80 kD and 55-60 kD similar to those observed by Ganju et al.1 The monoclonal antibodies used were MEM-57 (CD3; negative control), MEM-78 (CD10), MEM-102 (CD48), MEM-118 (CD55), and HH2 (CDw75). (B) The results of in vitro kinase assays on CD10 immunoprecipitates obtained from the detergent lysate of Raji cells size fractionated on Sepharose 4B column (fractions 3-9; L is the unfractionated lysate). Fractions 3 and 4 represent void volume where very large complexes or particles elute; elution volume of size standards IgM and IgG was in fractions 6 and 8, respectively. (C) Same as in (B) but the lysate was fractionated by sucrose density gradient ultracentrifugation (gradient from 40% to 5% sucrose). Fraction 4 (maximum of CD10-associated kinase activity) corresponds to buoyant density of 1.05 to 1.09 g/mL. An identical profile of separation was observed in the case of CD48 and CD55 immunoprecipitates (not shown).
(A) The results of in vitro kinase assays on the indicated immunoprecipitates obtained from NP40-solubilized Raji cells (analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE] and autoradiography). Note major in vitro phosphorylated zones of 75-80 kD and 55-60 kD similar to those observed by Ganju et al.1 The monoclonal antibodies used were MEM-57 (CD3; negative control), MEM-78 (CD10), MEM-102 (CD48), MEM-118 (CD55), and HH2 (CDw75). (B) The results of in vitro kinase assays on CD10 immunoprecipitates obtained from the detergent lysate of Raji cells size fractionated on Sepharose 4B column (fractions 3-9; L is the unfractionated lysate). Fractions 3 and 4 represent void volume where very large complexes or particles elute; elution volume of size standards IgM and IgG was in fractions 6 and 8, respectively. (C) Same as in (B) but the lysate was fractionated by sucrose density gradient ultracentrifugation (gradient from 40% to 5% sucrose). Fraction 4 (maximum of CD10-associated kinase activity) corresponds to buoyant density of 1.05 to 1.09 g/mL. An identical profile of separation was observed in the case of CD48 and CD55 immunoprecipitates (not shown).
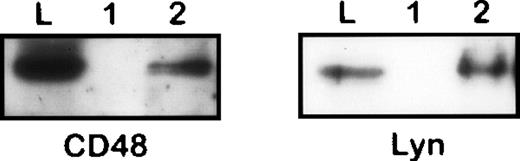
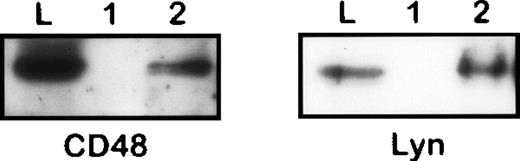
Coisolation of CD48 and Lyn with CD10 from 1% NP40 lysate of Nalm-6 cells. The immunoprecipitates obtained from an irrelevant control (1) or anti-CD10 (2) immunosorbents and the original lysate were subjected to SDS-PAGE/Western blotting and stained by antibodies to CD48 and Lyn, respectively.
Coisolation of CD48 and Lyn with CD10 from 1% NP40 lysate of Nalm-6 cells. The immunoprecipitates obtained from an irrelevant control (1) or anti-CD10 (2) immunosorbents and the original lysate were subjected to SDS-PAGE/Western blotting and stained by antibodies to CD48 and Lyn, respectively.
Western blotting analysis of the fractions obtained by sucrose gradient ultracentrifugation of Raji cell detergent lysate. The blots were immunostained by Abs to CD10, Lyn, and CD45 (a control protein that is not a component of the large buoyant complexes). (a) The buoyant fraction from the top of the gradient; (b) the bottom fraction. The buoyant fraction contained also large amounts of GPI-anchored proteins (CD48, CD55, and CD59; not shown).
Western blotting analysis of the fractions obtained by sucrose gradient ultracentrifugation of Raji cell detergent lysate. The blots were immunostained by Abs to CD10, Lyn, and CD45 (a control protein that is not a component of the large buoyant complexes). (a) The buoyant fraction from the top of the gradient; (b) the bottom fraction. The buoyant fraction contained also large amounts of GPI-anchored proteins (CD48, CD55, and CD59; not shown).
It can be speculated that the fraction of CD10 incorporated in the GPI-microdomains and thus potentially capable of making use of the associated signaling molecules may have unique functions in addition to the well-known ectoenzyme activity.
ACKNOWLEDGMENT
This work was supported by Grant No. 310/96/0673 from the Grant Agency of the Czech Republic. V.H. is supported by an International Research Scholar’s award from the Howard Hughes Medical Institute.

![Fig. 1. (A) The results of in vitro kinase assays on the indicated immunoprecipitates obtained from NP40-solubilized Raji cells (analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE] and autoradiography). Note major in vitro phosphorylated zones of 75-80 kD and 55-60 kD similar to those observed by Ganju et al.1 The monoclonal antibodies used were MEM-57 (CD3; negative control), MEM-78 (CD10), MEM-102 (CD48), MEM-118 (CD55), and HH2 (CDw75). (B) The results of in vitro kinase assays on CD10 immunoprecipitates obtained from the detergent lysate of Raji cells size fractionated on Sepharose 4B column (fractions 3-9; L is the unfractionated lysate). Fractions 3 and 4 represent void volume where very large complexes or particles elute; elution volume of size standards IgM and IgG was in fractions 6 and 8, respectively. (C) Same as in (B) but the lysate was fractionated by sucrose density gradient ultracentrifugation (gradient from 40% to 5% sucrose). Fraction 4 (maximum of CD10-associated kinase activity) corresponds to buoyant density of 1.05 to 1.09 g/mL. An identical profile of separation was observed in the case of CD48 and CD55 immunoprecipitates (not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/4/10.1182_blood.v93.4.1437a/5/m_blod40436004w.jpeg?Expires=1766114861&Signature=qf6C3bS8bL27HSefHJQ1H2G9VRObK1IacrZye9wxWyI3AK4H2-WdqVp~lTW7fS3qIHf1WqAOzpKa0FWNWla3MSblbALvoxBYAzFltVuQn8QwP4bmmty7BHZ4u165tcPiIxsvlbBYhX6dTxckzJMaNXzEHwvikLjwr0mpgHjkAdnIQEh5FadJ0~mRxgfzTOqyCekFKn~xw4Q0BmKNIb9i9wMBzVBEFbksthDPgXkIpO0chjvVGiT6yWB83oFQoz3dEsS2z~Tv3bLwfUwXooU~TP-FabgHPiRvibx-e1Tx4DnJBt2Hpw5ntIGNV5so189Ztc62y5iwyD9iHVsX9Kf3KA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 1. (A) The results of in vitro kinase assays on the indicated immunoprecipitates obtained from NP40-solubilized Raji cells (analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis [SDS-PAGE] and autoradiography). Note major in vitro phosphorylated zones of 75-80 kD and 55-60 kD similar to those observed by Ganju et al.1 The monoclonal antibodies used were MEM-57 (CD3; negative control), MEM-78 (CD10), MEM-102 (CD48), MEM-118 (CD55), and HH2 (CDw75). (B) The results of in vitro kinase assays on CD10 immunoprecipitates obtained from the detergent lysate of Raji cells size fractionated on Sepharose 4B column (fractions 3-9; L is the unfractionated lysate). Fractions 3 and 4 represent void volume where very large complexes or particles elute; elution volume of size standards IgM and IgG was in fractions 6 and 8, respectively. (C) Same as in (B) but the lysate was fractionated by sucrose density gradient ultracentrifugation (gradient from 40% to 5% sucrose). Fraction 4 (maximum of CD10-associated kinase activity) corresponds to buoyant density of 1.05 to 1.09 g/mL. An identical profile of separation was observed in the case of CD48 and CD55 immunoprecipitates (not shown).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/4/10.1182_blood.v93.4.1437a/5/m_blod40436004w.jpeg?Expires=1766114862&Signature=UdpeJJ~FFn16Zsi1Y4baTW9bb1VeUjZMpC3m4Del3hmauXzM49S9grtGqCboPPvMLBffKXhm91RmkbtbKmiZJ2t6k0CTs38w4Gfb~MmXu3lx9bzIRZilKXCbk-Hdlu2w96y3qSOvS8h6z-FZLTQy-ulrePPXzXTcBprY5sCk5jhNumV2RVYKZ3cZC6ulVctPxAJFa6ke~RFd3SMIsQFmTSBoQA8RrKqYDbFYg~poDG~ZcvdD0r9uutNQb6Ld7OSqYdd01sOhmHxyZ-zDUwChJXknLSoFUbb3HaBkVypB-YocYj2twJWZc5yXLijTq5NvnLj9ArAD3BmLk-RiQHlY7A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)